Abstract
Prior imaging studies of scoliosis patients attempted to demonstrate a relationship between plain radiographic curve patterns and curve progression and pain, or used magnetic resonance imaging (MRI) to focus on spinal cord abnormalities. Pain in scoliosis patients may differ from nondeformity patients, yet may still be discogenic. The purpose of this study was to assess the possible relationship of degenerative disc findings on MRI to scoliosis patients’ pain. This prospective study enrolled scoliosis and control patients, all of whom had assessment for back pain (visual analog scale) and disability (Oswestry Index) and spinal MRI to identify prevalence and distribution of degenerative disc findings. Specifically, we assessed 60 consecutive pediatric and adult idiopathic scoliosis patients who had progressed to surgical treatment, 60 age- and gender-matched asymptomatic controls, and 172 nondeformity symptomatic degenerative disc disease patients who had progressed to surgical treatment. All subjects had independent analysis of their preoperative MRI for disc degeneration, disc herniation, Schmorl’s nodes, and inflammatory end plate changes. Imaging findings of the scoliosis patients were compared to those from asymptomatic and symptomatic control groups. Our results found that both pediatric and adult scoliosis patients had significantly more pain and disability than did asymptomatic controls (P < 0.001). The adult idiopathic scoliosis patients had pain and disability similar to those of surgical degenerative disc disease control groups. Disc degeneration and herniation (contained) were not related to pain. However, in the pediatric scoliosis patients, those with Schmorl’s nodes often had greater pain than those without (P = 0.01). Adults with painful scoliosis, typically occurring at the apex of the scoliosis or at the lumbosacral junction, had a significantly higher frequency of inflammatory end plate changes on MRI than did controls (P < 0.001). Prior studies have demonstrated a correlation of inflammatory end plate changes to lumbar discogenic pain. In conclusions, scoliosis patients who have progressed to surgical intervention, pediatric patients have varying degrees of pain, and those with Schmorl’s nodes may be at greater risk for pain. Adult scoliosis patients have multifactorial pain of which one component may be related to degeneration of the lower lumbar discs similar to that in nondeformity patients. Additionally, adult scoliosis patients may have MRI findings consistent with discogenic pain at the apex of their curvature, most commonly at the proximal lumbar levels.
Keywords: Disc degeneration, Endplate changes, Modic, MRI, Schmorl’s nodes, Scoliosis
Introduction
The problem of pain and disability in scoliosis patients has received relatively little study compared to studies of pain in patients with lumbar degenerative disc disease (DDD) or stenosis. Classic teachings hold that adolescent idiopathic scoliosis patients do not present with pain, but rather that pain is typically associated with adult scoliosis [16, 33, 36, 64]. The etiology for painful scoliosis is thought to include muscular pain due to eccentric loading about the apex of a curvature, asymmetric facet joint loading resulting in facet arthritis or synovitis, discogenic pain, or a combination [67]. Animal scoliosis models and human studies have shown disc degeneration at the concave aspect of scoliotic discs [12, 13, 38, 39]. However, the progression from disc degeneration to discogenic pain is not fully understood.
Previous imaging studies have correlated plain radiographic scoliosis curve patterns to curve progression and also to pain [55]. Magnetic resonance imaging (MRI) is used to assess soft tissues of the spine and previous MRI studies of scoliosis patients have assessed abnormalities of the spinal canal or cord [15, 26, 49]. The MRI is also useful for assessment of degenerative changes in the disc, such as loss of hydration, herniation, Schmorl’s nodes, and inflammatory endplate changes; however, MRI has not yet been used to study these disc changes in scoliosis patients [24, 28, 43].
The authors anecdotally found that many adolescent idiopathic scoliosis patients identified themselves as having back pain. The authors were unable to correlate scoliosis curve magnitude on plain radiographs with pain severity. The purpose of this study was to quantify prospectively the degree of pain and disability in pediatric and adult scoliosis patients who had progressed to surgical intervention, and then to correlate these symptoms with degenerative changes on preoperative MRI scans. Specifically, MRI findings of disc degeneration, disc herniation, Schmorl’s nodes, and inflammatory endplate changes were analyzed. Comparative groups included age- and gender-matched asymptomatic and symptomatic controls (presurgical patients with severe pain due to DDD) to determine where in the MRI spectrum of degenerative disc changes the scoliosis patients fell.
Materials and methods
All pediatric and adult (pediatric onset) idiopathic scoliosis patients who presented at the author’s institution and had progressed to surgical intervention were prospectively evaluated using a reliable and valid outcomes survey for the assessment of pain and function. The survey included visual analog scales (VAS) for back and leg pain (0 = no pain; 10 = severe excruciating pain); a pain diagram assessing the area of pain involved over the surface of the body; and the Oswestry disability index (ODI, low score = no disability; high score = severe disability) [45]. An additional component to this survey assessed patients’ self-perception of appearance related to their spinal deformity using a VAS for deformity (0 = normal appearance; 10 = extremely deformed appearance).
This study enrolled idiopathic scoliosis patients to a total of 30 consecutive adults and 30 consecutive pediatric patients in each group. Inclusion criteria included a minimum curve magnitude of 40°, younger than age 66, and progression to surgical treatment (spinal arthrodesis). De novo, degenerative, and other non-idiopathic scoliosis patients, and idiopathics with prior surgical treatment of their deformity were excluded. Exclusion criteria also included pregnancy, medical conditions precluding surgical intervention, or acute disc herniation.
Surgical indications for the 30 pediatric idiopathic scoliosis patients included curve progression greater than 40° despite full-time bracing in an actively growing patient; curve magnitude greater than 45° in a patient with a Risser stage-2 or less; and curve magnitude of greater than 40° with progressive pain for more than 1 year and failure of full-time bracing, physical therapy, and nonsteroidal anti-inflammatory medication treatment. For the pediatric scoliosis group, 21 (70%) had been treated in a brace, 1 (3%) had physical therapy, and 4 (13%) had tried chiropractic prior to their orthopaedic referral. Surgical indications for the 30 adult idiopathic scoliosis patients included active curve progression to more than 50°; curve magnitude greater than 60° with presumed potential for progression; and curve magnitude greater than 40° with progressive pain for more than 1 year and failure of nonoperative treatment, including physical therapy, pain medications, thoracic or lumbar epidural steroid injections (or both), and bracing. Treatments for the adult scoliosis group included physical therapy in all 30 (100%), chiropractic in 4 (13%), bracing in 7 (23%), and epidural steroid injections in 28 (93%).
Age- and gender-matched controls who were identified (as shown later) considered themselves asymptomatic (for a minimum of 1 year), had never been treated for back pain by any health provider, and denied taking medication for pain. All controls completed identical surveys. All patients and control subjects had an MRI scan of their thoracic and lumbar spine. All MRIs included a T2-weighted sequence, with a TR typically in the 2,000–3,000 range, so that relative disc hydration could be adequately assessed. All patient and control group MRI scans were independently read by a neuroradiologist who had no knowledge of the subject’s symptoms and no knowledge as to whether the patients were being treated nonoperatively or were scheduled for surgery. Each MRI was specifically assessed for disc dehydration, herniated nucleus pulposus (HNP), Schmorl’s nodes, and inflammatory endplate changes. The study was approved by the institutional review board and informed consent was obtained in all subjects (or parents where appropriate).
After a pilot study of eight patients and eight control subjects, age- and gender-matched controls were recruited over the anticipated age range of the patients. Because the age range of the adult scoliosis patients was greater, more adult control subjects were recruited during the study period (56 pediatric and 68 adult controls). During recruitment of controls, two pediatric and four adult subjects were considered ineligible because they declined MRI scans. Control subjects completed their self-assessment questionnaires before their MRI scans and thus were blinded to the MRI scan findings at the time of questionnaire completion.
Additional control groups of symptomatic back pain patients were obtained from the author’s practice; they were concurrently treated with the scoliosis patients and had progressed to lumbar (n = 154) or thoracic (n = 18) fusion surgery for disabling axial back pain due to DDD. Surgical indications for the adult DDD control groups were progressive axial pain for more than 1-year symptom duration (mean 7.9 years) despite physical therapy, chiropractic, pharmacological treatment, epidural steroid injection, intradiscal steroid injections, and intradiscal electrothermal therapy treatments. All of these DDD patients had at least one level of concordant pain at the time of preoperative discography (Table 1). These patients were analyzed separately, as many did not undergo both thoracic and lumbar MRIs. These symptomatic control subjects are part of an ongoing fusion outcomes study in which they completed identical preoperative pain and disability questionnaires. The characteristics of the various study and control groups are shown in Table 1.
Table 1.
Study group characteristics
| Pediatric scoliosis | Pediatric control | Adult scoliosis | Adult control | Adulta lumbar DDD | Adultb thoracic DDD | |
|---|---|---|---|---|---|---|
| Number of subjects | 30 | 30 | 30 | 30 | 154 | 18 |
| Mean age (range) | 14 (11–17) | 14 (11–17) | 45 (25–64) | 45 (25–64) | 42 (18–65) | 37 (18–52) |
| Female/male | 20/6 | 20/6 | 25/1 | 25/1 | 103/51 | 12/6 |
| Vertebral levels fused, mean (standard deviation) | 8.4 (2.3) | – | 10.9 (2.6) | – | 1.6 (0.6) | 2.6 (1.1)c |
| Curve type (King et al. 1983) | 3 I, 15 II, 4 III, 1 IV, 6 V | – | 11 I, 12 II, 2 III, 1 V | – | – | – |
| Thoracic curve, mean degrees (range) | 51 (42–69) | – | 54 (40–72) | – | – | – |
| Lumbar curve, mean degrees (range) | 52 (44–62) | – | 57 (42–94) | – | – | – |
| Mean number of discs with dehydration on MRI per patient | 1.0 | 1.2 | 7.4 | 6.0 | 2.1 (1.0) | 3.4 (1.7) |
| Mean number of discs with Schmorl’s nodes on MRI per patient | 1.0 | 1.5 | 2.1 | 1.7 | – | 2.2 |
| Mean number of discs with HNP on MRI per patient | 0.0 | 0.0 | 0.2 | 0.5 | – | 0.4 |
| Mean number of discs with inflammatory end plate changes on MRI per patient | 0.0 | 0.0 | 1.5 | 0.2 | 0.5 | 0.3 |
| Levels of concordant pain on discography, mean (standard deviation) | – | – | – | – | 1.8 (0.7) | 2.4 (0.9) |
DDD, degenerative disc disease; HNP, herniated nucleus pulposus; MRI, magnetic resonance imaging
aMRI of lumbar spine only
bMRI of thoracic spine only
cIn two cases, an extra level was fused to avoid ending the fusion at the apex of the thoracic kyphosis
Statistical methods
Statistical analysis for the MRI and symptom variables for each group were calculated separately. Owing to the skewed distribution for most of these variables, nonparametric methods were used for analysis. The Kruskal–Wallis test was used to compare the MRI and symptom variables between the subject groups. Within the study and asymptomatic control groups, the association among age, MRI, and symptom parameters was assessed using the Spearman correlation. Any P values of less than 0.05 were considered statistically significant. In the graphs, the error bars represent plus or minus one standard deviation.
Results
Pain and disability
Outcomes surveys found that patients in the pediatric idiopathic scoliosis group had back pain significantly worse than those in the pediatric control group (P < 0.001; Fig. 1). The adult scoliosis patients had pain (VAS mean = 6.5; range 2.0–9.0) significantly worse than that of the asymptomatic adult controls and both pediatric groups (P < 0.001). The adult thoracic and lumbar DDD patients had back pain that was significantly worse than that of the asymptomatic adult and pediatric control patients (P < 0.0001), but it was not significantly different from that of the adult scoliosis patients for the number of subjects in this study. Leg pain was significantly worse in patients in the adult scoliosis group and lumbar DDD control group relative to those in the asymptomatic adult control, thoracic DDD, and both pediatric groups (P < 0.001; see Fig. 1). Leg pain was considered to be referred pain because, although many patients had central disc herniations (bulging), none had stenosis.
Fig. 1.
Back and leg pain severity measured by the visual analog pain scale among subjects in various study and control groups
Analysis of the pain-drawing scale found that pediatric idiopathic scoliosis patients had a pain area significantly greater than that found in the pediatric control group (P < 0.001; Fig. 2). The adult scoliosis patients had a pain area significantly greater than that of the adult asymptomatic controls and those in both pediatric groups (P < 0.001). Patients in both adult DDD groups had a pain area significantly greater than that of patients in the asymptomatic adult and pediatric control groups (P < 0.0001), but it was not significantly different from that seen in the adult scoliosis patients. Of the 16 pediatric scoliosis patients having pain (minimum VAS > 1.0), the location of the pain was over the apex of the primary curvature in 5; at the midline at levels corresponding to patients’ Schmorl’s nodes in 6; at the curve apex that included Schmorl’s nodes at the apex in 3; in the low back region in 1 with L45 disc degeneration; and in the interscapular region in 2 with normal discs throughout.
Fig. 2.
Size of pain area measured by pain drawing among subjects in various study and control groups
The adult scoliosis patients had more-complex pain patterns often encompassing thoracic and lumbar regions. In the interscapular region, pain coincided with disc degeneration (eight patients), and additional Schmorl’s nodes (three patients) and HNP (one patient). Thoracic curve apex pain in six patients coincided with disc degeneration and Schmorl’s nodes in two, degeneration with inflammatory endplates in three, and disc herniation in two. Pain about the thoracolumbar junction occurred in the midline in four patients, with two having disc degeneration and Schmorl’s nodes and two having degeneration with inflammatory endplates. Pain at the apex of a thoracolumbar curve was present in two who had disc degeneration with both Schmorl’s nodes and inflammatory endplates; one also had an HNP. Midline lumbar pain was most prominent in six patients, all of whom had disc degeneration with inflammatory endplates; of those patients, two also had Schmorl’s nodes, and one had an HNP. Lumbar pain over the curve apex in 11 patients coincided with disc degeneration and with additional inflammatory endplates (8 patients) and Schmorl’s nodes (3 patients). Midline lumbosacral pain was present in 14 patients, all of whom had disc degeneration; 10 of these also had inflammatory endplates, and 1 also had an HNP. One patient with severe pain at the concavity of a lumbosacral fractional curve had disc degeneration with inflammatory endplates.
Analysis of the ODI found that pediatric idiopathic scoliosis patients had scores significantly greater (i.e., worse) than those of patients in the pediatric control group (P = 0.001; Fig. 3). However, the overall degree of disability was clinically mild. The adult scoliosis patients had significantly greater disability than did patients in the asymptomatic adult control and both pediatric groups (P < 0.001). Patients in both adult DDD groups had disability significantly worse than the asymptomatic adult and pediatric control groups (P < 0.0001), but it was not significantly different from that of the adult scoliosis patients.
Fig. 3.
Degree of disability measured by the Oswestry disability index among subjects in various study and control groups
Spinal deformity VAS found that patients in both scoliosis groups considered their appearance significantly more deformed than did those in the asymptomatic and DDD control groups (P < 0.001; Fig. 4). Adult scoliosis patients had a perception of deformity significantly greater than that of the pediatric scoliosis patients (P < 0.01).
Fig. 4.
Effect of deformity on appearance measured by the visual analog scale among subjects in various study and control groups
MRI characteristics
The distribution of disc degeneration (dehydration and narrowing) for the scoliosis patients and their asymptomatic control subjects is shown in Fig. 5. The total number of degenerated discs per subject in the adult and pediatric scoliosis and respective asymptomatic control groups were similar. Although the adult scoliosis and control groups had a similar total number of degenerated discs the distribution found a greater number in the proximal lumbar levels (T12–L3) of the adult scoliosis group relative to all other groups (P < 0.001). Both adult groups had a significantly greater number of degenerated discs than found in the pediatric groups (P < 0.001). A significant correlation of disc degeneration with age was found at r = 0.52 and r = 0.63, respectively, in the adult asymptomatic control group and adult scoliosis group (P < 0.05). There was no significant difference in the rate of thoracic disc degeneration between the three adult groups.
Fig. 5.
Proportion of degenerated (dehydrated) discs in scoliosis patients and asymptomatic control subjects (n = 30 each)
Schmorl’s nodes in patients in the various groups was most common at the thoracolumbar junction (Fig. 6). A significant correlation of Schmorl’s nodes with age was found at r = 0.41 and r = 0.36, respectively, for patients in the asymptomatic pediatric control and those in the adult scoliosis groups (P < 0.05). No difference was found between the adult scoliosis and asymptomatic adult control groups in the total number of discs (per subject) with Schmorl’s nodes. The thoracic spine revealed no significant difference in the number of discs with Schmorl’s nodes among subjects in the adult scoliosis, asymptomatic control, and thoracic DDD groups.
Fig. 6.
Proportion of discs with Schmorl’s nodes in scoliosis patients and asymptomatic control subjects (n = 30 each)
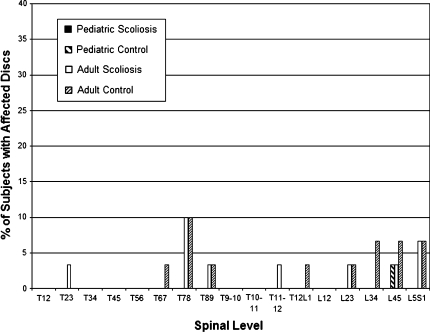
The number of disc herniations (all contained, none extruded or sequestered) was significantly greater in patients in the adult groups than in either pediatric group (P < 0.001; Fig. 7). Patients in the thoracic DDD group had more disc herniations within the thoracic spine per subject; however, owing to the small subject numbers, the difference relative to the adult asymptomatic control and the adult scoliosis groups was not significant (P = 0.06).
Fig. 7.
Proportion of discs with herniations (all contained) in scoliosis patients and asymptomatic control subjects (n = 30 each)
The rate of inflammatory endplate changes was highest in the adult scoliosis patients and had a bi-modal distribution with peaks at L23 and L5S1. The L23 peak corresponded to the apex of the lumbar curve, and in the T12–L3 region the number of affected discs was significantly greater than that found in the adult asymptomatic control or other groups (P < 0.001; Fig. 8a, b). In the adult lumbar DDD group, inflammatory endplate changes were identified at 84 discs in 154 patients. Unlike the bi-modal distribution of the adult scoliosis group, the adult DDD group had only nine discs with changes from T12–3; all the others were at L4–5 (26 discs) or L5–S1 (49 discs) levels. Patients in the adult scoliosis, lumbar DDD, and asymptomatic adult groups had significantly more inflammatory endplate changes than seen in patients in the pediatric groups (P < 0.001). Further comparisons restricted to only thoracic MRI found significantly higher rate of discs with inflammatory endplate changes in the thoracic DDD and adult scoliosis patients relative to those in the asymptomatic adult control group (P = 0.02).
Fig. 8.
a Proportion of discs with inflammatory end plate changes in scoliosis patients and asymptomatic control subjects (n = 30 each). b Coronal MRI demonstrating inflammatory end plate changes at the concave apex of scoliosis in a patient with pain in the corresponding proximal lumbar region of back. Note the relatively normal hydration of the convex aspect of the disc
Relationship of MRI parameters to symptoms
No significant relationship of MRI-revealed disc dehydration to outcome parameters was seen for any group. Statistical analysis found that pediatric scoliosis patients with Schmorl’s nodes had back pain significantly greater than those adolescent scoliosis patients without Schmorl’s nodes (mean VAS pain = 3.2 vs. 1.1, respectively; P = 0.01). Likewise, those pediatric patients with Schmorl’s nodes had an area of pain greater than those without Schmorl’s nodes (mean pain drawing score = 2.5 vs. 0.8, respectively; P < 0.01). The presence of Schmorl’s nodes in patients in the pediatric scoliosis group was significantly correlated with back pain (r = 0.53) and pain drawing (r = 0.60; P = 0.015). Within the adult scoliosis patients, inflammatory endplate changes were weakly correlated with back pain and significantly correlated with leg pain (r = 0.48; P = 0.03). In the combined adult scoliosis and control groups, inflammatory changes were significantly correlated with back pain (r = 0.48), leg pain (r = 0.69), pain area (r = 0.62), and ODI (r = 0.61), respectively (P = 0.03).
Discussion
Back pain is common in adults, and mild back pain is not uncommon in children, [3, 10, 19, 34, 41, 46, 57, 58, 62]. In scoliosis, pain is typically associated with adults and even those with mild curves have twice as much back pain as that experienced by nondeformity controls [6, 14, 54, 64]. A small proportion of scoliosis patients have greater curve magnitudes, and some have severe pain [33, 47]. The purpose of the current study was to place scoliosis patients who had progressed to surgical intervention into the spectrum of pain symptoms and disc degeneration by comparing them to “normal” and advanced symptomatic degenerative control subjects. The present study found that overall disc degeneration was similar in scoliosis and asymptomatic control groups, however, specific aspects of degeneration, such as Schmorl’s nodes and inflammatory endplate changes differed and suggested symptoms in the scoliosis patients may, in part, have a discogenic etiology.
To quantitate pain and disability in this study, a reliable and validated outcomes instrument, which included the pain VAS and ODI, was applied to both deformity and nondeformity patients. Currently, the SRS-22 instrument is often used in patients with deformity [53]. This disease-specific assessment instrument was not available at the onset of our study. Although the SRS-22 has many advantages, it also has limitations, including unknown applicability to patients without deformity and the necessity of reporting multiple subscales to clarify the meaning of baseline total scores and changes in total scores [2, 18]. If one considers the five function related questions of the SRS-22, there is a high correlation to the ODI in adult scoliosis [8]. A limitation of our study is the uncertain correlation of the ODI to the SRS-22 for pediatric patients or applicability of the ODI at all to the pediatric population.
The degree and variability of pain and disability in our patients seem valid as they generally agree with prior reports for both adult and pediatric scoliosis [2, 8, 14, 16, 35, 37, 40, 47, 48, 50, 53, 54, 56, 59]. One prior study allowed direct comparison of the back pain VAS to our study for presurgical pediatric scoliosis patients [48]. It rated the pain of those pediatric patients at a mean of 2.4, whereas our study recorded a mean score of 3.7 for our 16 symptomatic pediatric scoliosis patients. The location of pain in our pediatric patients was often at the apex (convexity) of the curve but, in others, the pain correlated with levels where Schmorl’s nodes were found to be present. In adults, there was a large range of VAS pain severity scores indicating that some patients had surgery primarily for pain and others primarily for curve progression. Within the adult group, pain was most common at the apex in the spine or (diffusely) in the lumbar-lumbosacral regions, which agrees with previous reports [7, 67]. Adult scoliosis back pain is complex and multifactorial. A component of the pain may be related to distal lumbar DDD, as is seen in patients without deformity or, additionally, it may be related to the discs at the apex of the curvature, which are in an abnormal biomechanical environment.
The degenerative findings on MRI of the asymptomatic control groups of the present study appear valid, because the frequency of degenerative disc changes falls into the ranges previously reported [5, 17, 30, 31, 65, 68]. Our study patients with deformity had an overall rate of disc degeneration similar to that of patients in the control group. However, within the proximal lumbar region (T12–L3), which was at the apex of the lumbar curve, there was a significantly higher prevalence of disc degeneration in the adult scoliosis relative to the control group. The lack of pain related to disc degeneration in the nondeformity pediatric population has been noted previously; however, not all studies have come to the same findings [51, 60]. Our study found no significant relationship of disc degeneration (dehydration and narrowing) to pain in either pediatric or adult scoliosis patients.
We found no disc herniation (only the contained type were studied) in our pediatric groups, although prior studies have found that the few herniations seen in the pediatric population may be symptomatic [30, 31, 51, 60]. A few of our adults had disc herniations with a rate similar to that found in prior studies [5]. Only the thoracic DDD patients had contained herniations with chronic pain symptoms.
Recent studies of subjects with lumbar MRI found a correlation to low back pain in subjects with inflammatory (Modic type 1) endplate changes [32, 52]. Additionally, prior studies of adult patients with pain due to lumbar DDD, have found a high correlation of concordant pain in discs with inflammatory endplate changes at provocative discography [11, 20, 42, 52, 61, 66]. Our study also found, as demonstrated in both the adult scoliosis and lumbar DDD patients, that inflammatory endplate changes were related to high pain and disability symptoms. A limitation of the present study is that even patients with inflammatory endplate changes may have other etiologies of back pain, and conversely many adult scolioisis patients without inflammatory endplate changes also had pain. This demonstrates the multifactorial etiology of pain for this group. These limitations could be addressed and our conclusions strengthened by increasing the subject number which would increase the statistical power of the study, and by confirming discogenic pain by discography (not routinely performed in our scoliosis patients). That is, a future study of scoliosis patients which includes the use of multi-level discography could confirm the presumption that inflammatory endplate changes found at the apex of the lumbar curve is related to symptomatic disc degeneration.
Schmorl’s nodes may have a role in pain symptoms. Patients without deformity had Schmorl’s nodes at the same rate as that in patients who had scoliosis; however, those patients with scoliosis and Schmorl’s nodes typically had pain even at a young age, as demonstrated in our pediatric groups and also found in previous studies [30, 50]. Additionally, a previous study of thoracic discography in asymptomatic individuals found intense pain on discography if a disc with Schmorl’s nodes was tested [69]. One can speculate that Schmorl’s nodes in a uniform loading environment may remain asymptomatic. However, under excessive disc-loading conditions, such as asymmetric loading (as seen in spinal deformity) or provocative discography, Schmorl’s nodes become symptomatic.
The present study found a similar rate of overall disc degeneration for the scoliosis and control subjects. Yet, two subgroups, those with Schmorl’s nodes and those with inflammatory endplates reported increased pain compared to controls. Unlike discogenic back pain related to a central disc herniation or posterior annuluar tear, perhaps pain related to Schmorl’s nodes and inflammatory endplates, both of which may occur in an abnormal loading environment combined with abnormal endplates, may be due to innervation of the endplates of vertebral bodies [1, 9, 18, 21, 22, 25, 27, 44, 63]. Specifically, there may be a type of “discogenic pain” that causes symptoms via interosseous nerve endings. This study also questions the notion that adult scoliosis patients are simply deformity patients with additional DDD, as seen in nondeformity patients. Our study findings suggest that this notion is only partly true. Indeed, many adult scoliosis patients have progressive pain in the distal lumbar spine as seen in nondeformity patients with typical chronic low back pain; however, many may also have additional discogenic pain related to the apex of their lumbar curve. Possibly, adult scoliosis patients have additional curvature (altered biomechanical environment) related discogenic pain.
Our study also found that adults with idiopathic lumbar scoliosis, particularly those whose symptoms progress, had greater pain severity than those with thoracic scoliosis, which is consistent with prior reports [23, 53]. This brings into question the role of the additive degeneration on a preexisting lumbar compensatory curve in type-2 curve-pattern scoliosis [29]. Most adult idiopathic scoliosis patients with progressive pain experience that pain in the lumbar region. Typically, these patients, when younger, had a thoracic curve magnitude greater than the lumbar curve but, by the time they progressed to arthrodesis surgery as adults, the preexisting lumbar curve had progressed often to a magnitude greater than that of the thoracic curve.
In conclusion, scoliosis patients who progress to surgery had increased symptoms relative to self-described, asymptomatic, age-matched control patients. Having established a difference in pain and disability, we subgrouped these patients on the basis of their MRI scans for possible correlations of disc abnormalities. In the surgically treated patients of this study, disc dehydration or small, contained herniations were not related to pain. However, pediatric scoliosis patients with Schmorl’s nodes may be at risk for varying degrees of back pain as their curve progresses. Adult idiopathic scoliosis patients in this study had pain and disability comparable to nondeformity patients with advanced DDD, but they had more complex pain patterns suggesting multiple etiologies for pain. Inflammatory endplate changes in the lumbar spine have been previously correlated to low back pain, and this study is the first to suggest that one etiology of pain in adult scoliosis is correlated to inflammatory endplate changes. Many adult scoliosis patients have inflammatory endplate changes but the distribution differs from that in nondeformity DDD patients in that inflammatory endplate changes occur at the apex of their lumbar scoliosis in addition to the distal lumbar spine.
The present study should be considered preliminary in nature. Further study with larger patient numbers and more sensitive testing for discogenic pain (such as multilevel discography) may further define the biomechanical, clinical, and degenerative relationships of pain at the apex of scoliotic deformities. Discographic validation of pain associated with inflammatory endplate changes would suggest that future scoliosis radiographic classifications include MRI in addition to plain radiographs [4].
References
- Antonacci MD, Mody DR, Heggeness MH. Innervation of the human vertebral body: a histologic study. J Spinal Disord. 1998;11:526–531. doi: 10.1097/00002517-199812000-00013. [DOI] [PubMed] [Google Scholar]
- Asher M, Lai SM, Burton D, Manna B. The influence of spine and trunk deformity on preoperative idiopathic scoliosis patients health-related quality of life questionnaire responses. Spine. 2004;29(8):861–868. doi: 10.1097/00007632-200404150-00008. [DOI] [PubMed] [Google Scholar]
- Balague F, Skovron ML, Nordin M, et al. Low back pain in schoolchildren. a study of familial and psychological factors. Spine. 1995;20(11):1265–1270. doi: 10.1097/00007632-199506000-00012. [DOI] [PubMed] [Google Scholar]
- Balderston RA, Albert TJ, McIntosh T, Wong L, Dolinskas C. Magnetic resonance imaging analysis of lumbar disc changes below scoliosis fusions. A prospective study. Spine. 1998;23(1):54–58. doi: 10.1097/00007632-199801010-00011. [DOI] [PubMed] [Google Scholar]
- Battie MC, Videman T. Parent E. lumbar disc degeneration: epidemiology and genetic influences. Spine. 2004;29(23):2679–2690. doi: 10.1097/01.brs.0000146457.83240.eb. [DOI] [PubMed] [Google Scholar]
- Berven S, Deviren V, Demir-Deviren S, et al. Studies in the modified scoliosis research society outcomes instrument in adults: validation, reliability, and discriminatory capacity. Spine. 2003;28(18):2164–2619. doi: 10.1097/01.BRS.0000084666.53553.D6. [DOI] [PubMed] [Google Scholar]
- Briard JL, Jegou D, Cauchiox J. Adult lumbar scoliosis. Spine. 1979;4:526–532. doi: 10.1097/00007632-197911000-00015. [DOI] [PubMed] [Google Scholar]
- Bridwell KH, Cats-Baril W, Harrast J, et al. The validity of the SRS-22 instrument in an adult spinal deformity population compared with the Oswestry and SF-12: a study of response distribution, concurrent validity, internal consistency, and reliability. Spine. 2005;30(4):455–461. doi: 10.1097/01.brs.0000153393.82368.6b. [DOI] [PubMed] [Google Scholar]
- Brown MF, Hukkanen MVI, McCarthy ID, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg. 1997;79B:147–153. doi: 10.1302/0301-620X.79B1.6814. [DOI] [PubMed] [Google Scholar]
- Burton AK, Clarke RD, McClune TD, Tillotson KM. The natural history of low back pain in adolescents. Spine. 1996;21(20):2323–2328. doi: 10.1097/00007632-199610150-00004. [DOI] [PubMed] [Google Scholar]
- Buttermann G. The effect of spinal steroid injections for degenerative disc disease. Spine J. 2004;4(5):495–505. doi: 10.1016/j.spinee.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Court C, Colliou OK, Chin JR, et al. The effect of static in vivo bending on the murine intervertebral disc. Spine. 2001;1(4):239–245. doi: 10.1016/S1529-9430(01)00056-0. [DOI] [PubMed] [Google Scholar]
- Crean JK, Roberts S, Jaffray DC, et al. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine. 1997;22(24):2877–2884. doi: 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- Danielsson AJ, Nachemson AL. Back pain and function 22 years after brace treatment for adolescent idiopathic scoliosis: a case–control study, part I. Spine. 2003;28(18):2078–2085. doi: 10.1097/01.BRS.0000084268.77805.6F. [DOI] [PubMed] [Google Scholar]
- Davids JR, Chamberlin E, Blackhurst DW. Indications for magnetic resonance imaging in presumed adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2004;86A(10):2187–2195. doi: 10.2106/00004623-200410000-00009. [DOI] [PubMed] [Google Scholar]
- Deviren V, Smith JA, Berven SH et al (2002) Treatment of thoracolumbar scoliosis with anterior instrumentation: adults versus adolescents. In: Proceedings of the sixty-ninth annual meeting, American Academy of Orthopaedic Surgeons, Dallas, TX, 13–17 February 2002
- Elfering A, Semmer N, Birkhofer D, et al. Risk factors for lumbar disc degeneration: a 5-year prospective MRI study in asymptomatic individuals. Spine. 2002;27(2):125–134. doi: 10.1097/00007632-200201150-00002. [DOI] [PubMed] [Google Scholar]
- Fagan A, Moore R, Vernon Roberts B, Blumbergs P, Fraser R. The innervation of the intervertebral disc: a quantitative analysis. Spine. 2003;28(23):2570–2576. doi: 10.1097/01.BRS.0000096942.29660.B1. [DOI] [PubMed] [Google Scholar]
- Fairbank JC, Pynsent PB, Poortvliet JA, Phillips H. Influence of anthropometric factors and joint laxity in the incidence of adolescent back pain. Spine. 1984;9(5):461–464. doi: 10.1097/00007632-198407000-00007. [DOI] [PubMed] [Google Scholar]
- Fayad F, Lefevre-Colau MM, Rannou F, Quintero N, Nys A, Mace Y, Poiraudeau S, Drape JL, Revel M (2007) Relation of inflammatory modic changes to intradiscal steroid injection outcome in chronic low back pain. Eur Spine J. Jan 10 EPub [DOI] [PMC free article] [PubMed]
- Fras C, Kravitz P, Mody DR, Heggeness MH. Substance P-containing nerves within the human vertebral body: an immunohistochemical study of the basivertebral nerve. Spine. 2003;3:63–67. doi: 10.1016/S1529-9430(02)00455-2. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Peacock TE, Goupille P, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178–181. doi: 10.1016/S0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- Glassman SD, Berven S, Bridwell K, Horton W, Dimar JR. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine. 2005;30(6):682–688. doi: 10.1097/01.brs.0000155425.04536.f7. [DOI] [PubMed] [Google Scholar]
- Hamanishi C, Kawabata T, Yosii T, Tanaka S. Schmorl’s nodes on magnetic resonance imaging. Their incidence and clinical relevance. Spine. 1994;19(4):450–453. doi: 10.1097/00007632-199402001-00012. [DOI] [PubMed] [Google Scholar]
- Hsu K, Zucherman JF, Derby R, et al. Painful lumbar endplate disruptions: a significant discographic finding. Spine. 1988;13:76–78. doi: 10.1097/00007632-198801000-00018. [DOI] [PubMed] [Google Scholar]
- Inoue M, Minami S, Nakata Y, et al. Preoperative MRI analysis of patients with idiopathic scoliosis: a prospective study. Spine. 2005;30(1):108–114. doi: 10.1097/01.brs.0000149075.96242.0e. [DOI] [PubMed] [Google Scholar]
- Jackson HC, II, Winkelmann RK, Bickel WH. Nerve endings in the human lumbar spinal column and related structures. J Bone Joint Surg. 1966;48A(7):1272–1281. [PubMed] [Google Scholar]
- Jones A, Clarke A, Freeman BJ, et al. The Modic classification: inter- and intraobserver error in clinical practice. Spine. 2005;30(16):1867–1869. doi: 10.1097/01.brs.0000173898.47585.7d. [DOI] [PubMed] [Google Scholar]
- King HA, Moe JH, Bradford DS, Winter RB. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am. 1983;65(9):1302–1313. [PubMed] [Google Scholar]
- Kjaer P, Leboeuf-Yde C, Sorensen JS, Bendix T. An epidemiologic study of MRI and low back pain in 13-year-old children. Spine. 2005;30(7):798–806. doi: 10.1097/01.brs.0000157424.72598.ec. [DOI] [PubMed] [Google Scholar]
- Kjaer P, Leboeuf-Yde C, Korsholm L, et al. Magnetic resonance imaging and low back pain in adults: a diagnostic imaging study of 40-year-old men and women. Spine. 2005;30(10):1173–1180. doi: 10.1097/01.brs.0000162396.97739.76. [DOI] [PubMed] [Google Scholar]
- Kjaer P, Korsholm L, Bendix T, Sorensen JS, Leboeuf-Yde C. Modic changes and their associations with clinical findings. Eur Spine J. 2006;15(9):1312–1319. doi: 10.1007/s00586-006-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostuik JP, Bentivoglio J. The incidence of low-back pain in adult scoliosis. Spine. 1981;6(3):268–273. doi: 10.1097/00007632-198105000-00009. [DOI] [PubMed] [Google Scholar]
- Leboeuf-Yde C, Kyvik KO. At what age does low back pain become a common problem? A study of 29,424 individuals aged 12–41 years. Spine. 1998;23(2):228–234. doi: 10.1097/00007632-199801150-00015. [DOI] [PubMed] [Google Scholar]
- Lerman JA, Sullivan E, Haynes RJ. The pediatric outcomes data collection instrument (PODCI) and functional assessment in patients with adolescent or juvenile idiopathic scoliosis and congenital scoliosis or kyphosis. Spine. 2002;27(18):2052–2058. doi: 10.1097/00007632-200209150-00016. [DOI] [PubMed] [Google Scholar]
- Lonstein J, et al. Patient evaluation. In: Bradford DS, Lonstein JE, Moe JH, et al., editors. Moe’s textbook of scoliosis and other spinal deformities. edn 2. Philadelphia: WB Saunders; 1987. [Google Scholar]
- Mayo NE, Goldberg MS, Poitras B, et al. The Ste-Justine adolescent idiopathic scoliosis cohort study: part 3. Back pain. Spine. 1994;19(14):1573–1581. doi: 10.1097/00007632-199407001-00005. [DOI] [PubMed] [Google Scholar]
- Melrose J, Gurr KR, Cole TC, et al. The influence of scoliosis and aging on proteoglycan heterogeneity in the human intervertebral disc. J Orthop Res. 1991;9(1):68–77. doi: 10.1002/jor.1100090110. [DOI] [PubMed] [Google Scholar]
- Mente PL, Stokes IAF, Spence H, Aronsson DD. Progression of vertebral wedging in an asymmetrically loaded rat tail model. Spine. 1997;22:1292–1296. doi: 10.1097/00007632-199706150-00003. [DOI] [PubMed] [Google Scholar]
- Merola AA, Haher TR, Brkaric M, et al. A multicenter study of the outcomes of the surgical treatment of adolescent idiopathic scoliosis using the scoliosis research society (SRS) outcome instrument. Spine. 2002;27:2046–2051. doi: 10.1097/00007632-200209150-00015. [DOI] [PubMed] [Google Scholar]
- Mierau D, Cassidy JD, Yong-Hing K. Low-back pain and straight leg raising in children and adolescents. Spine. 1989;14(5):526–528. doi: 10.1097/00007632-198905000-00010. [DOI] [PubMed] [Google Scholar]
- Mitra D, Cassar-Pullicino VN, McCall IW. Longitudinal study of vertebral type-1 end-plate changes on MR of the lumbar spine. Eur Radiol. 2004;14(9):1574–1581. doi: 10.1007/s00330-004-2314-4. [DOI] [PubMed] [Google Scholar]
- Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- Niv D, Gofeld M, Devor M. Causes of pain in degenerative bone and joint disease: a lesson from vertebroplasty. Pain. 2003;105(3):387–392. doi: 10.1016/S0304-3959(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Ohnmeiss DD, Gatchel, RJ, eds (2001) North American Spine Society compendium of outcome instruments for assessment and research of spinal disorders. North American Spine Society, La Grange
- Olsen TL, Anderson RL, Dearwater SR, et al. The epidemiology of low back pain in an adolescent population. Am J Public Health. 1992;82(4):606–608. doi: 10.2105/AJPH.82.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perennou D, Marcelli C, Herisson C, Simon L. Adult lumbar scoliosis: epidemiologic aspects in a low-back pain population. Spine. 1994;19(2):123–128. doi: 10.1097/00007632-199401001-00001. [DOI] [PubMed] [Google Scholar]
- Pratt RK, Burwell RG, Cole AA, Webb JK. Patient and parental perception of adolescent idiopathic scoliosis before and after surgery in comparison with surface and radiographic measurements. Spine. 2002;27(14):1543–1552. doi: 10.1097/00007632-200207150-00012. [DOI] [PubMed] [Google Scholar]
- Rajwani T, Bagnall KM, et al. Using magnetic resonance imaging to characterize pedicle asymmetry in both normal patients and patients with adolescent idiopathic scoliosis. Spine. 2004;29(7):E145–E152. doi: 10.1097/01.BRS.0000120507.36611.8D. [DOI] [PubMed] [Google Scholar]
- Ramirez N, Johnston CE, Browne RH. The prevalence of back pain in children who have idiopathic scoliosis. J Bone Joint Surg Am. 1997;79(3):364–368. doi: 10.2106/00004623-199703000-00007. [DOI] [PubMed] [Google Scholar]
- Salminen JJ, Erkintalo M, Laine M, Pentti J. Low back pain in the young. a prospective three-year follow-up study of subjects with and without low back pain. Spine. 1995;20(19):2101–2107. doi: 10.1097/00007632-199510000-00006. [DOI] [PubMed] [Google Scholar]
- Schenk P, Laubli T, Hodler J, Klipstein A. Magnetic resonance imaging of the lumbar spine: findings in female subjects from administrative and nursing professions. Spine. 2006;31(23):2701–2706. doi: 10.1097/01.brs.0000244570.36954.17. [DOI] [PubMed] [Google Scholar]
- Schwab FJ, Smith VA, Biserni M, et al. Adult scoliosis: a quantitative radiographic and clinical analysis. Spine. 2002;27(4):387–392. doi: 10.1097/00007632-200202150-00012. [DOI] [PubMed] [Google Scholar]
- Schwab F, Dubey A, Pagala M, et al. Adult scoliosis: a health assessment analysis by SF-36. Spine. 2003;28(6):602–606. doi: 10.1097/00007632-200303150-00016. [DOI] [PubMed] [Google Scholar]
- Schwab F, el-Fegoun AB, Gamez L, Goodman H, Farcy JP. A lumbar classification of scoliosis in the adult patient: preliminary approach. Spine. 2005;30(14):1670–1673. doi: 10.1097/01.brs.0000170293.81234.f0. [DOI] [PubMed] [Google Scholar]
- Shapiro GS, Taira G, Boachie-Adjei O. Results of surgical treatment of adult idiopathic scoliosis with low back pain and spinal stenosis. Spine. 2003;28(4):358–363. doi: 10.1097/00007632-200302150-00009. [DOI] [PubMed] [Google Scholar]
- Sjolie AN, Ljunggren AE. The significance of high lumbar mobility and low lumbar strength for current and future low back pain in adolescents. Spine. 2001;26(23):2629–2636. doi: 10.1097/00007632-200112010-00019. [DOI] [PubMed] [Google Scholar]
- Taimela S, Kujala UM, Salminen JJ, Viljanen T. The prevalence of low back pain among children and adolescents. a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22(10):1132–1136. doi: 10.1097/00007632-199705150-00013. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Delecrin J, Passuti N. Surgical treatment of idiopathic scoliosis in adults: an age-related analysis of outcome. Spine. 2002;27(16):1742–1748. doi: 10.1097/00007632-200208150-00011. [DOI] [PubMed] [Google Scholar]
- Tertti MO, Salminen JJ, Paajanen HE, et al. Low-back pain and disk degeneration in children: a case–control MR imaging study. Radiology. 1991;180(2):503–507. doi: 10.1148/radiology.180.2.1829844. [DOI] [PubMed] [Google Scholar]
- Toyone T, Takahashi K, Kitahara H, et al. Vertebral bone-marrow changes in degenerative lumbar disc disease. J Bone Joint Surg. 1994;76B:757–763. [PubMed] [Google Scholar]
- Turner PG, Green JH, Galasko CS. Back pain in childhood. Spine. 1989;14:812–814. doi: 10.1097/00007632-198908000-00007. [DOI] [PubMed] [Google Scholar]
- Dieen JH, Weinans H, Toussaint HM. Fractures of the lumbar vertebral endplate in the etiology of low back pain: a hypothesis on the causative role of spinal compression in aspecific low back pain. Med Hypotheses. 1999;53:242–252. doi: 10.1054/mehy.1998.0754. [DOI] [PubMed] [Google Scholar]
- Weinstein SL, Dolan LA, Spratt KF, et al. Health and function of patients with untreated idiopathic scoliosis: a 50-year natural history study. JAMA. 2003;289(5):559–567. doi: 10.1001/jama.289.5.559. [DOI] [PubMed] [Google Scholar]
- Weishaupt D, Zanetti M, Hodler J, Boos N. MR imaging of the lumbar spine: prevalence of intervertebral disk extrusion and sequestration, nerve root compression, endplate abnormalities, and osteoarthritis of the facet joints in asymptomatic volunteers. Radiology. 1998;209(3):661–666. doi: 10.1148/radiology.209.3.9844656. [DOI] [PubMed] [Google Scholar]
- Weishaupt D, Zanetti M, Hodler J, et al. Painful lumbar disk derangement: relevance of endplate abnormalities at MR imaging. Radiology. 2001;218(2):420–427. doi: 10.1148/radiology.218.2.r01fe15420. [DOI] [PubMed] [Google Scholar]
- Winter RB, Lonstein JE, Denis F. Pain patterns in adult scoliosis. Orthop Clin North Am. 1988;19(2):339–345. [PubMed] [Google Scholar]
- Wood KB, Garvey TA, Gundry C, Heitoff KB. Magnetic resonance imaging of the thoracic spine: evaluation of asymptomatic individuals. J Bone Joint Surg. 1995;77(11):1631–1638. doi: 10.2106/00004623-199511000-00001. [DOI] [PubMed] [Google Scholar]
- Wood KB, Schellhaus KP, Garvey TA, Asppli D. Thoracic discography in healthy individuals: a controlled prospective study of magnetic resonance imaging and discography in asymptomatic and symptomatic individuals. Spine. 1999;24(15):1548–1555. doi: 10.1097/00007632-199908010-00008. [DOI] [PubMed] [Google Scholar]