Abstract
The aim of this study was to undertake a detailed analysis of the structure of the inter and intra-lamellar regions of the annulus fibrosus. A total of 30 newborn to 6 year-old lumbar ovine intervertebral discs (IVDs) were fixed and decalcified en-bloc to avoid differential swelling artifacts during processing and vertical mid-sagittal, and horizontal 4 μm sections were cut. These were stained with toluidine blue to visualise anionic proteoglycan (PG) species, H & E for cellular morphology and picro-sirius red (viewed under polarized light) to examine collagenous organization. Immunolocalisations were also undertaken using anti-PG core-protein and glycosaminoglycan (GAG) side chain antibodies to native chondroitin sulphate (CS), Δ C-4-S and C-6-S unsaturated stubs generated by chondroitinase ABC digestion of CS, keratan sulphate (KS), and with antibodies to type I, II, VI, IX and X collagens. Trans-lamellar cross bridges (TLCBs), discontinuities in annular lamellae’s which provide transverse interconnections, stained prominently with toluidine blue in the adult IVDs but less so in the newborn IVDs. In adult discs TLCBs were evident in both the posterior and anterior AF where they extended from the outermost annular lamellae almost to the transitional zone extending across as many as eight lamellar layers displaying a characteristic circuitous, meandering, serpentine type course. There were significantly fewer TLCBs in 2 week-old compared with skeletally mature sheep and there was a further increase from 2 to 6 years. Immunolocalisation of perlecan delineated blood vessels in the TLBs of the newborn but not adult IVDs extending into the mid AF. In contrast adult but not 2 week-old TLCBs were immunpositive for C-4-S, C-6-S, KS, aggrecan, versican and type VI collagen. The change in number and matrix components of the trans-lamellar cross bridges with skeletal maturity and ageing suggest that they represent an adaptation to the complex biomechanical forces occurring in the annulus fibrosus.
Keywords: Annular cross bridges, Type VI collagen, Aggrecan, Versican
Introduction
The intervertebral disc (IVD) is the largest predominantly avascular, alymphatic and aneural structure in the human body. It is composed of several connective tissues of dissimilar composition and structure and it is the dynamic interplay between these in the biocomposite IVD which equips it with its unique biomechanical properties and ability to act as a viscoelastic and hydrodynamic weight bearing cushion absorbing and dissipating axial spinal compressive loads [5, 7, 16, 22, 40, 49, 50]. The outermost region of the IVD, the annulus fibrosus (AF) is composed of collagenous lamellae which convey tensile properties and along with the superior and inferior hyaline-like cartilaginous endplates, prevent the inner proteoglycan rich region of the IVD, the nucleus pulposus (NP) being extruded anteriorly or posteriorly during axial and torsional spinal loading.
The type I and II collagen fibre networks are arranged at a 60° angle relative to one another in adjacent lamellae [16–19, 22, 40, 49, 50]. Elastic fibres traverse between lamellae where they have a role to play in the recoil properties of these tissues aiding lamellae collagen crimp patterns to return to their pre-loaded dimensions and orientations [4, 6, 24–26, 43, 54–57]. These fibre networks couple adjacent lamellae together such that they work co-operatively during dynamic loading and prevent separation of lamellae during torsional compressive loading. A further adaptation in the AF is the recently described translamellar cross-bridge [41, 42]. This is a discontinuity in the lamellae which traverses and interconnects several annular lamellae. Little is known about the structure and composition of these annular structures. Differential interference contrast microscopy demonstrates that these cross bridges have a high fibre component and both elastin and fibrillin-1 have recently been immunolocalized to these structures [56]. In the present study we have investigated the morphology and the collagen and proteoglycan composition of the translamellar cross-bridges in normal ovine discs of different ages to provide further insights as to their functional roles.
Materials and methods
All histology consumables were purchased from DAKO-Australia, Botany, Sydney, Australia. Positively charged SuperFrost Plus glass microscope slides were purchased from Menzel-Glaser, GmbH, Germany. Details of the antibodies used and epitope retrieval steps required for detection are provided in table 1 and suppliers are provided below. Monoclonal antibody (Mab) 5-D-5 to versican G3 domain [1, 30, 48] was a gift from Dr Firoz Rahemtulla, University of Alabama, Birmingham, AL, USA. Mab A7L6, rat anti perlecan domain IV [14, 15] and MAb CS-56 to native CS [23] were purchased from Abcam, Cambridge, UK. Mab 12C5 to versican G1 domain originally developed by Dr R Asher [2, 3] was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA, USA. Affinity purified rabbit polyclonal antibodies to aggrecan G1 and G3 domains [39, 46, 51] were gifts from Dr Peter Roughley and Dr John Mort, Genetics Unit, Shriners Hospital for Children, Montreal, Quebec, Canada. Rabbit polyclonal anti bovine type VI collagen was a gift from Dr Betty Reinboth, Department of Pathology, University of Adelaide, Adelaide, Australia. Mabs 3-B-3, 2-B-6 and 5-D-4 [9–13] were gifts from Prof. Bruce Caterson, School of Molecular and Medical Biosciences, University of Cardiff, Cardiff, UK.
Table 1.
Antibodies used for immunolocalisation of intervertebral disc proteoglycans
| Ab clone (IgG sub type) specificity | Epitope specificity | Dilution used, pre-treatments | Ref. |
|---|---|---|---|
| Ab 2194 (rabbit polyclonal) aggrecan G1 | Ab raised against four peptides corresponding to conserved regions in the G1 domain of human, canine and bovine aggrecan but not in the G2 domain of aggrecan or G1 domain of versican. Peptides used: (1) HDNSLSVSIPQPS, (2) RVLLGTSLTIPCYFIDPMHPVTTAPS, (3) TEGRVRNSAYQDK and (4) SSRYDAICYTG | 1/1,000 Ch’ase ABC 50 mU/ml, 2 h, 37°C | [46, 51] |
| (rabbit polyclonal) aggrecan G3 | Ab raised against five peptides to regions within the G3 domain including the sequences (1) GWNKYQGHCYRHFPDR, (2) TWVDAERRCREQQSHLSS, (3)VEHARTGQKKDRYGGC, (4) CQPSGHWEEPRIT and (5) CGGTTYKRRLQKRSSR | 1/1,000 Ch’ase ABC 50 mU/ml, 2 h | [38] |
| 5D5 (IgG) versican G3 | G3 carboxyl domain of versican | 1/1,000 Ch’ase ABC 50 mU/ml, 2 h | [48] |
| 12C5 (IgG1) versican G1 | G1 domain of versican | 1/1,000 Ch’ase ABC 50 mU/ml, 2 h | [2, 3] |
| A7L6 (IgG) Perlecan domain IV | Perlecan domain IV | 1/400 Ch’ase ABC 0.25 U/ml, 1 h, 37°C | [14, 15] |
| Rabbit polyclonal anti bovine type VI collagen | Type VI collagen | 1/1,000 Ch’ase ABC 0.25 U/ml, 2 h Hy’ase . | [32, 33, 36] |
| CS-56 (native CS) | Native CS-A and CS-C but not CS-B | 1/1,000, none | [23] |
| 3-B-3 (IgM) | Δ Chondroitin-6-sulphate stub epitopes generated by chondroitinase ABC | 1/3,000 Ch’ase ABC 0.25 U/ml, 1 h | [9–13] |
| 2-B-6 (IgG) | Δ Chondroitin-4-sulphate stub epitopes generated by chondroitinase ABC | 1/3,000 Ch’ase ABC 0.25 U/ml, 1 h | [9–13] |
| 5-D-4 (IgG) | Keratan sulphate | 1/5,000, none | [9–13] |
Histology
Three lumbar IVDs (L2L3, L3L4 and L4L5) from two 2 day-old lambs and 27 lumbar IVDs from groups of sheep aged 2, 4 and 6 years (nine IVDs from each age, three L2L3, three, L3L4 and three L5L6 IVDs) were examined. The IVDs with superior and inferior vertebral bodies attached, were fixed en-bloc in 10% neutral buffered formalin for 1 week then decalcified in several changes of 10% formic acid in 5% formalin for 2 weeks with constant agitation. Vertical mid-sagittal tissue slabs (∼5 mm) of the vertebral body-IVD specimens were cut and dehydrated in graded alcohols then double embedded in paraffin-celloidin. Sections (4 μ) were cut using a rotary microtome, and mounted on SuperFrost Plus glass slides. These were dried at 85°C for 30 min followed by 55°C in an oven overnight. Horizontal slices (2 mm) of selected IVDs devoid of vertebral body and hence requiring no decalcification were fixed directly in 10% neutral buffered formalin for 24 h, dehydrated in graded alcohols, embedded in paraffin wax and sections prepared as outlined above. Following de-paraffinisation in xylene and re-hydration through graded ethanol washes (100–70% v/v) to water, the tissue sections were stained with toluidine blue/fast green [20] or picro-sirius red [45], the latter stain was examined by polarised light microscopy. The picrosirius red staining method of Sweat et al. [45] was used after initial removal of tissue proteoglycans as advocated by Junqueira et al. [27] by pre-digestion with bovine testicular hyaluronidase (1,000 U/ml) in 0.1 M phosphate buffer pH 5.0 for 2 h at 37°C. The tissue sections were initially stained in Wiegert’s Iron haematoxylin for 30 min, then in 0.1% Sirius red F3BA in saturated aqueous picric acid for 2 h at room temp. The sections were not rinsed in water since sirius red is soluble in water but were washed rapidly in several changes of iso-propanol, cleared in xylene and mounted in Eukitt. Tissue proteoglycans were stained in 0.04% w/v toluidine blue O in 0.1 M sodium acetate buffer pH 4.0 for 10 min and the sections counterstained with 0.1% w/v aqueous fast green FCF for 2 min.
Immunohistology
Four micron tissue sections were cut and adhered to positively charged microscope slides as indicated earlier. Incubations with primary antibodies (Table 1) were performed using a Sequenza vertical cover-plate immunostaining system [32–37]. Endogenous peroxidase activity was blocked by incubating the tissue sections with 3% H2O2 for 5 min, washed in water then non-specific binding sites were blocked with a serum free protein block (Dako x0909) or 10% swine serum for 10 min. The perlecan sections were pre-digested with chondroitinase ABC (0.25 U/ml) for 1 h at 37°C in 0.1 M Tris–HCl, 0.03 M sodium acetate buffer pH 8.0, the aggrecan and versican sections were pre-digested for 2 h at 37°C with 0.05 U/ml chondroitinase ABC prior to undertaking the localisations. The tissue sections were incubated overnight at 4°C with anti-perlecan domain-IV antibody A7L6 (5 μg/ml), anti aggrecan G1 or G3 (1:1,000), Mabs 5D5 or 12C5 to versican (1.25 μg/ml) or anti CS (MAb CS-56) 1/1,000 diln diluted in 2% BSA in TBS. Selected tissue sections were pre-digested with chondroitinase ABC (0.25 U/ml) for 2 h prior to undertaking the 3-B-3 and 2-B-6 localisations, these were both used at a dilution of 1/3,000, Mab 5-D-4 to keratan sulphate required no pre-treatment step and was used at a dilution of 1/5,000 [9–13]. After washing in 50 mM Tris–HCl, pH 7.6 containing 150 mM NaCl 0.05% Tween 20 (TBS–Tween) the slides were incubated with appropriate biotinylated goat anti-rabbit, mouse or rat IgG for detection of primary antibody and horse–radish peroxidase or alkaline phosphatase conjugated streptavidin for visualisation using nova red, new fuchsin or diaminobenzidene substrate for colour development. Negative control sections were developed using an isotype matched mouse monoclonal IgG (clone DAK-GO1) against Aspergillus niger glucose oxidase, an enzyme which is neither present nor inducible in mammalian tissues or for the rabbit and rat primary antibodies were undertaken either omitting primary antibody or using concentration matched non-immune rabbit or rat serum instead of authentic primary antibody. The tissue specimens were examined by bright field, Nomarsky DIC or polarised light microscopy using a Leica photomicroscope linked to a DFC 480 digital camera.
Results
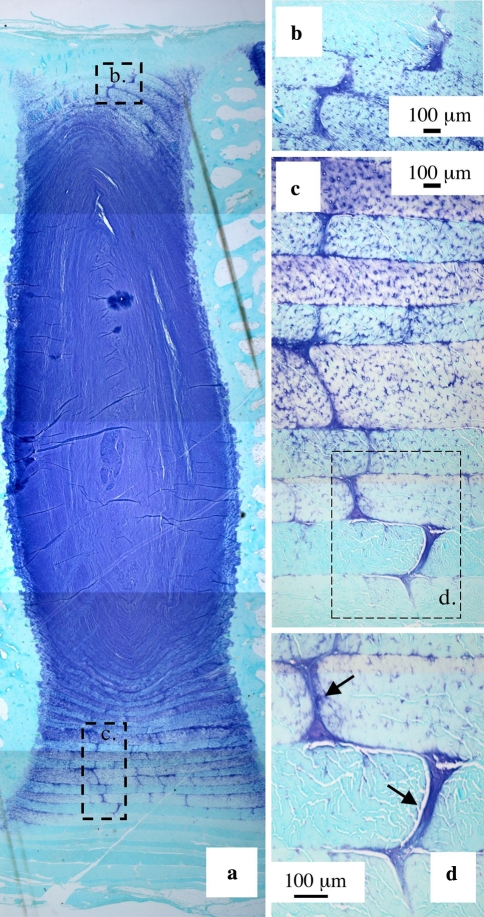
Preliminary examination of toluidine blue stained vertical mid-sagittal sections of IVD revealed discretely stained discontinuities in the AF annular lamellae in all IVDs examined (Fig. 1). We have called these discontinuities translamellar cross-bridges (TLCBs) since they span in some cases up to eight annular lamellae in a meandering serpentine type fashion (Fig. 2). The annular cross-bridges were more numerous and extended over longer distances in the anterior than in the posterior AF (Figs. 1, 2). These toluidine blue stained cross-bridges were predominantly confined to the mid AF, but a few were also discernable in the outermost annular lamellae in adult IVDs. The cross bridges however were not observed in the transitional zone between the AF and NP. The cross-bridges were contiguous with the interlamellar junctions between adjacent lamellae which also stained with toluidine blue but ran circumferentially around the disc (Fig. 1b–d).
Fig. 1.
Translamellar annular cross-bridge arrangements in the anterior a and posterior AF b of a 2 year-old ovine lumbar IVD b The boxed areas in a are reproduced at higher magnification in b and c, and the boxed area in c further reproduced at higher magnification in d. Toluidine blue-fast green stain. Most cells associated with the cross bridges arrows have a rounded morphology and fibrillar material is also clearly associated with this structure
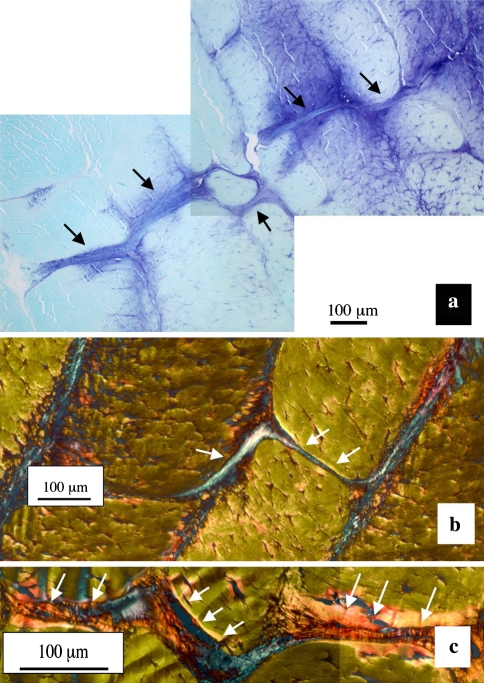
Fig. 2.
A posterior cross-bridge stained with toluidine blue/fast green extending over several lamellae (black arrows) in a 4 year-old sheep a with details of two cross bridge arrangements b, c stained with picrosirius red viewed under polarised light, the cross bridge structures are highly refractile white arrows
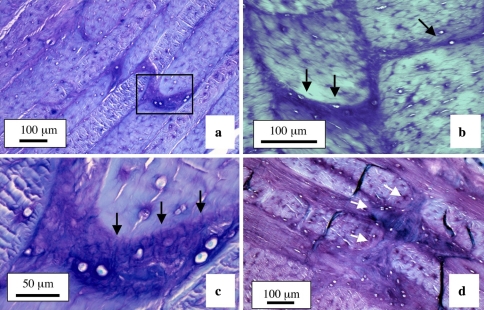
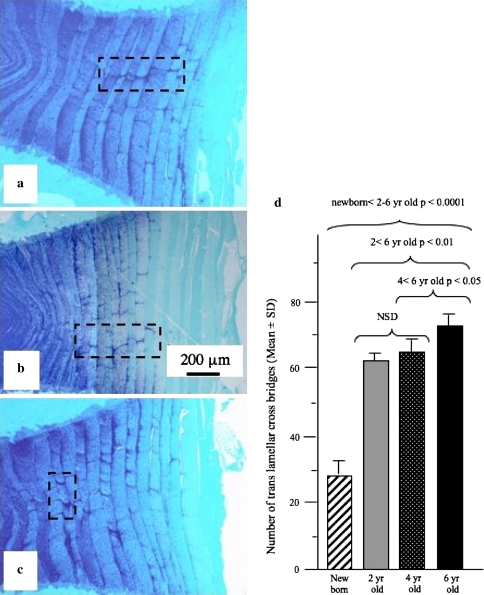
The cross-bridges contained anionic proteoglycan which stained strongly with toluidine blue compared to the adjacent annulus (Fig. 2a). Picrosirius red staining of IVD sections viewed under polarised light also clearly delineated the cross bridges extending through a number of annular lamellae (Fig. 2b, c). Many of the cross-bridges contained strongly refractile fibrous material indicative of highly organised collagenous fibrils, however, these appeared to be separate from the collagenous lamellae through which the cross bridges penetrated, indicating that these are discrete independent structures (Figs. 1d; 2b, c; 3). Toluidine blue stained annular cross-bridges were readily demonstrated in IVDs from 2, 4, and 6 year-old animals (Fig. 4a–c). The number of cross-bridges per lamellae and the number of lamellae with cross-bridges (i.e. depth from the disc exterior) increased with age (Fig. 4d) and displayed a statistically significant increase when the newborn IVDs were compared with all other sample groups (p < 0.001). The 2 year-old IVDs had less cross bridges than the 4 year-old group but this difference was not statistically significant, and the 4 year-old IVD group also had less cross bridges than the 6 year-old IVD group (p < 0.05). In contrast, in newborn IVDs annular cross bridges were not as readily identified since they had less associated anionic proteoglycan, particularly those in the outer AF (Fig. 5a–c). However, immunolocalisation for perlecan demonstrated the presence of blood vessels in the translamellar spaces which penetrated deep within the AF in newborn IVDs (Fig. 5d, e).
Fig. 3.
Annular cross bridges in horizontal sections of 4 a, c and 6 year-old b, d ovine IVDs. In a the cross bridge within the boxed area is reproduced at higher magnification in segment b. The fibrous nature of the cross bridge in d is clearly evident as are interconnections to several annular lamellae. Toluidine blue fast green stain
Fig. 4.
Vertical mid sagittal sections of the anterior AF of three sheep IVDs aged 2 a, 4 b and 6 years c stained with toluidine blue-fast green to visualise tissue anionic proteoglycan species. Cross bridge arrangements are evident in each of the boxed areas. d The cross bridges were counted in single mid-sagittal sections of newborn (n = 3), 2 year-old (n = 9), 4 year-old (n = 9) and 6 year-old (n = 9) lumbar IVDs. These sections were stained with toluidine blue as depicted in (a, b, c) and the cross bridges counted in low magnification views encompassing the entire anterior AF. This data were compared statistically using a Student’s t -test
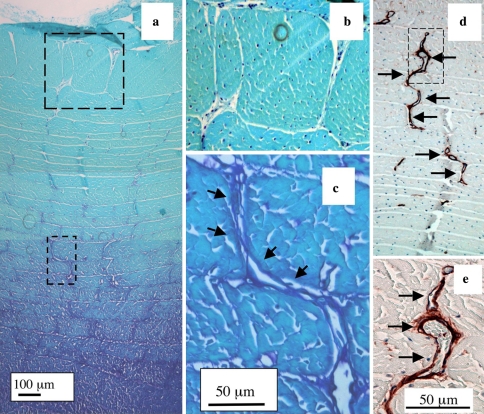
Fig. 5.
Annular cross bridges are not easily discernable in newborn IVDs stained with toluidine blue since anionic proteoglycans are not prominently associated with them a. The boxed areas in segment a are reproduced at higher magnification in b and c, respectively. The outermost regions of the IVD are at the top of each photosegment. Blood vessels in the outer AF of a newborn IVD immunolocalised with anti-perlecan domain-IV Mab A7L6 showing the circuitous meandering serpentine course these often take along a series of interconnecting annular cross-bridges. The vessels in the boxed area in d is reproduced at higher magnification in e using DIC Nomarsky optics to visualise the entrapped red blood cells within the vessels
Immunolocalisation studies using antibodies to specific core protein and GAG side chain epitopes were undertaken to characterise the proteoglycans in the translamellar bridges. Positive staining for chondroitinase-generated chondroitin-4 and -6 sulphate (3-B-3 and 2-B-6, respectively) and keratan sulphate (5-D-4) was evident in cross-bridges of adult (Fig. 6a) but not new-born discs. The presence of native chondroitin-sulphate in adult cross-bridges was demonstrated with CS-56 antibody (Fig. 6b). This native chondroitin-sulphate co-localised with the G1 and G3 domains of both aggrecan and versican in adult discs (Fig. 6b). The chondroitin and keratan sulphate epitopes were not confined to the cross bridges but were also immunolocalised to finer fibrillar material emerging from the circumferential interlamellar regions and were also readily visualised in the inner AF and NP in association with positive toluidine blue staining in these same areas (Fig. 7). Cross bridges were not stained with antibodies to type II, IX or X collagen although other disc regions were positive for these proteins, while type I immunostaining was similar to surrounding lamellae (data not shown). In contrast, type VI collagen was specifically localized to the annular cross-bridges along with the circumferential interlamellar junctions in adult (Fig. 8) but not newborn IVDs (data not shown). Immunolcalisation of type VI collagen indicated that it was not confined to pericellular regions but was diffusely distributed throughout the cross-bridge assemblies, a prominent pericellular component of the fibrocartilaginous cells interspersed within the main substance of the annular lamellae and in the matrix of the NP in both adult and newborn discs.
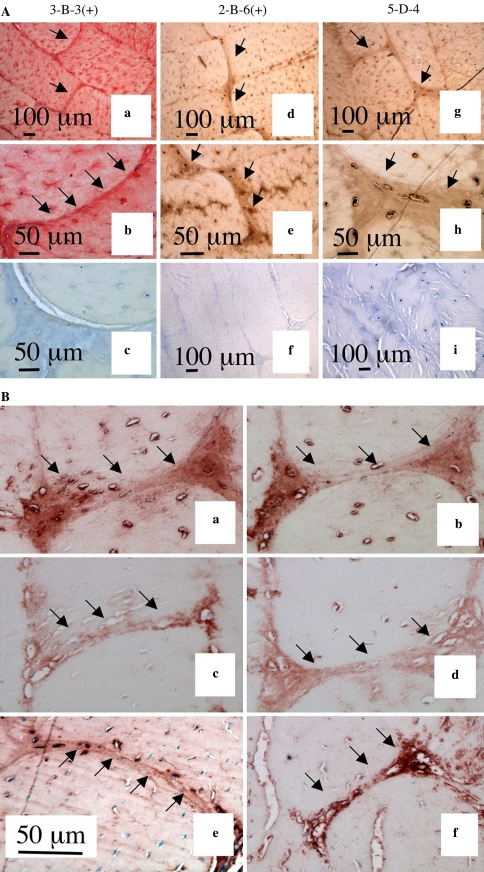
Fig. 6.
(1) Immunolocalisation of chondroitin-6-sulphate a, b, c and chondroitin-4-sulphate Δ-stub epitopes d, e, f generated by chondroitinase ABC and detected using Mabs 3-B-3(+) and 2-B-6(+) and keratan sulphate detected with Mab 5-D-4 g, h, and i. Controls for Mabs 3-B-3, 2-B-6 and 5-D-4 are presented in segments c, f, and i, respectively. (2) Immunolocalisation of CS-proteoglycans a, b aggrecan c, d and versican e, f in annular cross bridge structures in ovine anterior lamellae, vertical sagittal sections. The antibodies used were MAb CS-56 to native CS a, b anti-aggrecan G1 domain c anti-aggrecan G3 domain d, Mab 12C5 to versican G1 domain e and Mab 5D5 to versican G3 domain f
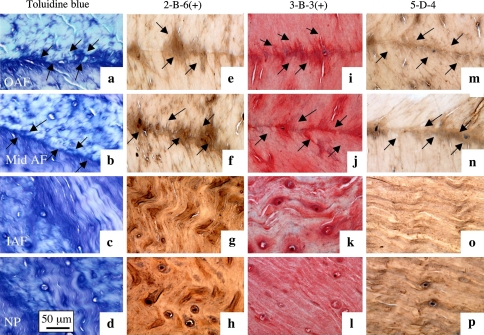
Fig. 7.
The junctional regions arrows between adjacent annular lamellae (4 year-old ovine lumbar IVD) are proteoglycan rich, toluidine blue stain a, b, c, d and contain fibrous material which may represent type VI collagen and elastin. The outer AF a, e, i, m; mid AF b, f, j, n, inner AF c, g, k, o and NP d, h, l, p are depicted. Immunolocalisations were undertaken with Mab 2-B-6(+) e, f, g, h, 3-B-3(+) i, j, k, l to chondroitin-4 and 6 sulphate stub epitopes generated by chondroitinase ABC (+) on CS ; and with Mab 5-D-4 to KS m, n, o, p
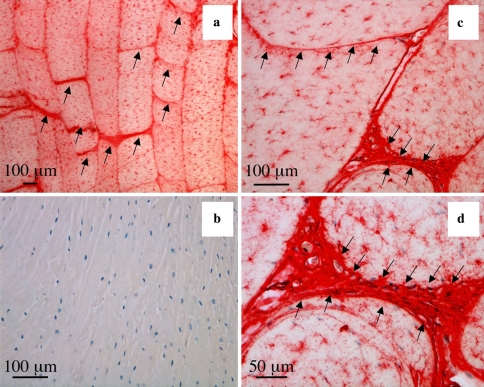
Fig. 8.
Immunolocalisation of type VI collagen in lamellar cross bridges arrows in lumbar 4 year-old ovine IVDs, vertical sagittal sections. A negative control is presented in segment b. Type VI collagen is also associated pericellularly with the fibrochondrocytes within the annular lamellae
Discussion
The translamellar cross-bridges we have described in the present study are discrete discontinuities in the normal lamellar layers of the AF, they occur from birth to old age but are less readily visualised in newborn IVDs. Proteoglycans are not obviously associated with the translamellar structures in newborn IVDs however small blood vessels penetrate in a serpentine fashion along these structures into the mid AF and can be visualised using antibodies to specific basement membrane components laid down by the endothelial cells (perlecan) to form these vessel networks [35]. Nomarski DIC optics further confirmed the functional status of these vessels by demonstrating they contained red blood cells. These annular blood vessels are transient entities in the developing IVD and they regress with neonatal maturational developmental changes when an increase in proteoglycans occurs concomitant with an increase in the internal hydrostatic pressure in the IVD as it becomes a weight bearing spinal structure [21, 35]. With increasing age the annular cross-bridges become prominent due to the localisation of anionic proteoglycans in these structures which are readily stained by common anionic dyes such as toluidine blue. These toluidine blue stained cross-lamellar bridges were more numerous per lamella and extended a greater depth into the AF with skeletal maturity suggesting that this may represent a functional adaptation to intrinsic biomechanical forces experienced by the disc.
We have shown for the first time that aggrecan and versican are localised in the translamellar cross-bridges. However, the cross bridges are distinct from surrounding collagenous lamellae in having a matrix rich in type VI collagen in adult IVDs but no discernable type VI collagen in newborn IVDs, again suggestive of a mechanically driven, age dependant, adaptive change in these connective tissues. The cross-bridges also contain some type I collagen but no detectable type II, IX or X collagen. The progressive increase in GAG containing cross-bridges with age also suggests that this is an adaptation to mechanical loading in the IVD. However, the lack of expression of type II collagen (but abundant type VI collagen) makes the cross bridges distinct from other tensional tissue adaptations to loading such as evident in the compressed regions of tendons or inner meniscus where aggrecan and type II and IX collagen are increased [33].
A role for aggrecan and versican in the lubrication of collagen bundles to facilitate the sliding of tendon fascicles over one another has been suggested to prevent fibril damage during twisting movements under extreme load [52, 53]. Connective tissues containing collagen bundles which occur at variable angles to one another are subject to focal transverse compressive load even in tissues which are generally considered to be tensional. The small islands of rounded cells of a chondrocytic phenotype in the mid-meniscus in a matrix rich in anionic proteoglycan (aggrecan) and type II collagen, may be one such example of focal compressive load leading to phenotypic adaptation of intrinsic cell populations [33]. Type VI collagen accumulates in regions of deep flexor digital tendon around nests of cells experiencing focal compressive load and has been suggested as a marker of fibrocartilage differentiation [8]. The supraspinatus tendon of the human rotator cuff contains a number of structurally independent fascicles which can be variably and independently loaded depending on joint angulation. Inter-fascicular proteoglycan rich regions play a role in the lubrication of these units minimising shear stresses. The annular lamellae of the IVD are also variably loaded with a lamella under tension occurring adjacent to one that is relaxed [41, 42, 47]. The interlamellar regions of the IVD are also proteoglycan-rich. Versican is localised in such annular junctional regions where it may play a similar role to the fascicle associated proteoglycans in tendon [34]. The proposal of versican and aggrecan acting as lubricating agents is a new role for these proteoglycans in the IVD but consistent with roles proposed for CS–PGs in tendon. Furthermore, type VI collagen is capable of interacting with hyaluronan, a well known lubricating component of synovial fluids [28, 29, 31, 44, 52, 53] and this association may also facilitate the lubrication of collagen bundles.
Trans lamellar cross-bridges have also been observed in the human AF [55, 56]. We hypothesis that these cross-bridges represent an adaptation to the complex tensional and compressional loading of the annular lamellae. Therefore any change in disc mechanics that may occur with NP degeneration may also influence the cross-bridges. Future studies will attempt to examine the relationship between the cross bridges and NP degeneration in animal models.
Contributor Information
James Melrose, Phone: +61-2-99266535, FAX: +61-2-99266539, Email: jmelrose@med.usyd.edu.au.
Susan M. Smith, Email: sues@staff.usyd.edu.au
Richard C. Appleyard, Email: appleyardr@med.usyd.edu.au
Christopher B. Little, Email: cblittle@med.usyd.edu.au
References
- 1.Abiko Y, Nishimura M, Rahemtulla F, Mizoguchi I, Kaku T. Immunohistochemical localisation of large chondroitin sulphate proteoglycan in porcine gingival epithelia. Eur J Morphol. 2001;39:99–104. doi: 10.1076/ejom.39.2.99.7372. [DOI] [PubMed] [Google Scholar]
- 2.Asher RA, Perides G, Vanderhaeghen J-J, Bignani A. Extracellular matrix of central bervous system white matter: demonstration of an hyaluronate-protein complex. J Neurosci Res. 1991;28:410–421. doi: 10.1002/jnr.490280314. [DOI] [PubMed] [Google Scholar]
- 3.Asher RA, Schiebe RJ, Keiser HD, Bignami A. On the existence of a cartilage like proteoglycan and link protein in the central nervous system. Glia. 1995;13:294–308. doi: 10.1002/glia.440130406. [DOI] [PubMed] [Google Scholar]
- 4.Boszczyk BM, Boszczyk AA, Putz R, Buttner A, Benjamin M, Milz S. An immunohistochemical study of the dorsal capsule of the lumbar and thoracic facet joints. Spine. 2001;26:E338–E343. doi: 10.1097/00007632-200108010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Buckwalter JA, Cooper RR, Maynard JA. Elastic fibers in human intervertebral discs. J Bone Joint Surg Am. 1976;58:73–76. [PubMed] [Google Scholar]
- 7.Buckwalter JA, Woo SL, Goldberg VM, Hadley EC, Booth F, Oegema TR, Eyre DR. Soft-tissue aging and musculoskeletal function. J Bone Joint Surg Am. 1993;75:1533–1548. doi: 10.2106/00004623-199310000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho HF, Felisbino SL, Keene DR, Vogel KG. Identification, content, and distribution of type VI collagen in bovine tendons. Cell Tissue Res. 2006;325:315–324. doi: 10.1007/s00441-006-0161-0. [DOI] [PubMed] [Google Scholar]
- 9.Caterson B, Baker JR, Christner JE, Couchman JR. Immunological methods for the detection and determination of connective tissue proteoglycans. J Invest Dermatol. 1982;79(Suppl 1):45s–50s. doi: 10.1111/1523-1747.ep12545740. [DOI] [PubMed] [Google Scholar]
- 10.Caterson B, Calabro T, Hampton A. Monoclonal antibodies as probes for elucidating proteoglycan structure and function. In: Wright T, Mecham R, editors. Biology of Proteoglycans. New York: Academic; 1987. pp. 1–26. [Google Scholar]
- 11.Caterson B, Christner JE, Baker JR, Couchman JR. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc. 1985;44:386–393. [PubMed] [Google Scholar]
- 12.Caterson B, Christner JE, Baker JR, Couchman JR. Production and characterisation of monoclonal antibodies against connective tissue proteoglycans. Fed Proc Fed Am Soc Exp Biol. 1985;44:386–393. [PubMed] [Google Scholar]
- 13.Caterson B, Mahmoodian F, Sorrell JM, Hardingham TE, Bayliss MT, Carney SL, Ratcliffe A, Muir H. Modulation of native chondroitin sulphate structure in tissue development and in disease. J Cell Sci. 1990;97:411–417. doi: 10.1242/jcs.97.3.411. [DOI] [PubMed] [Google Scholar]
- 14.Couchman JR, Ljubimov AV. Mammalian tissue distribution of a large heparan sulfate proteoglycan detected by monoclonal antibodies. Matrix. 1989;9:311–321. doi: 10.1016/s0934-8832(89)80007-1. [DOI] [PubMed] [Google Scholar]
- 15.Couchman JR, Ljubimov AV, Sthanam M, Horchar T, Hassell JR. Antibody mapping and tissue localization of globular and cysteine rich regions of perlecan domain III. J Histochem Cytochem. 1995;43:955–963. doi: 10.1177/43.9.7543915. [DOI] [PubMed] [Google Scholar]
- 16.Eyre DR. Biochemistry of the intervertebral disc. Int Rev Connect Tissue Res. 1979;8:227–291. doi: 10.1016/b978-0-12-363708-6.50012-6. [DOI] [PubMed] [Google Scholar]
- 17.Eyre DR, Muir H. Collagen polymorphism: two molecular species in pig intervertebral disc. FEBS Lett. 1974;42:192–196. doi: 10.1016/0014-5793(74)80783-0. [DOI] [PubMed] [Google Scholar]
- 18.Eyre Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem J. 1976;157:267–270. doi: 10.1042/bj1570267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eyre DR, Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977;492:29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- 20.Getzy LL, Malemud CJ, Goldberg VM, Moskowitz RW. Factors influencing metachromatic staining in paraffin embedded sections of rabbit and human articular cartilage: a comparison of the Safranin O and toluidine blue techniques. J Histotechnol. 1982;5:111–116. [Google Scholar]
- 21.Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20:107–121. doi: 10.1016/S0945-053X(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 22.Humzah MD, Soames RW. Human intervertebral disc: structure and function. Anat Rec. 1988;220:337–356. doi: 10.1002/ar.1092200402. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y, Hikino M, Yajima Y, Mikami T, Sirko S, Holst A, Faissner A, Fukui S, Sugahara K. Structural characterization of the epitopes of the monoclonal antibodies 473HD, CS-56, and MO-225 specific for chondroitin sulfate D-type using the oligosaccharide library. Glycobiology. 2005;15:593–603. doi: 10.1093/glycob/cwi036. [DOI] [PubMed] [Google Scholar]
- 24.Johnson Elastic fibres in the anulus fibrosus of the adult human lumbar intervertebral disc. A preliminary report. J Anat. 1985;143:57–63. [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson EF, Caldwell RW, Berryman HE, Miller A, Chetty K. Elastic fibers in the anulus fibrosus of the dog intervertebral disc. Acta Anat (Basel) 1984;118:238–242. doi: 10.1159/000145851. [DOI] [PubMed] [Google Scholar]
- 26.Johnson EF, Chetty K, Moore IM, Stewart A, Jones W. The distribution and arrangement of elastic fibres in the intervertebral disc of the adult human. J Anat. 1982;135:301–309. [PMC free article] [PubMed] [Google Scholar]
- 27.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 28.Kielty CM, Whittaker SP, Grant ME, Shuttleworth CA. Type VI collagen forms a structural association with hyaluronan in vivo. Biochem Soc Trans. 1991;19:384S. doi: 10.1042/bst019384s. [DOI] [PubMed] [Google Scholar]
- 29.Kielty CM, Whittaker SP, Grant ME, Shuttleworth CA. Type VI collagen microfibrils: evidence for a structural association with hyaluronan. J Cell Biol. 1992;118:979–990. doi: 10.1083/jcb.118.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzucato M, Cozzi MR, Pradella P, Perissinotto D, Malmstrom A, Morgelin M, Spessotto P, Colombatti A, Marco L, Perris R. Vascular PG-M/versican variants promote platelet adhesion at low shear rates and cooperate with collagens to induce aggregation. Faseb J. 2002;16:1903–1916. doi: 10.1096/fj.02-0382com. [DOI] [PubMed] [Google Scholar]
- 31.McDevitt CA, Marcelino J, Tucker L. Interaction of intact type VI collagen with hyaluronan. FEBS Lett. 1991;294:167–170. doi: 10.1016/0014-5793(91)80660-U. [DOI] [PubMed] [Google Scholar]
- 32.Melrose J, Smith S, Cake M, Read R, Whitelock J. Perlecan displays variable spatial and temporal immunolocalisation patterns in the articular and growth plate cartilages of the ovine stifle joint. Histochem Cell Biol. 2005;123:561–571. doi: 10.1007/s00418-005-0789-y. [DOI] [PubMed] [Google Scholar]
- 33.Melrose J, Smith S, Cake M, Read R, Whitelock J. Spatial and temporal immunolocalisation of perlecan in the ovine meniscus. Histochem Cell Biol. 2005;124:225–235. doi: 10.1007/s00418-005-0005-0. [DOI] [PubMed] [Google Scholar]
- 34.Melrose J, Smith S, Ghosh P. Differential expression of proteoglycan epitopes by ovine intervertebral disc cells. J Anat. 2000;197(Pt 2):189–198. doi: 10.1046/j.1469-7580.2000.19720189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melrose J, Smith S, Ghosh P, Whitelock J. Perlecan, the multidomain heparan sulfate proteoglycan of basement membranes, is also a prominent component of the cartilaginous primordia in the developing human fetal spine. J Histochem Cytochem. 2003;51:1331–1341. doi: 10.1177/002215540305101010. [DOI] [PubMed] [Google Scholar]
- 36.Melrose J, Smith S, Knox S, Whitelock J. Perlecan, the multidomain HS-proteoglycan of basement membranes, is a prominent pericellular component of ovine hypertrophic vertebral growth plate and cartilaginous endplate chondrocytes. Histochem Cell Biol. 2002;118:269–280. doi: 10.1007/s00418-002-0449-4. [DOI] [PubMed] [Google Scholar]
- 37.Melrose J, Smith S, Whitelock J. Perlecan immunolocalises to perichondral vessels and canals in human foetal cartilagenous promordia in early vascular and matrix remodelling events associated with diarthrodial-joint development. J Histochem Cytochem. 2004;52:1405–1413. doi: 10.1369/jhc.4A6261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mort JS, Buttle DJ. The use of cleavage site specific antibodies to delineate protein processing and breakdown pathways. Mol Pathol. 1999;52:11–18. doi: 10.1136/mp.52.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mort JS, Roughley PJ. Production of antibodies against degradative neoepitopes in aggrecan. Methods Mol Med. 2004;100:237–250. doi: 10.1385/1-59259-810-2:237. [DOI] [PubMed] [Google Scholar]
- 40.Oegema TR., Jr Biochemistry of the intervertebral disc. Clin Sports Med. 1993;12:419–439. [PubMed] [Google Scholar]
- 41.Pezowicz CA, Robertson PA, Broom ND. Intralamellar relationships within the collagenous architecture of the annulus fibrosus imaged in its fully hydrated state. J Anat. 2005;207:299–312. doi: 10.1111/j.1469-7580.2005.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pezowicz CA, Robertson PA, Broom ND. The structural basis of interlamellar cohesion in the intervertebral disc wall. J Anat. 2006;208:317–330. doi: 10.1111/j.1469-7580.2006.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith LJ, Fazzalari NL. Regional variations in the density and arrangement of elastic fibres in the anulus fibrosus of the human lumbar disc. J Anat. 2006;209:359–367. doi: 10.1111/j.1469-7580.2006.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Specks U, Mayer U, Nischt R, Spissinger T, Mann K, Timpl R, Engel J, Chu M. Structure of recombinant N-terminal globule of type VI collagen alpha 3 chain and its binding to heparin and hyaluronan. Embo J. 1992;11:4281–4290. doi: 10.1002/j.1460-2075.1992.tb05527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sweat F, Puchtler H, Rosenthal SI. Sirius red F3ba as a stain for connective tissue. Arch Pathol. 1964;78:69–72. [PubMed] [Google Scholar]
- 46.Sztrolovic R, White RJ, Roughley PJ, Mort JS. The mechanism of aggrecan release from cartilage differs with tissue origin and the agent used to stimulate catabolism. Biochem J. 2002;362:465–472. doi: 10.1042/0264-6021:3620465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor TK, Melrose J, Burkhardt D, Ghosh P, Claes LE, Kettler A, Wilke HJ. Spinal biomechanics and aging are major determinants of the proteoglycan metabolism of intervertebral disc cells. Spine. 2000;25:3014–3020. doi: 10.1097/00007632-200012010-00008. [DOI] [PubMed] [Google Scholar]
- 48.Toriya N, Takuma T, Arakawa T, Abiko Y, Sasano Y, Takahashi I, Sakakura Y, Rahemtulla F, Mizoguchi I. Expression and localisation of versican during postnatal development of rat temporomandibular joint disc. J Histochem Cell Biol. 2006;125:205–214. doi: 10.1007/s00418-005-0020-1. [DOI] [PubMed] [Google Scholar]
- 49.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Urban JPG, Roberts S, Ralphs JR. The nucleus of the intervertebral disc from development to degeneration. Am Zool. 2000;40:53–61. doi: 10.1668/0003-1569(2000)040[0053:TNOTID]2.0.CO;2. [DOI] [Google Scholar]
- 51.Valiyaveettil M, Mort JS, McDevitt CA. The concentration, gene expression, and spatial distribution of aggrecan in canine articular cartilage, meniscus, and anterior and posterior cruciate ligaments: a new molecular distinction between hyaline cartilage and fibrocartilage in the knee joint. Connect Tissue Res. 2005;46:83–91. doi: 10.1080/03008200590954113. [DOI] [PubMed] [Google Scholar]
- 52.Vogel KG. What happens when tendons bend and twist? Proteoglycans. J Musculoskel Neuron Interact. 2004;4:202–203. [PubMed] [Google Scholar]
- 53.Vogel KG, Peters JA. Histochemistry defines a proteoglycan-rich layer in bovine flexor tendon subjected to bending. J Musculoskelet Neuronal Interact. 2005;5:64–69. [PubMed] [Google Scholar]
- 54.Yu J. Elastic tissues of the intervertebral disc. Biochem Soc Trans. 2002;30:848–852. doi: 10.1042/BST0300848. [DOI] [PubMed] [Google Scholar]
- 55.Yu J, Fairbank JC, Roberts S, Urban JP. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine. 2005;30:1815–1820. doi: 10.1097/01.brs.0000173899.97415.5b. [DOI] [PubMed] [Google Scholar]
- 56.Yu J, Tirlapur U, Fairbank J, Handford P, Roberts S, Winlove CP, Cui Z, Urban J. Microfibrils, elastin fibres and collagen fibres in the human intervertebral disc and bovine tail disc. J Anat. 2007;210:460–471. doi: 10.1111/j.1469-7580.2007.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu J, Winlove PC, Roberts S, Urban JP. Elastic fibre organization in the intervertebral discs of the bovine tail. J Anat. 2002;201:465–475. doi: 10.1046/j.1469-7580.2002.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]