Abstract
Women with germ-line mutations of the BRCA1 tumor suppressor gene are highly susceptible to breast and ovarian cancer. The protein product of BRCA1 is involved in a broad spectrum of biological processes and interacts with many diverse proteins. One of these, BARD1, associates with BRCA1 to form a heterodimeric complex that is enzymatically active as an ubiquitin E3 ligase. Although the BRCA1/BARD1 heterodimer has been implicated in several aspects of BRCA1 function, its role in tumor suppression has not been evaluated. To address this question, we generated mouse strains carrying conditional alleles of either Bard1 or Brca1 and used Cre recombination to inactivate these genes in mammary epithelial cells. Significantly, the conditional Bard1- and Brca1-mutant mice developed breast carcinomas that are indistinguishable from each other (and from those of double conditional Bard1/Brca1-mutant animals) with respect to their frequency, latency, histopathology, and cytogenetic features. Reminiscent of the basal-like breast carcinomas seen in human BRCA1 mutation carriers, these tumors are “triple negative” for estrogen and progesterone receptor expression and HER2/neu amplification. They also express basal cytokeratins CK5 and CK14, have an elevated frequency of p53 lesions, and display high levels of chromosomal instability. The remarkable similarities between the mammary carcinomas of Bard1-, Brca1-, and Bard1/Brca1-mutant mice indicate that the tumor suppressor activities of both genes are mediated through the BRCA1/BARD1 heterodimer.
Keywords: basal-like breast cancer, mammary carcinogenesis
Germ-line mutations of the BRCA1 tumor suppressor gene serve as predisposing lesions in women with a familial susceptibility to breast and ovarian cancer (1). The breast tumors of BRCA1 mutation carriers typically display “basal-like” features that define a subtype of breast cancer with a distinct histopathology and gene expression profile (2–5). Basal-like breast carcinomas have been described as “triple negative” because they often lack expression of estrogen receptor (ER) α, the progesterone receptor (PR), and the HER2/neu protooncogene. Patients with basal-like breast cancer face a poor prognosis and reap little benefit from current therapies that target ER- or HER2-expressing tumor cells.
Although the mechanism of BRCA1-mediated tumor suppression is unclear, its protein product has been implicated in a remarkably broad range of cellular processes, some of which serve to maintain genome stability (6–8). Consistent with its pleiotropic nature, the BRCA1 polypeptide has been reported to interact with a large and diverse group of proteins. One of these, BARD1, is structurally related to BRCA1 in that it harbors an N-terminal RING motif and two C-terminal BRCT domains (9), and the heterodimer complex formed by these proteins functions as a potent ubiquitin E3 ligase (10). Although it has been proposed that the tumor suppression activity of BRCA1 is mediated by the BRCA1/BARD1 heterodimer (11), experimental evidence to support this hypothesis is lacking.
Early attempts to develop animal models of BRCA1-linked breast cancer were unsuccessful (reviewed in ref. 12). Tumor formation was not observed in heterozygous mice bearing null or hypomorphic Brca1 alleles, whereas early embryonic lethality precluded tumor development in Brca1 nullizygous mice. Nevertheless, homozygous mice with certain hypomorphic Brca1 alleles can survive to adulthood, on which they display heightened susceptibility to a range of tumors, including mammary carcinomas (13). In addition, tumors can also be induced by conditional inactivation of Brca1 in breast epithelial cells through cre/loxP-mediated recombination (14), and recent studies show that the mammary tumors generated by conditional Brca1 inactivation resemble the basal-like breast tumors of human BRCA1 mutation carriers (15, 16).
Although many BRCA1 functions are executed by the BRCA1/BARD1 heterodimer, the role of this complex in BRCA1-mediated tumor suppression has not been tested. If the heterodimer is required for tumor suppression, one would expect that BARD1 deficiency, in either clinical or experimental settings, would promote the formation of tumors that resemble those arising in BRCA1 mutation carriers. Tumor-specific lesions of BARD1 have been observed in human cancers, including breast and ovarian carcinomas (17–19), and although these mutations are rare, they are often associated with tumor-specific loss of the other BARD1 allele (18). Nevertheless, unlike the cancer-predisposing lesions of BRCA1, which are mostly frameshift or nonsense mutations that grossly disrupt its protein coding potential, the known tumor-specific lesions of BARD1 are all missense mutations (17–19). To test the tumor suppression potential of BARD1 experimentally, we have generated mouse strains that undergo mammary-specific inactivation of the Bard1 and/or Brca1 genes. Analysis of these strains revealed that conditional inactivation of Bard1 induces basal-like mammary carcinomas with a frequency, latency, and histopathology that are indistinguishable from those that develop in conditional Brca1-mutant mice and double conditional Bard1/Brca1-mutant mice. These results establish that BARD1 is itself a tumor suppression gene and that BRCA1-mediated tumor suppression is mediated by the BRCA1/BARD1 heterodimer.
Results
Mammary-Specific Inactivation of Bard1 and Brca1.
Because the tumor suppressor activity of BRCA1 can be demonstrated experimentally by disrupting the mouse Brca1 gene (13–16), we used a similar strategy to ascertain whether BARD1 also functions as a tumor suppressor. To bypass the embryonic lethality of Bard1 nullizygous (Bard1−/−) mice (20), a conditional Bard1 allele (Bard1flex1) was generated by homologous recombination in ES cells. Two loxP sites were positioned within the Bard1flex1 allele such that a DNA fragment encompassing the promoter (≈2 kbp of 5′ flanking sequence) and exon 1 (which encodes the initiator methionine and part of the RING domain) of the Bard1 gene would be deleted on Cre-mediated recombination [supporting information (SI) Fig. S1]. To compare the consequences of mammary-specific ablation of Bard1 and Brca1, we also generated a conditional Brca1 allele by flanking exon 2, which encodes most of the RING domain, with loxP sites (Fig. S2). In contrast to the early embryonic lethality of Bard1- and Brca1-nullizygous embryos (20), homozygous Bard1flex1/flex1 and Brca1flex2/flex2 mice are healthy, fertile, and have a normal lifespan, indicating that the loxP sites of the conditional Bard1flex1 and Brca1flex2 alleles do not interfere with normal function of these genes. To assess the functions of the Cre-recombined products of the conditional alleles (i.e., Bard1Δflex1 and Brca1Δflex2), Bard1flex1/flex1 and Brcaflex2/flex2 mice were mated, respectively, with Bard1+/− and Brca1+/− animals that carry a ubiquitously expressed cre transgene (Hscre) driven by the mouse Hsp70-1 gene promoter (21). Notably, the Bard1Δflex1/−/Hscre and Brca1Δflex2/−/Hscre offspring of these crosses suffered an early embryonic lethality (data not shown) similar to that of nullizygous (i.e., Bard1−/− or Brca1−/−) mice. These results indicate that the Bard1Δflex1 and Brca1Δflex2 alleles that arise, respectively, on Cre-mediated recombination of Bard1flex1 and Brca1flex2 are nonfunctional.
To inactivate the conditional alleles in mammary glands, Bard1flex1/flex1 and Brca1flex2/flex2 animals were crossed with Bard1+/−, Brca1+/−, and Wapcre/+ knockin mice that express cre under control of regulatory elements from the whey acidic protein (Wap) gene, expression of which is restricted to mammary epithelial cells during late pregnancy and lactation (22). These crosses, and additional crosses of the relevant progeny, generated conditional Bard1 (Bard1flex1/flex1/Wapcre/+ and Bard1flex1/−/Wapcre/+) and Brca1 (Brca1flex2/flex2/Wapcre/+ and Brca1flex2/−/Wapcre/+) females, all of whom appeared normal in that they were fertile, had normal litter sizes, and were able to nurse their pups.
Mammary Tumor Development in Conditional Bard1, Brca1, and Bard1/Brca1 Females.
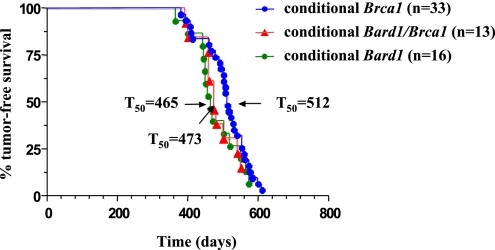
Experimental cohorts of conditional Bard1 (n = 16; 10 Bard1flex1/flex1/Wapcre/+ and 6 Bard1flex1/−/Wapcre/+) and conditional Brca1 (n = 33; 20 Brca1flex2/flex2/Wapcre/+ and 13 Brca1flex2/−/Wapcre/+) females were mated to induce at least one round of pregnancy and lactation, and then monitored for tumor formation. As shown in Fig. 1, mammary tumors developed with high incidence and long latency in the experimental cohorts, whereas all control animals [Bard1flex1/+/Wapcre/+ (n = 10) and Brca1flex2/+/Wapcre/+ (n = 11)] remained tumor-free over the entire observation period (data not shown). A total of 21 mammary tumors developed in 15 of 16 conditional Bard1/Wapcre/+ females with a median tumor-free survival (T50) of 465 days (Fig. 1). Notably, 35 tumors appeared in 31 of 33 conditional Brca1/Wapcre/+ females with a latency (T50 = 512 days) that is statistically indistinguishable from that of the Bard1/Wapcre/+ mice (P = 0.1977, log-rank test) (Fig. 1). Southern blot analysis confirmed that the conditional Bard1 and Brca1 alleles were recombined in these mammary tumors (Figs. S1C and S2C).
Fig. 1.
Survival curves of conditional Bard1, Brca1, and Bard1/Brca1 mutant mice. Kaplan–Meier curves of tumor-free survival for conditional Bard1/Wapcre/+ (n = 16), conditional Brca1/Wapcre/+ (n = 33), and double conditional Brca1/Bard1/Wapcre/+ (n = 13) animals. The differences between the tumor-free survivals of the three experimental cohorts are statistically insignificant. For each cohort, the median tumor-free survival (T50) is shown in days.
To examine the consequences of concomitant inactivation of Bard1 and Brca1, we also monitored a cohort of double conditional Bard1flex1/flex1/Brca1flex2/flex2/Wapcre/+ females and observed 18 tumors develop in 11 of 13 experimental females with a T50 of 473 days. As shown in Fig. 1, the survival curves of the three experimental cohorts are overlapping and statistically indistinguishable (P = 0.2660, log-rank test with df = 2). Furthermore, all pairwise comparisons of the cohort survival curves yielded statistically insignificant results (Bard1 vs. Brca1, P = 0.1977; Bard1 vs. Bard1/Brca1, P = 0.8643; and Brca1 vs. Bard1/Brca1, P = 0.1479), indicating that the mutant alleles of these genes are epistatic with respect to mammary carcinogenesis. These results support the hypothesis that Bard1 and Brca1 promote tumor suppression through common genetic/biochemical pathways.
The Basal-Like Phenotype of Bard1-, Brca1-, and Bard1/Brca1-Mutant Mammary Tumors.
The mammary tumors of the conditional Bard1 and conditional Brca1 females were invasive adenocarcinomas that formed bulky, round, solid nests and invaded with a broad front the surrounding fat and underlying pectoral muscle (Fig. 2 B and D). These solid cancer nodules were separated by highly vascular but delicate stroma, and each contained densely packed syncytial-appearing tumor cells. No lymphocytic infiltrate was seen along the border of the neoplasms. Residual ductal/tubular differentiation was consistently identified, but it constituted the dominant pattern (>75%) in only 14.3 or 8.6% of the Bard1- or Brca1-mutant malignancies, respectively (Table 1). Both Bard1- and Brca1-deficient cancer cells were strikingly similar, with irregularly shaped nuclei that featured coarsely granular chromatin and conspicuous eosinophilic nucleoli (Fig. 2 B and D, Insets). Mitotic figures were abundant and often atypical with distorted and/or multipolar spindles. The majority of the Bard1- or Brca1-mutant tumors were Dunn type B adenocarcinomas with a medullary growth pattern that displayed variable necrosis and lacked peripheral lymphocytic infiltrate. As such, these tumors were highly reminiscent of the human atypical medullary carcinomas that are prevalent in human BRCA1 mutation carriers (2). Sarcomatous, adenoid-cystic, and myoepithelial histological patterns, which are also considered to be basaloid in human neoplasms, were not observed in the mammary tumors of these cohorts. However, metaplastic cancers with squamous differentiation occurred in 4 of 21 Bard1-mutant and 8 of 35 Brca1-mutant carcinomas, although the squamous component was never the dominant histological architecture.
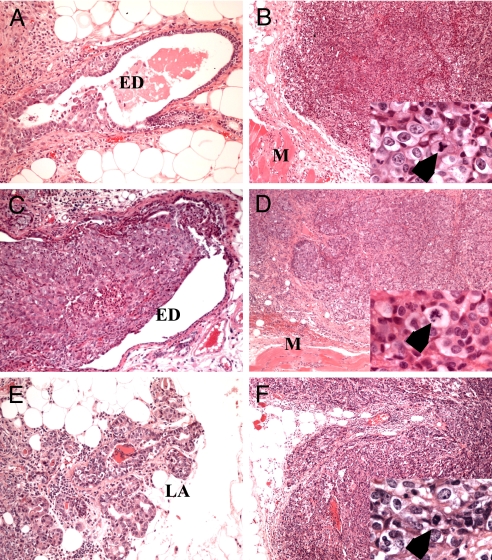
Fig. 2.
Histological analysis of mammary tumors. H&E-stained sections of Bard1-mutant (A and B), Brca1-mutant (C and D), and Bard1/Brca1-double-mutant (E and F) mammary carcinomas display virtually identical pathohistological characteristics. MIN (A, C, and E), which probably arose from lobuloalveolar units (LA), commonly extended into extralobular breast ducts (ED). Invasive carcinomas (B, D, and F) were composed of large solid nests of markedly pleomorphic cells with numerous mitotic figures including tripolar mitotic figures and those with lagging chromosomes (B, D, and F, Insets, arrows). Such neoplasms invaded along a broad front and pushed toward the underlying pectoral muscle (M). (Original magnification: A–F, ×200; Insets, ×2,000.)
Table 1.
Summary of histopathological observations in mammary tumors
| Genotype (mutant) | Total no. of animals | Total no. of tumors | Patterns |
CK14* | ER-negative† | ||
|---|---|---|---|---|---|---|---|
| Tubular | Solid | Squamous | |||||
| Bard1 | 15 | 21 | 3 (14.3%) | 18 (85.7%) | 4 (19.1%) | 19/21 (90.5%) | 19/21 (90.5%) |
| Brca1 | 31 | 35 | 3 (8.6%) | 32 (91.4%) | 8 (22.9%) | 34/35 (97.1%) | 30/35 (85.7%) |
| Bard1/Brca1 | 11 | 18 | 4 (22.2%) | 14 (77.8%) | 2 (11.1%) | 14/18 (77.8%) | 18/18 (100%) |
*At least 15% of cancer cells had to be positive for CK14 for a neoplasm to be considered to have basaloid features.
†Neoplasms were considered to be negative for ER only when <5% of tumor cells stained positive.
Murine intraepithelial neoplasia (MIN) was detected both adjacent and distal to each invasive carcinoma examined, and within separate mammary glands, suggesting that multiple MIN foci developed in each mouse and that one or a few of these foci progressed to invasive carcinoma. The distinction between in situ vs. invasive lesions was aided by immunostaining for p63, which highlights the myoepithelial layer (data not shown). MIN consistently presented as a solid growth of large highly pleomorphic cells in both the lobuloalveolar unit and the extralobular breast ducts (Fig. 2 A and C). Because central necrosis was commonly observed, this form of MIN is comparable with the solid and comedo subtypes of human ductal carcinoma in situ.
By immunohistochemistry, most Bard1-mutant (19 of 21) (Fig. 3 B and C) and Brca1-mutant (34 of 35) (Fig. 3 F and G) invasive neoplasms stained for the cytoskeletal markers of basal-like breast cancer, including CK5 (data not shown), CK14, and vimentin (Table 1). Vimentin is also a marker of the epithelial to mesenchymal transition. However, as is typical for epithelial rather than mesenchymal cells, all Bard1- and Brca1-mutant murine breast cancers retained E-cadherin staining along the cell surface, including cells at the invasive front (data not shown). Although staining for ER was readily observed in the normal epithelial cells of adjacent or entrapped glands (Fig. 3E), most Bard1-mutant (19 of 21) (Fig. 3A) and Brca1-mutant (30 of 35) (Fig. 3E) invasive neoplasms were ER-negative (Table 1). In addition, none of the tumors of either genotype stained positive for PR (both α and β) (data not shown). Interestingly, high-grade MIN was also usually negative for both ER and PR. In addition, none of the tumors analyzed showed HER2/neu amplification as determined by Southern analysis of tumor DNA compared to tail DNA from the same animal (R.S. and T.L., unpublished data). Applying a stringent definition of the basal-like phenotype that includes positive staining for both CK5 and -14, negative expression of both ER and PR, and the absence of HER2/neu gene amplification, 90.5 and 85.7% of Bard1- and Brca1-mutant mammary tumors, respectively, were categorized as basal-like breast carcinomas.
Fig. 3.
The immunohistochemical phenotypes of mammary carcinomas. The Bard1- (A–D), Brca1- (E–H), or Bard1/Brca1- (I–L) mutant mammary carcinomas shared a common basal-like phenotype that included negative staining for estrogen receptor (ERα) (A, E, and I) when compared to entrapped nonneoplastic mammary epithelial (E, arrow), and strong immunolabeling for vimentin (B, F, and J) and CK14 (C, G, and K). Note CK14 immunolabeling is already present in the MIN (C, Inset). Nuclear p53 immunostaining was often detected in the tumors (D, H, and L) and MIN (D, Inset), and correlated well with the presence of p53 missense mutations (see Table S2).
On coinactivation of Bard1 and Brca1, the double conditional mice developed invasive adenocarcinomas (Figs. 2 E and F and 3 I–L) similar to those seen in the single conditional Bard1- and Brca1-mutant animals (Figs. 2 A–D and 3 A–H). All of the Bard1/Brca1-mutant tumors analyzed were “triple negative” for ER (Fig. 3I), PR, and HER2/neu gene amplification (R.S. and T.L., unpublished data), and most stained positive for CK5, CK14, and vimentin (Fig. 3 J and K and Table 1). Thus, according to the aforementioned definition of basal-like breast cancer, 77.8% of Bard1/Brca1-mutant tumors were classified as basal-like carcinomas. Again, the in situ phase (MIN) was multifocal and widespread, appearing in both lobuloalveolar units (LA) and extralobular breast ducts, and featured a solid or comedo-type growth pattern (Fig. 2E). In summary, the histopathologic and immunohistochemical analyses indicate that the tumors arising on conditional inactivation of the Bard1, Brca1, or Bard1/Brca1 genes in mice exhibit an atypical medullary phenotype strongly reminiscent of the basal-like breast tumors that arise in human BRCA1 mutation carriers.
p53 Lesions in Bard1-Mutant Mammary Tumors.
Relative to other subtypes of human breast cancer, lesions of the p53 gene are especially prevalent in basal-like tumors, including those of BRCA1 mutation carriers (23, 24). By immunohistochemistry, we observed strong p53 nuclear staining in the majority of cancer cells from 70% (7 of 10) of the Bard1-mutant, 78% (7 of 9) of the Brca1-mutant, and 67% (8 of 12) of the double Bard1/Brca1-mutant mammary carcinomas examined (Fig. 3). To evaluate the pattern of p53 mutation in Bard1-mutant mammary carcinomas, p53 exons 2–11 were PCR amplified and subjected to DNA sequence analysis (Table S1). Six of the 10 Bard1-mutant tumors (60%) harbored detectable p53 gene mutations, and in each of these six tumors the wild-type p53 allele was lost. Four of the six mutations led to amino acid substitutions (Y217D, M234I, R270H, and C272F) that resulted in nuclear p53 immunostaining. The two remaining p53 mutations did not result in p53 immunostaining: a G331W missense mutation and a 46-bp deletion within exon 6 that causes a frameshift and premature termination. Interestingly, the amino acid altered by the R270H mutation corresponds to a mutation hot spot in the human p53 sequence (human residue R273), and this same residue was also found to be mutated in two of the Brca1-mutant tumors (data not shown) and a reported Brca2-deficient mouse mammary tumor (22).
Of note, MIN foci that lie adjacent to invasive breast carcinomas frequently displayed the same p53 labeling properties as the invasive carcinoma. For example, immunostaining of a tumorous mammary gland from a conditional Bard1-mutant mouse (Fig. 3D Inset) showed comparable levels of p53 overexpression in malignant cells harboring a p53 mutation (R270H) and in the cells of an adjacent MIN lesion. This observation supports the hypothesis that p53 mutations can manifest early in Bard1- and/or Brca1-deficient tumor cells and that inactivation of p53 checkpoint function can be a critical step in tumorigenesis (25).
Chromosomal Instability in Bard1- and Brca1-Mutant Tumor Cells.
Although abnormalities of chromosome number and structure are prevalent in breast cancer, early work established that chromosomal instability is especially severe in the tumors of BRCA1 mutation carriers (26) and that cultured tumor cells from mouse mammary carcinomas induced by Brca1 inactivation have elevated levels of chromosome instability (14, 27). To determine whether Bard1-mutant mammary tumors display a similar pattern of chromosomal instability, cell lines were derived from mammary tumors of conditional Bard1-mutant and Brca1-mutant female mice, and early passage (2–6) cultures of these lines were examined by spectral karyotyping (SKY). Each of the three Bard1-mutant mammary tumor lines displayed a triploid (3N; 58–66 chromosomes) karyotype with complex structural rearrangements (Fig. 4A). In accord with previous studies (14, 27), a similar array of numerical and structural lesions was observed in cultured cells from three independent mammary carcinomas that arose in conditional Brca1/Wapcre/+ animals (Fig. 4B). To assess the extent of genomic instability, we calculated an index of chromosome instability (CIN) based on the number of chromosome breaks per haploid genome and found a similar range of CIN ratios in the Bard1-mutant (41–74 breaks per haploid genome) and Brca1-mutant (35–67 breaks per haploid genome) carcinoma cells (Table S2). To determine whether this high level of CIN is unique to Bard1- and Brca1-mutant mammary carcinomas, we also examined cells of mammary tumors derived on breast-specific inactivation of the p53 gene (in p53flex7/flex7/Wapcre/+ mice, to be described elsewhere). Although the p53-mutant tumors displayed extensive aneuploidy, with chromosome numbers in the range of 3N–6N (Table S2), very few structural chromosome aberrations were observed (Fig. 4C). Thus, the CIN indices of the p53-mutant tumors were nearly 10-fold lower than those seen in the Bard1- and Brca1-mutant mammary carcinomas (Table S2).
Fig. 4.
Karyotype analysis of primary mammary carcinoma cells. Chromosomal instability identified by spectral karyotype analysis in Bard1-, Brca1-, and p53-mutant mammary tumor cells. (A) Mutant Bard1 tumor cell line. The left image shows spectral classification of a metaphase exhibiting high frequency of multiple complex structural chromosome abnormalities. The right image showing spectral-based display color (Upper) and the corresponding DAPI image in black and white (Lower) of partial metaphases exhibiting various chromosomal abnormalities. The most visible abnormalities are indicated on DAPI-stained images. (Left) a, Translocations involving two different chromosomes; b, formation of metacentric chromosome involving centromeres of two different chromosomes; c, dicentric chromosome involving translocation of two chromosomes; d, dicentric chromosome formation by intrachromosomal rearrangement; e, acentric chromosome. (Center) a, A large marker chromosome with multiple centromeres involving four different chromosomes. (Right) a, Tricentric chromosome involving translocation of five different chromosomes; b, duplication of centromeric region of chromosome 18; c, translocation involving two chromosomes. (B) Mutant Brca1 tumor cell line. The left and right images are as in A. (Left) a–d, Translocations involving two (a and d), three (c), and four (b) chromosomes; e and f, centromere duplication; g, dicentric formation due to telomeric fusion. (Center) Chromosome translocations involving two (b–e and g) and three (a and f) chromosomes. (Right) a, Formation of a dicentric chromosome involving three different chromosomes; b–f, simple chromosome translocations involving two chromosomes. (C) A hyper-triploid SKY karyotype of a tumor derived by mammary gland specific inactivation of p53. Note very few chromosomal translocations are seen in these tumors. Translocation partners are indicated.
Discussion
BRCA1 has been implicated in a remarkably diverse array of cellular processes, many of which serve in some capacity to preserve genome integrity. Its pleiotropic nature is consistent with biochemical evidence that BRCA1 can interact with many different proteins and, as an ubiquitin E3 ligase, can potentially catalyze covalent modification of these proteins. Thus, it will be important to define which of the various BRCA1 functions, or which combinations of these functions, are required for tumor suppression—especially in those settings, such as mammary and ovarian epithelial cells, that are relevant to human disease. Previous studies have shown that conditional disruption of the mouse Brca1 gene induces mammary carcinomas that resemble the basal-like breast tumors of human BRCA1 mutation carriers (14–16). Here, we report that conditional inactivation of Bard1, the heterodimeric partner of Brca1, elicits basal-like mammary tumors in mice that are indistinguishable from those that arise on Brca1 inactivation. These results establish that BARD1 is itself a tumor suppressor. More significantly, the striking similarities between the Brca1- and Bard1-mutant mammary carcinomas indicate that the tumor suppressor functions of both proteins are mediated by the BRCA1/BARD1 heterodimer.
A major challenge of cancer modeling is to generate experimental tumors that recapitulate both the responsible genetic lesion(s) and consequent neoplastic phenotype of the relevant human malignancy. The mammary carcinomas of the Bard1-mice, like those of Brca1-mutant mice, are highly reminiscent of the basal-like atypical medullary breast tumors that arise in human BRCA1 mutation carriers. Thus, the Bard1-mutant tumors are “triple negative” in that they lack expression of the estrogen and progesterone receptors and show no signs of HER2/neu gene amplification. They also express basal cytokeratins CK5 and CK14, have an elevated frequency of p53 lesions, and display extensive chromosomal instability. Interestingly, although all mammary tumors induced by conditional inactivation of Bard1, Brca1, and p53 were aneuploid, elevated levels of chromosomal breaks were only observed in the Bard1- and Brca1-mutant tumors (Table S2), perhaps reflecting the known requirements for BRCA1 and BARD1 in double-strand DNA break repair (28–31).
Our data demonstrate that BARD1, like BRCA1, is a tumor suppressor that normally functions to inhibit neoplastic transformation in mammary epithelial cells. As such, the tumor-specific BARD1 mutations observed in patients with breast, ovarian, and endometrial carcinomas may be oncogenic lesions that disrupt BRCA1/BARD1-mediated tumor suppression. However, it should be noted that the Bard1 defect induced in our mouse model is a null mutation, whereas all of the BARD1 lesions reported to date in human tumors are missense mutations (17–19). Thus, further studies are required to ascertain whether these human BARD1 missense mutations are truly oncogenic.
The existing biochemical and cellular data are consistent with the conclusion that BRCA1 and BARD1 polypeptides function in vivo as an obligate heterodimer (11). Coimmunoprecipitation studies in mammalian cells and Xenopus egg extracts indicate that most, if not all, of the cellular pool of endogenous BRCA1 exists in complex with BARD1 (32, 33). Moreover, the two proteins remain associated as a heterodimer and colocalize within common nuclear structures both before and after genotoxic stress (34, 35), and their steady-state levels fluctuate in parallel during cell cycle progression (36). Significantly, homozygous mice bearing null alleles of either Brca1 or Bard1 display phenotypes that appear to be indistinguishable from one another, and from that of double Brca1/Bard1 nullizygous animals (20). In particular, these mice become developmentally retarded and die before gastrulation [between embryonic day 7.5 (E7.5) and E8.5] from a severe defect in cell proliferation that is at least partly p53 dependent. Likewise, indistinguishable phenotypes have also been described for nematodes bearing mutations in the Caenorhabditis elegans orthologs of BRCA1 and BARD1 (37), and for plants harboring mutations in the corresponding genes of Arabidopsis thaliana (38). Although it has been proposed that BARD1 executes cellular functions that are independent of BRCA1 (39), the impact of these on the phenotypes of BARD1 mutant organisms has not yet been discerned (20, 37, 38). In any event, the data presented here suggest that BRCA1-independent functions of BARD1, if they exist, are not required for tumor suppression in mammary epithelial cells.
Given the diverse functions attributed to BRCA1, it will be intriguing to know whether all or only some of these are dependent on the BRCA1/BARD1 heterodimer. Because the latency of tumor formation in the double conditional Bard1/Brca1-mutant mice is statistically indistinguishable from those of the single conditional Bard1- and Brca1-mutant animals (Fig. 1), the Bard1 and Brca1 genes appear to be fully epistatic with respect to tumor suppression in mammary epithelial cells. This observation, together with the common phenotype of the Brca1- and Bard1-mutant tumors, strongly suggests that the tumor suppression activities of both proteins are mediated through the BRCA1/BARD1 heterodimer. As an E3 ligase, the heterodimer has the potential to modulate multiple downstream pathways by ubiquitin conjugation of itself and other substrates. It is also conceivable that BRCA1/BARD1 has functions relevant to tumor suppression that are independent of its E3 ligase activity. Future studies should resolve whether the enzymatic activity of the BRCA1/BARD1 heterodimer is required for tumor suppression and identify which of its diverse functions serve to inhibit human carcinogenesis.
Materials and Methods
Targeted Mutagenesis.
The conditional Bard1 targeting vector consisted of a 7.4-kb fragment containing the 5′ flanking region and exon 1. The loxP-flanked PGK-neo cassette was inserted 2 kb upstream of the transcriptional initiation site and a single loxP site was introduced in intron 1. The conditional Brca1 vector consisted of a 6.1-kb fragment containing exons 1 and 2. A FRT-flanked PGK-neo cassette together with a single loxP site was cloned into intron 1 and a second loxP site was introduced in intron 2. Gene targeting in 129Sv ES cells and blastocyst injections were performed following standard techniques.
Mouse Breeding.
Experiments involving mice were performed according to Columbia University Institutional Animal Care and Use Committee-approved protocols. All of the mice used in the breeding program were on a mixed background of 129/Sv × C57BL/6J.
Molecular Analysis.
For genotyping by Southern blot analysis, genomic DNA was prepared from tails and mammary tumors of mice (for details, see Figs. S1 and S2). For mutational analysis of p53 in the mammary tumors, exons 2–11 were amplified with intron-specific primers by using mammary tumor DNA as template and PCR products were directly sequenced.
Histological Analysis.
Mice were killed 2 weeks after mammary neoplasms were detected by palpation and tissues were processed for histopathological evaluation and immunohistochemistry. Both in situ and invasive neoplasms were evaluated for their growth pattern, distribution, cellular and nuclear morphology, and mitotic rate. For immunophenotyping, all specimens were labeled with antibodies against p63 and E-cadherin (both BD Pharmingen), vimentin (RDI), CK5 and CK14 (both Covance), ER (Santa Cruz), PR (ABR), and p53 (Vector Laboratories).
Cytogenetic Analysis.
Metaphase preparations were made from logarithmically growing tumor cells. Hybridization and detection of SKY probes was performed following manufacturer's protocol (Applied Spectral Imaging). DAPI-counterstained metaphases were captured by using the SD300-C SpectraCube and analyzed by using Skyview software. A total of 10–20 metaphases were analyzed in each tumor line.
Supplementary Material
Acknowledgments.
We thank Qiong Li and Xi Sun for expert technical assistance and Dr. Shuang Wang for help with the statistical analysis. This work was supported by National Cancer Institute Program Grant 1P01-CA97403 [Projects 4 (R.B.) and 5 (T.L.)], a grant to the Herbert Irving Comprehensive Cancer Center from the Avon Products Foundation Breast Cancer Research and Care Program (to T.L.), and National Institutes of Health Cancer Biology Training Grant T32-CA09503 (to E.M.). R.S. was supported by a fellowship from the Susan G. Komen Breast Cancer Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711032105/DCSupplemental.
References
- 1.Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med. 2003;348:2339–2347. doi: 10.1056/NEJMra012284. [DOI] [PubMed] [Google Scholar]
- 2.Honrado E, Benitez J, Palacios J. Histopathology of BRCA1- and BRCA2-associated breast cancer. Crit Rev Oncol Hematol. 2006;59:27–39. doi: 10.1016/j.critrevonc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Foulkes WD, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 4.Sorlie T, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner NC, Reis-Filho JS. Basal-like breast cancer and the BRCA1 phenotype. Oncogene. 2006;25:5846–5853. doi: 10.1038/sj.onc.1209876. [DOI] [PubMed] [Google Scholar]
- 6.Johnson RD, Jasin M. Double-strand-break-induced homologous recombination in mammalian cells. Biochem Soc Trans. 2001;29:196–201. doi: 10.1042/0300-5127:0290196. [DOI] [PubMed] [Google Scholar]
- 7.Nagaraju G, Scully R. Minding the gap: The underground functions of BRCA1 and BRCA2 at stalled replication forks. DNA Repair (Amsterdam) 2007;6:1018–1031. doi: 10.1016/j.dnarep.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scully R, Livingston DM. In search of the tumour-suppressor functions of BRCA1 and BRCA2. Nature. 2000;408:429–432. doi: 10.1038/35044000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu LC, et al. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat Genet. 1996;14:430–440. doi: 10.1038/ng1296-430. [DOI] [PubMed] [Google Scholar]
- 10.Hashizume R, et al. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J Biol Chem. 2001;276:14537–14540. doi: 10.1074/jbc.C000881200. [DOI] [PubMed] [Google Scholar]
- 11.Baer R, Ludwig T. The BRCA1/BARD1 heterodimer, a tumor suppressor complex with ubiquitin E3 ligase activity. Curr Opin Genet Dev. 2002;12:86–91. doi: 10.1016/s0959-437x(01)00269-6. [DOI] [PubMed] [Google Scholar]
- 12.Evers B, Jonkers J. Mouse models of BRCA1 and BRCA2 deficiency: Past lessons, current understanding and future prospects. Oncogene. 2006;25:5885–5897. doi: 10.1038/sj.onc.1209871. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig T, Fisher P, Ganesan S, Efstratiadis A. Tumorigenesis in mice carrying a truncating Brca1 mutation. Genes Dev. 2001;15:1188–1193. doi: 10.1101/gad.879201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy A, et al. A mouse model of basal-like breast carcinoma with metaplastic elements. J Pathol. 2007;211:389–398. doi: 10.1002/path.2124. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc Natl Acad Sci USA. 2007;104:12111–12116. doi: 10.1073/pnas.0702969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghimenti C, et al. Germline mutations of the BRCA1-associated ring domain (BARD1) gene in breast and breast/ovarian families negative for BRCA1 and BRCA2 alterations. Genes Chromosomes Cancer. 2002;33:235–242. doi: 10.1002/gcc.1223. [DOI] [PubMed] [Google Scholar]
- 18.Hoa Thai T, et al. Mutations in the BRCA1-associated RING domain (BARD1) gene in primary breast, ovarian and uterine cancers. Hum Mol Genet. 1998;7:195–202. doi: 10.1093/hmg/7.2.195. [DOI] [PubMed] [Google Scholar]
- 19.Sauer MK, Andrulis IL. Identification and characterization of missense alterations in the BRCA1 associated RING domain (BARD1) gene in breast and ovarian cancer. J Med Genet. 2005;42:633–638. doi: 10.1136/jmg.2004.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy EE, Celebi JT, Baer R, Ludwig T. Loss of Bard1, the heterodimeric partner of the Brca1 tumor suppressor, results in early embryonic lethality and chromosomal instability. Mol Cell Biol. 2003;23:5056–5063. doi: 10.1128/MCB.23.14.5056-5063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietrich P, Dragatsis I, Xuan S, Zeitlin S, Efstratiadis A. Conditional mutagenesis in mice with heat shock promoter-driven cre transgenes. Mamm Genome. 2000;11:196–205. doi: 10.1007/s003350010037. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig T, Fisher P, Murty V, Efstratiadis A. Development of mammary adenocarcinomas by tissue-specific knockout of Brca2 in mice. Oncogene. 2001;20:3937–3948. doi: 10.1038/sj.onc.1204512. [DOI] [PubMed] [Google Scholar]
- 23.Tischkowitz MD, Foulkes WD. The basal phenotype of BRCA1-related breast cancer: Past, present and future. Cell Cycle. 2006;5:963–967. doi: 10.4161/cc.5.9.2713. [DOI] [PubMed] [Google Scholar]
- 24.Yehiely F, Moyano JV, Evans JR, Nielsen TO, Cryns VL. Deconstructing the molecular portrait of basal-like breast cancer. Trends Mol Med. 2006;12:537–544. doi: 10.1016/j.molmed.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Brugarolas J, Jacks T. Double indemnity: p53, BRCA and cancer. p53 mutation partially rescues developmental arrest in Brca1 and Brca2 null mice, suggesting a role for familial breast cancer genes in DNA damage repair. Nat Med. 1997;3:721–722. doi: 10.1038/nm0797-721. [DOI] [PubMed] [Google Scholar]
- 26.Tirkkonen M, et al. Distinct somatic genetic changes associated with tumor progression in carriers of BRCA1 and BRCA2 germ-line mutations. Cancer Res. 1997;57:1222–1227. [PubMed] [Google Scholar]
- 27.Weaver Z, et al. Mammary tumors in mice conditionally mutant for Brca1 exhibit gross genomic instability and centrosome amplification yet display a recurring distribution of genomic imbalances that is similar to human breast cancer. Oncogene. 2002;21:5097–5107. doi: 10.1038/sj.onc.1205636. [DOI] [PubMed] [Google Scholar]
- 28.Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 29.Moynahan ME, Cui TY, Jasin M. Homology-directed DNA repair, mitomycin-c resistance, and chromosome stability is restored with correction of a Brca1 mutation. Cancer Res. 2001;61:4842–4850. [PubMed] [Google Scholar]
- 30.Snouwaert JN, et al. BRCA1 deficient embryonic stem cells display a decreased homologous recombination frequency and an increased frequency of non-homologous recombination that is corrected by expression of a brca1 transgene. Oncogene. 1999;18:7900–7907. doi: 10.1038/sj.onc.1203334. [DOI] [PubMed] [Google Scholar]
- 31.Westermark UK, et al. BARD1 participates with BRCA1 in homology-directed repair of chromosome breaks. Mol Cell Biol. 2003;23:7926–7936. doi: 10.1128/MCB.23.21.7926-7936.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu X, Baer R. Nuclear localization and cell cycle-specific expression of CtIP, a protein that associates with the BRCA1 tumor suppressor. J Biol Chem. 2000;275:18541–18549. doi: 10.1074/jbc.M909494199. [DOI] [PubMed] [Google Scholar]
- 33.Joukov V, et al. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell. 2006;127:539–552. doi: 10.1016/j.cell.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 34.Jin Y, et al. Cell-cycle dependent colocalization of BARD1 and BRCA1 in discrete nuclear domains. Proc Natl Acad Sci USA. 1997;94:12075–12080. doi: 10.1073/pnas.94.22.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scully R, et al. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–435. doi: 10.1016/s0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 36.Choudhury AD, Xu H, Baer R. Ubiquitination and proteasomal degradation of the BRCA1 tumor suppressor is regulated during cell cycle progression. J Biol Chem. 2004;279:33909–33918. doi: 10.1074/jbc.M403646200. [DOI] [PubMed] [Google Scholar]
- 37.Boulton SJ, et al. BRCA1/BARD1 orthologs required for DNA repair in Caenorhabditis elegans. Curr Biol. 2004;14:33–39. doi: 10.1016/j.cub.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 38.Reidt W, Wurz R, Wanieck K, Chu HH, Puchta H. A homologue of the breast cancer-associated gene BARD1 is involved in DNA repair in plants. EMBO J. 2006;25:4326–4337. doi: 10.1038/sj.emboj.7601313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irminger-Finger I, et al. Identification of BARD1 as mediator between proapoptotic stress and p53- dependent apoptosis. Mol Cell. 2001;8:1255–1266. doi: 10.1016/s1097-2765(01)00406-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.