Abstract
The mechanisms by which bone marrow (BM)-derived stem cells might contribute to angiogenesis and the origin of neovascular endothelial cells (ECs) are controversial. Neovascular ECs have been proposed to originate from VEGF receptor 2-expressing (VEGFR-2+) stem cells mobilized from the BM by VEGF or tumors, and it is thought that angiogenesis and tumor growth may depend on such endothelial precursors or progenitors. We studied the mobilization of BM cells to circulation by inoculating mice with VEGF polypeptides, adenoviral vectors expressing VEGF, or tumors. We induced angiogenesis by syngeneic melanomas, APCmin adenomas, adenoviral VEGF delivery, or matrigel plugs in four different genetically tagged universal or endothelial cell-specific chimeric mouse models, and subsequently analyzed the contribution of BM-derived cells to endothelium in a wide range of time points. To study the existence of circulating ECs in a nonmyeloablative setting, pairs of genetically marked parabiotic mice with a shared anastomosed circulatory system were created. We did not observe specific mobilization of VEGFR-2+ cells to circulation by VEGF or tumors. During angiogenesis, abundant BM-derived perivascular cells were recruited close to blood vessel wall ECs but did not form part of the endothelium. No circulation-derived vascular ECs were observed in the parabiosis experiments. Our results show that no BM-derived VEGFR-2+ or other EC precursors contribute to vascular endothelium and that cancer growth does not require BM-derived endothelial progenitors. Endothelial differentiation is not a typical in vivo function of normal BM-derived stem cells in adults, and it has to be an extremely rare event if it occurs at all.
Keywords: angiogenesis, cancer, progenitor, stem cell, differentiation
Postnatal neovascularization was originally considered to result from the proliferation, migration, and remodeling of differentiated endothelial cells (ECs) derived from preexisting blood vessels at the site of angiogenesis (1). However, a contemporary belief about the source of ECs responsible for vascular growth in adults is that a significant and crucial part of neovascular ECs originates from circulating stem and progenitor cells that are first mobilized from the bone marrow (BM) and subsequently differentiate to mature bona fide ECs and incorporate in the vasculature. According to this idea, BM-derived circulating ECs contribute to vascular endothelium in various situations of postnatal angiogenesis. This concept has become a common theme in modern vascular biology (2–12). Importantly, it has been proposed that the recruitment of BM-derived endothelial progenitors is important for tumor angiogenesis (13–16) and that BM-derived ECs could constitute as much as 50% (17) or 38% (14) of all ECs in tumor neovessels.
EC precursors were originally defined to be circulating VEGF receptor 2-expressing (VEGFR-2+) cells that are mobilized from the BM by VEGF (3, 13, 18–20). It has been suggested that BM-derived vascular ECs differentiate from common myeloid progenitors and granulocyte/macrophage progenitors and that ECs are an intrinsic component of myeloid lineage differentiation (21). BM-derived EC precursors would provide a powerful approach to block tumor angiogenesis (22). Correspondingly, therapeutic endothelial precursor cell transplantation would be a promising approach to restore tissue vascularization after ischemic events (23). Clinical trials with human patients are already ongoing based on the circulating EC progenitor concept (24, 25).
However, in some reports, the BM contribution to endothelium has been estimated to be very low, averaging from 4.9% to undetectable (26–30). Thus, the mechanisms by which BM-derived cells may contribute to angiogenesis and the origin of neovascular ECs remain controversial. We therefore wanted to rigorously study the mobilization and differentiation of stem and precursor cells from the adult BM during angiogenesis and tumor growth. First, we analyzed the effects of VEGF or tumors on the mobilization of BM cells to circulation. We also created chimeric mice in three different mouse backgrounds by transplanting to lethally irradiated wild-type (WT) recipients BM cells derived from transgenic mice expressing reporter genes GFP or DsRed.T3 under the universal chicken β-actin promoter or lacZ under endothelial cell-specific (VEGFR-2 or Tie-1) promoters. After recovery, angiogenesis was induced in the engrafted chimeric recipients by s.c. injections with adenoviral vectors expressing VEGF (AdVEGF) or matrigel plugs supplemented with murine VEGF. Alternatively, angiogenesis was induced by s.c. inoculation of syngeneic B16 melanomas or by using the APCmin spontaneous adenoma tumor model (31). After allowing the new blood vessels to grow from 1 day to 6 months, the tissues were analyzed for the presence of cells expressing β-gal, GFP, or DsRed.T3. To study the possible existence of circulating ECs in a nonmyeloablative setting, we used the parabiotic system to establish a shared anastomosed circulatory system (32) between young APCmin mice (31) and mice ubiquitously expressing GFP under the β-actin promoter (33). Our results demonstrate that BM-derived circulating EC precursors do not contribute to vascular endothelium and are not required for tumor growth.
Results
No VEGFR-2+ Cells Are Mobilized from the BM by Systemic VEGF or Tumors, and All BM-Derived Cells in Angiogenic Tissues Are Perivascular in Location.
We first studied the effects of VEGF on BM cell mobilization by treating WT mice with murine VEGF164 polypeptides or with i.v. administration of adenoviral vectors expressing murine VEGF164 or lacZ (34). Recombinant VEGF164 was administered to C57BL/6 mice (n = 6) by i.p. injection daily for 5 days. The dosing scheme (10 μg VEGF per mouse per day, i.p) and the mouse strain used (C57BL/6) were the same as in the original work describing circulating EC progenitors (3). The adenoviral vectors were used within the same concentration range (AdVEGF, AdLacZ, 1 × 108–1 × 109 pfu) as reported in earlier papers describing the mobilization of VEGFR-2+ EC precursors in mice by adenoviral VEGF delivery (13, 18, 19). Adenovirus titers were measured according to international ARMWG standard adenovirus prep, with routines suggested by the adenovirus standardization working group (35). Replication competent adenovirus levels were measured in A546 cells with a standard viral cytopathic effect assay (36). VEGFR-2+ or VEGFR-2+/CD11b− circulating cells that were earlier proposed to be EC precursors (2, 3, 13, 16, 18, 20, 37–42) were detected by using flow cytometry [see supporting information (SI) Materials and Methods]. Detection of VEGFR-2+ cells was controlled by FACS analysis for VEGFR-2 and CD31 by using murine MS-1 ECs as positive control (Fig. S1). We observed no mobilization of total WBCs, VEGFR-2+ cells, or VEGFR-2+/CD11b− cells in response to VEGF protein (Fig. 1A). Because the levels of the supposed EC precursors have been suggested to vary between different mouse strains (43), we performed the adenoviral experiments in both C57BL/6 and BALB/c mice (n = 6 in each treatment group). BALB/c mice were treated with AdVEGF at a dose of 1 × 109 pfu (Fig. 1B). The (fatal) biological action of expressing systemic VEGF was well demonstrated because the 1 × 109 pfu dose was lethal in C57BL/6 mice (data not shown). This finding is in agreement with earlier findings by Thurston et al., who described dose-dependent reduction in the survival of C57BL/6 mice after AdVEGF and a 100% lethality in doses >108 pfu (44). Thus, C57BL/6 mice received 1 × 108 pfu of AdVEGF (Fig. S1). In both BALB/c and C57BL/6 mice, a single i.v. administration of AdVEGF or AdLacZ promoted a transient unspecific mobilization of haematopoietic cells from the BM, as can be seen in WBC counts. We found no evidence of mobilization of VEGFR-2+ or VEGFR-2+/CD11b− cells in AdVEGF-treated mice. A statistically significant mobilization of VEGFR-2+ cells was observed in BALB/c mice treated with AdLacZ (Fig. 1B). We also used s.c. injections of AdVEGF to induce local angiogenesis in chimeric mice with GFP-tagged BM and studied the localization of BM-derived cell populations in the angiogenic tissues. Because putative EC precursors are described as VEGFR-2+ cells (2, 3, 13, 16, 18, 20, 37–42), we used two different monoclonal antibodies against VEGFR-2 (clones AVAS 12α1 and DC101) to detect these cells (Fig. 1B and Fig. S2). The tissues were typically analyzed at 14 days after the AdVEGF injection, but time points from 7 days to 6 months also were studied. The AdVEGF-treated tissues exhibited enhanced angiogenesis and contained large numbers of infiltrating BM-derived GFP+ cells (Fig. S2). All BM-derived cells were periendothelial, having a stromal or perivascular location (Fig. 1B and Fig. S2). After analyzing AdVEGF-treated tissues from >25 chimeric mice, we found no BM-derived vascular ECs expressing VEGFR-2+ or other EC markers (Fig. 2).
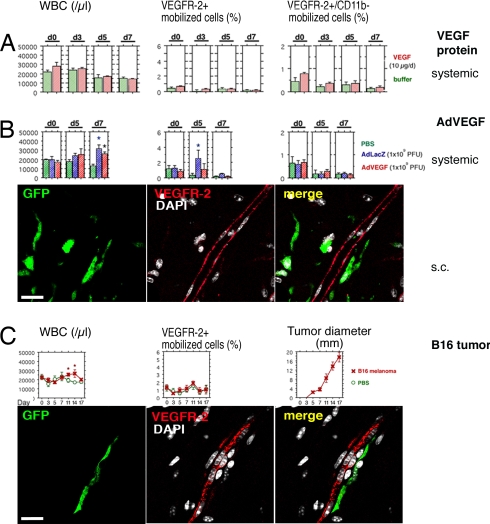
Fig. 1.
Systemic VEGF or tumors do not promote the mobilization of VEGFR-2+ BM cells to circulation, and BM-derived VEGFR-2+ cells do not form part of the growing endothelium. Peripheral blood cells were isolated on indicated days, counted, and analyzed by FACS. Day 0 (d0) shows baseline levels before inoculation. The results are given as mean ± SE. The asterisks indicate statistical significance (P < 0.05). The s.c. angiogenesis was analyzed in chimeric mice with transgenic GFP-tagged BM. VEGFR-2+ cells were detected (antibody clone AVAS 12α1) and analyzed by multichannel confocal scanning. The nuclei are stained with DAPI (white) to recognize individual cells. (A) No mobilization of BM cell populations was observed in response to systemic treatment with VEGF polypeptides. (B) Systemic adenoviral administration caused nonspecific mobilization of cells from the BM, as can be seen in the WBC counts. The results on WBC mobilization were identical by using AdLacZ or AdVEGF. Statistically significant mobilization of VEGFR-2+ cells was observed on day 5 in the control mice treated with AdLacZ. Numerous GFP-tagged BM-derived perivascular cells (green) were recruited to the site of angiogenesis after s.c. AdVEGF. No VEGFR-2+ (red) ECs originating from the BM were discovered in the blood vessel endothelium. (Scale bar: 20 μm.) (C) The s.c. B16 tumors caused mobilization of hematopoietic cells from the BM on days 11–14, simultaneously to the highest growth rate of the tumors. Large numbers of BM-derived perivascular cells (green) were infiltrating the angiogenic tumor stroma. No VEGFR-2+ (red) vascular ECs originating from the BM were discovered. (Scale bar: 20 μm.)
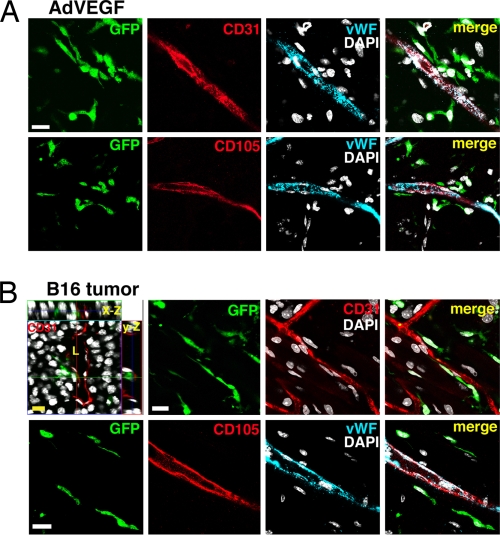
Fig. 2.
All BM-derived cells recruited during AdVEGF- or tumor-induced angiogenesis are perivascular. ECs were detected using confocal scanning against CD31, CD105, or vWF. Transgenic GFP reporter is expressed in BM-derived cells. The nuclei are stained with DAPI (white) to recognize individual cells. No vascular ECs originating from the BM were detected. (Scale bars: 20 μm.) (A) Angiogenesis was induced in the chimeric hosts by s.c. AdVEGF. A time point of 14 days after administration is shown. (B) Angiogenesis was induced in the chimeric hosts by s.c. inoculation of B16 melanomas. A late time point of 21 days is shown. The 3D orthogonal projections (x–z and y–z axes) also are shown, and tumor blood vessel lumen (L) is indicated.
Mobilization of VEGFR-2+ endothelial precursors from the BM to circulation and their incorporation to vascular endothelium has been reported to occur during tumor growth in C57BL/6 mice (13). We therefore inoculated C57BL/6 mice with syngeneic B16 melanomas or PBS (n = 12 in both groups). B16 tumors promoted a mobilization of hematopoietic cells from the BM (Fig. 1C). This mobilization was evident at days 11–14 simultaneously to the highest growth rate of the tumors (Fig. 1C). No differences were observed between the tumor mice and the controls in mobilized VEGFR-2+ cells or cells expressing the adult stem cell markers CD117 (c-Kit) or Sca-1 that also have been suggested to be expressed on EC precursors (4, 16, 19, 20, 39, 41, 42, 45, 46) (Fig. 1C and Fig. S1). The tumor vasculature was analyzed at different time points after 7–21 days of growth. We used longitudinal, sagittal, and cross-sectional confocal scanning and 3D orthogonal projections with various endothelial markers as well as lectin perfusion staining (47) of functional blood vessels. Correct detection of the endogenous GFP signal was controlled by staining part of the samples with an anti-GFP antibody (data not shown). Tumor tissues persistently contained high numbers of periendothelial BM-derived cells (Fig. 1C and Fig. S2). No BM-derived vascular ECs expressing VEGFR-2+ were found (Fig. 1C and Fig. S2). Correspondingly, no BM-derived ECs were detected by using other endothelial-specific markers von Willebrand Factor (vWF), CD31/PECAM-1, and CD105/endoglin (Fig. 2) or functional lectin perfusion (tumor tissues from >30 chimeric mice were analyzed) (Fig. S2). The close proximity of some of the BM-derived perivascular cells and blood vessel wall ECs is demonstrated by optical sectioning in Fig. S2.
All BM-Derived Cells in Matrigel Plug Neovasculature Are Perivascular.
Next, we injected s.c. matrigel basement membrane matrix plugs (supplemented with VEGF164) into chimeric GFP+ BM-engrafted C57BL/6 mice. All blood vessels observed later within the plugs must be novel, neoangiogenic vessels. BM-derived EC precursors have earlier been described to be essential for the formation of neovasculature within matrigel plugs in mice (13). We analyzed the plugs typically at 7 or 14 days after the matrigel inoculation, but time points from 1 day to 6 months also were studied. A high number of new blood vessels could be observed within the plugs when they were removed (Fig. 3A). The ECs of the plug neovasculature stained readily for VEGFR-2, CD105, vWF, and CD31, and the plugs were infiltrated by high numbers of BM-derived GFP+ cells (Fig. 3 and Fig. S3). The infiltrating BM-derived cells could be observed 1 day after the implantation, and the first ECs could be seen within the plugs in <3 days (data not shown). By 1 week, the forming neovasculature could be seen in very close contact with the numerous BM-derived cells (Fig. 3B). We also wanted to confirm detection of possible GFP+ ECs. Therefore, we implanted plugs in transgenic C57BL/6-Tg(ACTB-EGFP)1Osb/J mice where all tissues, including the blood vessels, are GFP+ (positive control) (Fig. 3C). GFP+ ECs in the positive control mice colocalized with VEGFR-2 signal (Fig. 3C). After analyzing matrigel plugs from >40 chimeric mice with engrafted GFP+ BM, we did not detect any VEGFR-2+ BM-derived vascular ECs (Fig. 3C and Fig. S3A). Similarly, no GFP+ vascular endothelia were detected when antibodies against vWF, CD31, or CD105 were used (Fig. S3A). However, we constantly observed high numbers of BM-derived perivascular cells (Fig. 3 and Fig. S3). Corresponding experiments also were performed with 129S6/B6-F1 mice engrafted with DsRed.T3+ BM (48). DsRed.T3 was used because it has been proposed that GFP by itself might selectively induce certain genes in ECs and thus perhaps affect their functions (49). Again, no BM-derived VEGFR-2+ or other vascular ECs were observed (Fig. S3B) (data not shown).
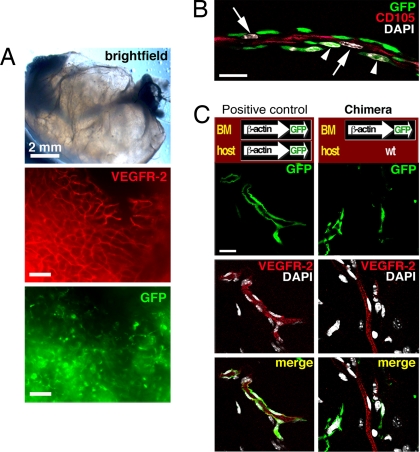
Fig. 3.
No BM-derived vascular ECs are present in matrigel plugs. All blood vessels observed within the plugs must be novel. (A) A high number of VEGFR-2+ (red; antibody clone AVAS 12α1) blood vessels and abundant BM-derived GFP+ cells can be seen. (Scale bars: 100 μm.) (B) At 1 week after the implantation of plug, the ECs (arrows) of the forming neovasculature (stained for CD105, red) could be seen in very close contact with the infiltrating BM-derived perivascular cells (arrowheads) when studied by multichannel confocal scanning. The nuclei are stained with DAPI (white). (Scale bar: 20 μm.) (C) Confocal scans of the whole mounts. The positive control is a transgenic C57BL/6-Tg(ACTB-EGFP)1Osb/J mouse, where all of the tissues including the blood vessel vessels are GFP+. The positive control confirms successful detection of GFP+ ECs. The ECs stain readily for VEGFR-2 (AVAS 12α1). In chimeric mice, where the WT C57BL/6 hosts are engrafted with transgenic GFP+ BM, no BM-derived VEGFR-2+ endothelium can be found. (Scale bar: 20 μm.)
Endothelial Cell-Specific Reporter Gene Systems Detect No BM-Derived VEGFR-2+ or Tie-1+ ECs in Angiogenic Neovasculature.
We next wanted to test our findings by using two different endothelial cell-specific genetic models for the lacZ marker gene expression. BM from transgenic donor mice expressing β-gal under the VEGFR-2 [C57BL/6J-Kdrtm1Jrt (50); VEGFR-2-promoter-lacZ mice] or the Tie-1 promoter [CD-1/129Sv-tielcz (51); Tie-1-promoter-lacZ mice] were transplanted to syngeneic WT recipients to specifically detect VEGFR-2+ or Tie-1+ BM-derived cells, respectively (Fig. 4A). The chimeric hosts were implanted with matrigel plugs, and we also implanted plugs to transgenic donors and WT recipients to serve as controls. At the end of the experiments, the plugs and BM were examined for the presence of β-gal-expressing cells. Sections of the plugs were stained with Nuclear fast red to visualize the nuclei, thus also confirming equal cellularity within plugs from the different groups. Plugs from the transgenic VEGFR-2-promoter-lacZ donor mice or from the transgenic Tie-1-promoter-lacZ donor mice had abundant β-gal+ neoangiogenic vessels (Fig. 4 B and C), and their BMs contained constant, low numbers (<0.001% of total BM cells) of β-gal+ cells (Fig. S4), presumably ECs of the BM vasculature or haematopoietic stem cells expressing VEGFR-2 or Tie-1 (52, 53). The plugs or BM samples from the WT recipients contained no β-gal+ cells (Fig. 4 B and C and Fig. S4). Identically to the transgenic BM of the donor animals, the BMs from the chimeric recipients contained constant, low numbers (<0.001% of total BM cells) of β-gal+ cells, confirming the engraftment and detection of the VEGFR-2+ or Tie-1+ cells from the BM transplants (Fig. S4). However, in >40 matrigel plugs from the chimeric mice, we detected no β-gal+ vascular ECs, demonstrating that BM-derived cells did not differentiate to endothelium (Fig. 4 B and C).
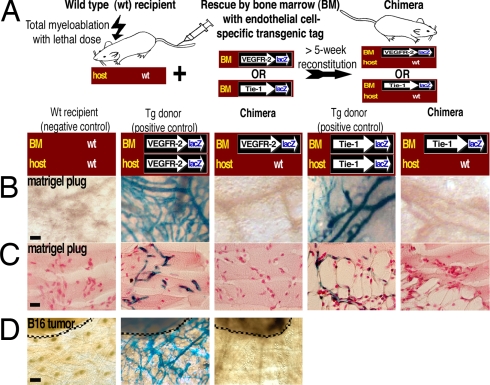
Fig. 4.
Endothelial cell-specific genetic reporter systems identify no BM-derived VEGFR-2+ or Tie-1+ ECs in angiogenic neovasculature. (A) Strategy for the two EC-specific gene tag and BM-transplantation models. Transgenic β-gal expression is driven by the promoter for the endothelial cell-specific gene VEGFR-2 or Tie-1. WT mice serve as negative controls. Transgenic donor mice serve as positive controls and confirm successful detection of β-gal+ cells. (B) No BM-derived β-gal+ vascular ECs can be found in the chimeric mice, demonstrating that BM-derived cells do not differentiate to VEGFR-2+ or Tie-1+ vascular ECs. (Scale bar: 50 μm.) (C) Sections of the plugs were stained with Nuclear fast red to visualize the nuclei, thus also confirming equal cellularity within the matrigel plugs. (Scale bar: 20 μm.) (D) The mice were implanted with B16 melanomas (dashed lines). No BM-derived β-gal+ endothelium can be found in the chimeric mice, demonstrating that tumor angiogenesis or growth do not involve or require contribution from VEGFR-2+ circulating EC precursors. (Scale bar: 100 μm.)
Tumor Angiogenesis and Growth Do Not Require BM-Derived Progenitors for Vascular ECs.
Importantly, we wanted to study the origin of tumor neovasculature in the lacZ-tagged reporter system for VEGFR-2 expression. We implanted B16 melanomas to transgenic VEGFR-2-promoter-lacZ donor mice, WT C57BL/6 recipient mice, and chimeric C57BL/6 mice with engrafted VEGFR-2-promoter-lacZ BM. The tumors were allowed to grow from 7 to 21 days. Tumor vasculature in the transgenic VEGFR-2-promoter-lacZ donor mice (positive controls) contained abundant β-gal+ vessels, whereas no β-gal+ vessels or vascular ECs were detected in the WT recipient mice (negative controls) or in the chimeric mice with VEGFR-2-promoter-lacZ BM (n = 16) (Fig. 4D). The results decisively show that BM-derived cells did not differentiate to VEGFR-2+ tumor endothelium, and therefore tumor angiogenesis or growth do not require contribution from BM-derived VEGFR-2+ progenitors for vascular ECs.
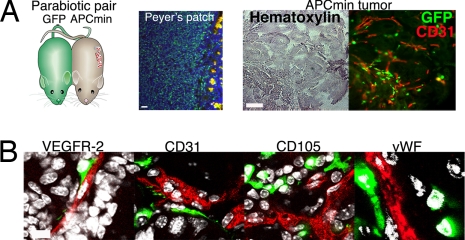
No Circulation-Derived Vascular ECs Are Observed in the Parabiosed APCmin Tumors.
To study the possible existence of circulating ECs in a nonmyeloablative setting, we created pairs of genetically marked parabiotic mice that have a shared anastomosed circulatory system (33). APCmin tumor mice were surgically conjoined to mice expressing GFP under the β-actin promoter at 7–9 weeks of age (before the onset of tumorigenesis) and then killed at 22–24 weeks (Fig. 5A). Upon being killed, the parabiosed APCmin mice presented 20–55 adenomas per mouse. Chimerism of lymphoid cells in the Peyer's patch of the APCmin partner mice was used to monitor the 50% blood cell chimerism in the system (Fig. 5A). No GFP+ vascular ECs were observed in the parabiosed APCmin tumors (Fig. 5B), thus confirming the results obtained with myeloablative BM transplantations.
Fig. 5.
No circulation-derived vascular ECs were observed in the parabiosed APCmin tumors. (A) Pairs of parabiotic mice that have a shared anastomosed circulatory system were created by cojoining GFP-expressing mice with young APCmin mice. Chimerism of lymphoid cells in the Peyer's patch of the APCmin mouse demonstrates the 50% blood cell chimerism in the system. Upon killing at 22–24 weeks of age, the parabiosed APCmin mice presented 20–55 adenomas per mouse. Overview pictures of the APCmin tumor are shown in brightfield and fluorescence. Note close association of the GFP+ circulating cells to the vascular endothelium (CD31 stain, red). (Scale bars: 50 μm.) (B) Confocal scans show that circulating cells did not contribute to host vascular endothelium. GFP-tagged cells in angiogenic APCmin tumor tissues are shown in green. The nuclei are stained with DAPI (white). (Scale bars: 20 μm.)
Discussion
Our present experiments demonstrate that large numbers of BM-derived perivascular cells are recruited to angiogenic sites during neovascularization or tumor growth. Especially during the early phases of angiogenesis, the perivascular BM-derived cells were numerous and often in very close contact with the underlying vascular wall ECs. It is evident from previous studies that BM may contribute to angiogenesis through various hematopoietic cell populations such as monocytes or other myeloid blood cells. These proangiogenic hematopoietic cells can release angiogenic factors or create permissive conditions that favor the growth of locally derived blood vessels (10, 26, 28, 54–60).
Earlier studies reported mobilization of VEGFR-2+ or VEGFR-2+/CD11b− EC precursors to circulation by systemic treatment with VEGF protein or adenoviral VEGF (3, 13, 18–20). In our hands, mobilization of these cells to circulation was not observed. It has been proposed that recruitment of BM-derived endothelial progenitors is important during tumor angiogenesis (13–16), and studies with C57BL/6 mice with tagged BM have estimated that the BM-derived ECs constitute as much as half of all ECs in tumor neovessels (17). We were not able to reproduce these earlier findings. Putative EC precursors have been defined as VEGFR-2+ cells (2, 3, 13, 16, 18, 20, 37–42), but we did not detect any BM-derived VEGFR-2+ vascular ECs by the various tumor or angiogenesis models and reporter systems that we used, including a specific genetic reporter model for VEGFR-2 expression in BM-derived cells. No BM-derived vascular ECs were detected by using any other endothelial cell-specific marker. Neither did we find any indication of mobilization of EC precursors to circulation during tumor growth. There has been controversy concerning the time during neovascularization at which the proposed contribution of EC precursors to neovascular vessels might occur. Some studies imply that EC precursors have a significant effect on vascular growth during the early stages of neoangiogenesis or tumor growth (15, 61), whereas other studies have reported them to have a role in late-stage tumor vascularization or dissemination (14, 41, 62). GFP-tagged BM-derived ECs were estimated to represent 38% of all tumor ECs in late-stage insulinomas, thus suggesting that a substantial number of BM-derived EC precursors predominantly contribute to later stages of vessel remodeling (14). We analyzed angiogenic tissues in a wide range of time points from 1 day to 6 months after the induction of angiogenesis by matrigel plugs, AdVEGF, or tumors. The results were the same at any time point studied, early or late.
We also used careful high-resolution multichannel (sequential) confocal scanning of whole mounts capable of visualizing 3D vascular structures and identifying single cells. The very close proximity of BM-derived perivascular cells and blood vessel wall ECs is demonstrated by optical sectioning in Fig. S2. Conventional histological analyses of thin tissue sections cannot distinguish marker colocalization from superimposition of ECs and the numerous closely adjacent hematopoietic cells that infiltrate tissues, as demonstrated in Fig. S5. Earlier studies describing EC precursors may therefore have suffered from technical difficulties in distinguishing hematopoietic cells from the adjacent vessel wall ECs. Furthermore, subpopulations of monocytes, macrophages, T cells, B cells, and/or primitive hematopoietic progenitor cells share markers such as CD31, Tie-2, CD105, VE-cadherdin (CD144), VEGFR-1, and VEGFR-2 with ECs, and they also bind lectins and take up acetylated LDL (28, 63–67). In our samples, single BM-derived cells were occasionally seen expressing CD31, VEGFR-1, or VEGFR-2, but these cells were always stromal or perivascular in location (data not shown). These perivascular hematopoietic cell populations sporadically expressing EC markers may earlier have been misinterpreted as BM-derived ECs.
Initial reports described contributions of BM cells to several nonhematopoietic lineages, including vascular ECs, encouraging clinicians to move into patient trials (24, 68–72). However, it is by now obvious from multiple studies that the contribution of BM to nonhematopoietic cell types such as myocardium, skeletal muscle, or liver hepatocytes is exceedingly rare (73–76). Similarly, although the capacity of adult BM stem cells to differentiate to vascular ECs in other selective in vivo models or in vitro may still be undetermined, our current results plainly demonstrate that endothelial differentiation is not a typical in vivo function of normal BM-derived stem cells and that it has to be an extremely rare event if it occurs at all. Importantly, we now also show that angiogenesis during tumor growth does not involve or require contribution from BM-derived circulating progenitors for vascular ECs.
Materials and Methods
Genetically Tagged Mice.
The following strains were used as donors in the BM transplantations: B6.129S7-Gt(ROSA)26Sor/J, C57BL/6-Tg(ACTB-EGFP)1Osb/J, C57BL/6J-Kdrtm1Jrt (all from Jackson Laboratory), 129S6/B6-F1-TgN(ACTB-DsRed.T3) Nagy (48), and CD-1/129Sv-tielcz (51).
Supplementary Material
Acknowledgments.
We thank Dr. Ian Kasman (Genentech, South San Francisco, CA) for tissue preparation of the parabiotic mice. This work was supported by the Finnish Academy of Sciences, the Sigrid Juselius Foundation, the Finnish Cancer Organizations, and the European Union (Tumor-Host Genomics LSHC-CT-2005-518198).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710516105/DCSupplemental.
References
- 1.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi T, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruzinova MB, et al. Effect of angiogenesis inhibition by Id loss and the contribution of bone-marrow-derived endothelial cells in spontaneous murine tumors. Cancer Cell. 2003;4:277–289. doi: 10.1016/s1535-6108(03)00240-x. [DOI] [PubMed] [Google Scholar]
- 7.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: Towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 8.Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2:805–810. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaked Y, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 10.De Palma M, Naldini L. Role of haematopoietic cells and endothelial progenitors in tumour angiogenesis. Biochim Biophys Acta. 2006;1766:159–166. doi: 10.1016/j.bbcan.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Craig N, Li J, Lindahl T, Maynard O, West S. Histology: The lives and deaths of cells in tissues. In: Albers BJA, Lewis J, Raff M, Roberts KPW, editors. Molecular Biology of the Cell. New York and London: Garland Science; 2002. p. 1280. [Google Scholar]
- 12.Folkman J, Kalluri R. Tumor angiogenesis. In: Kufe D, Pollock R, Weichselbaum R, Bast RJ, Gansler T, Holland J, Frei E, editors. Cancer Medicine. Hamilton, Canada: BC Decker; 2003. pp. 165–166. [Google Scholar]
- 13.Lyden D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 14.Spring H, Schuler T, Arnold B, Hammerling GJ, Ganss R. Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci USA. 2005;102:18111–18116. doi: 10.1073/pnas.0507158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nolan DJ, et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21:1546–1558. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciarrocchi A, et al. Id1 restrains p21 expression to control endothelial progenitor cell formation. PLoS ONE. 2007;2:e1338. doi: 10.1371/journal.pone.0001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Barros M, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 18.Hattori K, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heissig B, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aicher A, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 21.Bailey AS, et al. Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci USA. 2006;103:13156–13161. doi: 10.1073/pnas.0604203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: Novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 23.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 24.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: Part II: Cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 25.Kawamoto A, Losordo DW. Endothelial progenitor cells for cardiovascular regeneration. Trends Cardiovasc Med. 2008;18:33–37. doi: 10.1016/j.tcm.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 27.Gothert JR, et al. Genetically tagging endothelial cells in vivo: Bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104:1769–1777. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- 28.De Palma M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Peters BA, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 30.Rajantie I, et al. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 32.Bunster E, Meyer RK. An improved method of parabiosis. Anat Rec. 1933;339:339–343. [Google Scholar]
- 33.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 34.Vajanto I, et al. Evaluation of angiogenesis and side effects in ischemic rabbit hindlimbs after intramuscular injection of adenoviral vectors encoding VEGF and LacZ. J Gene Med. 2002;4:371–380. doi: 10.1002/jgm.287. [DOI] [PubMed] [Google Scholar]
- 35.Hutchins B, et al. Working toward an adenoviral vector testing standard. Mol Ther. 2000;2:532–534. doi: 10.1006/mthe.2000.0217. [DOI] [PubMed] [Google Scholar]
- 36.Dion LD, Fang J, Garver RI., Jr Supernatant rescue assay vs. polymerase chain reaction for detection of wild type adenovirus-contaminating recombinant adenovirus stocks. J Virol Methods. 1996;56:99–107. doi: 10.1016/0166-0934(95)01973-1. [DOI] [PubMed] [Google Scholar]
- 37.Modarai B, Burnand KG, Sawyer B, Smith A. Endothelial progenitor cells are recruited into resolving venous thrombi. Circulation. 2005;111:2645–2653. doi: 10.1161/CIRCULATIONAHA.104.492678. [DOI] [PubMed] [Google Scholar]
- 38.Vasa M, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 39.Iwakura A, et al. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 40.Urbich C, et al. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med. 2005;11:206–213. doi: 10.1038/nm1182. [DOI] [PubMed] [Google Scholar]
- 41.Gao D, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 42.Bertolini F, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–4346. [PubMed] [Google Scholar]
- 43.Shaked Y, et al. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis: Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 44.Thurston G, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 45.Bailey AS, et al. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood. 2004;103:13–19. doi: 10.1182/blood-2003-05-1684. [DOI] [PubMed] [Google Scholar]
- 46.Chavakis E, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang YS, et al. Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA. 2000;97:14608–14613. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vintersten K, et al. Mouse in red: Red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis. 2004;40:241–246. doi: 10.1002/gene.20095. [DOI] [PubMed] [Google Scholar]
- 49.Zhang F, et al. Green fluorescent protein selectively induces HSP70-mediated up-regulation of COX-2 expression in endothelial cells. Blood. 2003;102:2115–2121. doi: 10.1182/blood-2003-01-0049. [DOI] [PubMed] [Google Scholar]
- 50.Shalaby F, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 51.Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14:5884–5891. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziegler BL, et al. KDR receptor: A key marker defining hematopoietic stem cells. Science. 1999;285:1553–1558. doi: 10.1126/science.285.5433.1553. [DOI] [PubMed] [Google Scholar]
- 53.Yano M, et al. Expression and function of murine receptor tyrosine kinases, TIE and TEK, in hematopoietic stem cells. Blood. 1997;89:4317–4326. [PubMed] [Google Scholar]
- 54.Salven P, Hattori K, Heissig B, Rafii S. Interleukin-1alpha promotes angiogenesis in vivo via VEGFR-2 pathway by inducing inflammatory cell VEGF synthesis and secretion. FASEB J. 2002;16:1471–1473. doi: 10.1096/fj.02-0134fje. [DOI] [PubMed] [Google Scholar]
- 55.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grunewald M, et al. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 57.Salven P, Orpana A, Joensuu H. Leukocytes and platelets of patients with cancer contain high levels of vascular endothelial growth factor. Clin Cancer Res. 1999;5:487–491. [PubMed] [Google Scholar]
- 58.Fazel S, et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okamoto R, et al. Hematopoietic cells regulate the angiogenic switch during tumorigenesis. Blood. 2005;105:2757–2763. doi: 10.1182/blood-2004-08-3317. [DOI] [PubMed] [Google Scholar]
- 60.Takakura N, et al. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102:199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 61.Stoll BR, Migliorini C, Kadambi A, Munn LL, Jain RK. A mathematical model of the contribution of endothelial progenitor cells to angiogenesis in tumors: Implications for antiangiogenic therapy. Blood. 2003;102:2555–2561. doi: 10.1182/blood-2003-02-0365. [DOI] [PubMed] [Google Scholar]
- 62.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clauss M, et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 64.Udani VM, et al. Hematopoietic stem cells give rise to perivascular endothelial-like cells during brain tumor angiogenesis. Stem Cells Dev. 2005;14:478–486. doi: 10.1089/scd.2005.14.478. [DOI] [PubMed] [Google Scholar]
- 65.Rohde E, et al. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357–367. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 66.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 67.Case J, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Strauer BE, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 69.Stamm C, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 70.Schachinger V, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 71.Assmus B, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 72.Seeger FH, Zeiher AM, Dimmeler S. Cell-enhancement strategies for the treatment of ischemic heart disease. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S110–S113. doi: 10.1038/ncpcardio0734. [DOI] [PubMed] [Google Scholar]
- 73.Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nat Med. 2001;7:393–395. doi: 10.1038/86439. [DOI] [PubMed] [Google Scholar]
- 74.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 75.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 76.Balsam LB, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.