Abstract
Many cytotoxic compounds of therapeutic interest have been isolated from marine invertebrates, and some of them have been reported to be of microbial origin. Pyridoacridine alkaloids are the main compounds extracted from the ascidian Cystodytes dellechiajei. Here we describe the in vitro antiproliferative activity against different tumor cell lines of the ascidian extracts and provide some insights on the role of the microbial community associated with the tunicate in the production of these compounds. C. dellechiajei extracts showed remarkably high antiproliferative activity (IC50 ≤5 μg/mL) in human lung carcinoma A-549, colon adenocarcinoma H-116, pancreatic adenocarcinoma PSN-1 and breast carcinoma SKBR3 cell lines. Moreover, we found that the maximum activity was located in the tunic tissue of the colony, which harbours a microbial community. In order to ascertain the involvement of this community in the synthesis of the bioactive compounds different approachs that included culture and culture independent methods were carried out. We undertook a screening for antiproliferative activities of the bacterial isolates from the ascidian, as well as a comprative analysis of the cytotoxic activities and the microbial communities from two color morphs of the ascidian, green and blue. In addition, the changes of the antiproliferative activities and the composition of the microbial communities were studied from ascidians kept in aquaria and treated with antibiotics for one month. Our data obtained from the different experiments did not point out to bacteria as the source of the cytotoxic compounds, suggesting thus an ascidian origin.
Keywords: Ascidian, cytotoxicity, tumor, Cystodytes, bacteria
1. Introduction
The number of natural products isolated from marine organisms increases rapidly, and now exceeds 18,000 [1], with hundreds of new compounds being discovered every year [2, 3]. A large proportion of these natural compounds have been extracted from marine invertebrates, especially sponges, ascidians, bryozoans and molluscs, and some of them are currently in clinical trials [4]. Most marine invertebrates are sessile soft bodies that inhabit benthic rock environments. In the sea, rock substrate is limited, and benthic organisms have to compete for the space to live and develop. Invertebrate organisms have evolved defence strategies based on the synthesis of cytotoxic compounds in order to avoid predation and epibiosis [5–7]. Many invertebrate animals, like sponges, tunicates, bryozoans, molluscs and oligochaetes are symbiotically associated with microorganisms belonging to the Bacteria and Archaea domains [8–15]. In some cases, the source of the cytotoxic compounds isolated from marine invertebrates are the symbiont bacteria. For instance, the tunicate Lissoclinum patella is simbiotically associated with the cyanobacteria Prochloron sp. [13], which produces the cytotoxic compounds patellamides A and C, each with clinical potential [16, 17]. Davidson et al. [18] provided evidence in the bryozoan Bugula neritina that its symbiont “Candidatus Endobugula sertula” is the source of bryostatins, which show excellent potential as therapeutic agents against leukemias, lymphomas, melanomas and solid tumors [19].
The colonian ascidian Cystodytes dellechiajei (Della Valle, 1877) (Aplousobranchiata, Polycitoridae) inhabits benthonic rock environments in tropical and temperate waters in the Atlantic, Pacific and Indian oceans, and in the Mediterranean sea. Its life cycle has two phases, as an adult sessile colony and as free-living larva. Larvae exhibit all the characteristic chordate features: a notocord, a dorsal, hollow nerve cord, pharyngal gill slits and a muscular post-anal tail. Colony and larva are surrounded by a protective tunic, which is analogous to a mesenchymal tissue, formed by a matrix of acidic mucopolysaccharides and diverse eukaryotic cell lines [20, 21]. C. dellechiajei stores acid substances in the vacuoles of the bladder cells of tunic tissue. These cells break upon aggression and release the vacuolar content into the tunic, transiently lowering the local pH down to 1–2 [20]. In addition, the tunic contains calcium carbonate spicules that protect the zooids of the colony and accumulate different cytotoxic compounds: mainly pyridoacridine alkaloids [20, 22], as well as diterpenes [23], sphingosines and ceramides [24]; some of which have antileukemic properties [25–29]. These cytotoxicity and acidity mechanisms of the tunic of C. dellechiajei are defence strategies to deter predators and competitors [21, 30–32].
The aim of the present study was, first, to analyze the antiproliferative activity against different tumor cell lines of tissue extracts from the two colour morphs (blue and green) of C. dellechiajei that inhabit the southeastern Mediterranean sea. In addition, since this tunicate harbours a microbial community associated with the tunic tissues [33], our second aim was to analyze the involvement of these bacteria in the synthesis of bioactive compounds, by both culture of bacterial isolates and culture independent methods based on denaturing gradient gel electrophoresis of 16S rRNA genes, a widely used molecular approach for the description of microbial community composition [34].
2. Results and Discussion
2.1. In vitro antitumor activity from natural samples
The organic crude extracts from the 11 blue colonies analyzed showed high inhibitory activity against breast SKBR3, colorectal H-116, lung A-549, and pancreas PSN-1 cancer cell lines and displayed essentially no antiproliferative activity against the glioblastoma T98G cancer cell line (Table 1). Previous studies proved that the ascidian showed cytotoxicity against HL-60 and P338 leukemic cells, and the MCF7 breast cancer cell line [25, 27, 35, 36]. Morover, when the blue ascidians were kept in aquarium for up to 75 days, the antiproliferative activities were conserved with high levels, (IC50 <5 μg/ml in A-549, H-116, PSN-1 and SKBR3 cancer lines).
Table 1.
In vitro antitumor activity from organic crude extract obtained from C. dellechiajei.
| IC50 for cancer cell line (μg/ml) | |||||
|---|---|---|---|---|---|
| Samples | aA-549 | bH-116 | cPSN-1 | dSKBR3 | eT98G |
| Blue colony 1 | 5 | <5 | 5 | 10 | 25 |
| Blue colony 2 | 5 | <5 | 5 | 5 | 10 |
| Blue colony 3 | 5 | <5 | 5 | 5 | 25 |
| Blue colony 4 | 5 | <5 | <5 | 5 | 10 |
| Blue colony 5 | 5 | <5 | <5 | 5 | 25 |
| Blue colony 6 | 5 | <5 | 5 | 5 | 25 |
| Blue colony 7 | 5 | <5 | 5 | 5 | 25 |
| Blue colony 8 | 5 | <5 | <5 | 5 | 25 |
| Blue colony 9 | 5 | <5 | 5 | 5 | 25 |
| Blue colony 10 | 5 | <5 | 5 | 5 | 25 |
| Blue colony 11 | <2.5 | 2.5 | 5 | 5 | 10 |
| Green colony 1 | 25 | 5 | - | - | - |
| Green colony 2 | 25 | 25 | - | - | - |
| Green colony 3 | 25 | 25 | - | - | - |
| Blue colony tunic | 2.5 | 2.5 | - | - | - |
| Blue colony zooids | 12.5 | 12.5 | - | - | - |
| Larva | 12.5 | 25 | - | - | - |
Human lung carcinoma
Colon adenocarcinoma
Pancreatic adenocarcinoma
Breast carcinoma
Caucasian glioblastoma
In good agreement with toxicity data from the larvae reported by Tarjuelo et al. [31], we found that larva crude extracts did not display antiproliferative activity against human lung carcinoma A549 or colon adenocarcinoma H116. The lack of antitumor activity in larva extracts could be due to the absence of cytotoxic compounds in the tunic tissue of the larvae. Adults and larvae of a given species may use distinct chemical and physical defence strategies [31, 37–41] and unpalatable larvae do not always come from unpalatable adults [39]. Larval defence could thus be associated with the reproductive strategy of the species rather than the chemical and physical defence mechanisms [37, 38, 40, 42].
On the other hand, our results showed a significative difference between antiproliferative activities between green and blue ascidian colonies. The cytotoxic activity detected in the green colonies was very low, IC50 ≈ 25 μg/mL, and only the blue pigmented colonies showed high values. Within the population of C. dellechiajei in the sampling area, the blue pigmented colonies were much more abundant (around 3 fold) than the green colonies. It could thus be possible that the high cytotoxicty of the blue colonies provide them with an adaptative advantage compared to green colonies.
Although we have not analyzed the chemical nature of the active compounds, it has been widely reported that the main compounds extracted from C. dellechiajei are pyridoacridine alkaloids. These compounds are ascididemins, 11-hydroxyascididemin, cystodytins A-I, shermilamine B, kuanoniamine D, and sebastianines A and B [20, 22, 25, 28, 32, 35, 43–45], some of which showed antitumor properties [25–29]. However, only ascididemin, 11-hydroxyascididemin and sebastianines A and B have been detected in the blue colonies [20, 21, 29]. Therefore, the cytotoxic activities we have observed could be due to these products. In other hand, Rottmayr et al. [20] and Turon et al. [21] reported that the pyridoacridine compounds were located in the pigmented cells of the tunic tissue in purple color colony, and, in addition, López-Legentil et al. [46] showed seasonal variations in the production of these compounds, with minimum values in summer, attributable to sexual exhaustion and seasonally varying biotic interaction or abiotic parameters. Therefore, according to the data from the location of these compounds inside the ascidian tissues [20, 21], our results provided from the different parts of the ascidian analyzed, zooid, tunic and larva, suggest that the cytotoxic compounds could be also located inside the pigmented cells of the tunic tissue.
2.2. Antibiotic treatment of the C. dellechiajei colonies
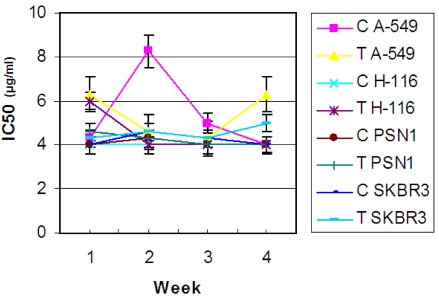
Antibiotic treatment did not produce any apparent detrimental effect on the ascidian growth, as no morphology and/or pigmentation differences were observed between treated and untreated colonies. In vitro antitumor activities were very similar in treated and control colonies in triplicate samples. For both control and treated colonies, the antitumor activity remained constant during the experiment (Figure 1), showing a range of IC50 values from 4 to 6 μg/mL, except for the control sample after 2 weeks, which showed a higher value, IC50 ≈ 8 μg/mL against A-549 cell line, since one specimen of the three replicates samples at that time displayed a low antiproliferative activity. Although, as shown in Figure 1, treated samples during two weeks showed the IC50 value lower than the control samples.
Figure 1.
In vitro antitumor activity against lung A-549, colorectal H-116, pancreas PSN-1 and breast SKBR3 cancer cell lines of extracts from ascidians treated with antibiotics. Results are expressed in IC50 (μg/mL), the concentration required to inhibit growth by 50%, obtained from crude extracts from control and treated colonies in triplicate samples during four weeks. IC50 (μg/mL) values for control colonies (C) and treated colonies (T) are shown for each cancer cell line.
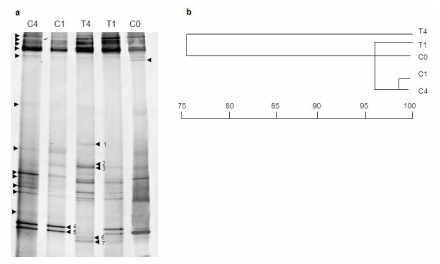
The data provided by DGGE analysis showed that bacterial communities associated with sample controls (sample C0, before starting the experiment, and samples C1 and C4, cultured during one and four weeks respectively) were similar (above 95% similarity, Figure 2), indicating that the community underwent few changes when the tunicate was maintained out of its natural environment.
Figure 2.
Antibiotic treatment of ascidia kept in aquaria; (a) DGGE gel patterns representing the bacterial communities in different samples: control sample before starting the experiment (C0), sample treated for 1 week (T1), control sample without treatment cultured for 1 week (C1), sample treated for 4 weeks (T4) and control sample without treatment cultured for 4 weeks (C4). Arrows point to bands included in the similarity analysis. Numbered arrows point to bands excised, reamplified and sequenced that showed different pattern among the samples. (b) Dendrogram based on the Jaccard index calculated from band patterns in panel (a) showing the relationship among the microbial communities of different samples.
After 4 weeks of antibiotic treatment (T4), the bacterial community in the tunicate had changed remarkably, and displayed 75% similarity (Figure 2b) to the rest of the samples, which were all above 95% similar to each other. T4 DGGE pattern displayed changes in the intensity of some bands present in the rest of the samples (2, 3, 4 and 5 in Figure 2a) as well as some new bands (bands 1, 6 and 7).
These bands were reamplified and sequenced, and they were found to be related to the bacterial 16S rRNA gene sequences shown in Table 2. The uppermost gel bands belong to 16S rRNA sequences of chloroplast of photosynthetic epibionts of C. dellechiajei, like diatoms and rhodophyte algae [33]. The rest of bands corresponded to 16S rRNA sequences of bacteria. Most of the DGGE bands were present in all samples without significative variation, representing bacteria that were either resistant or inaccesible to gentamicine and bacitracine at the assayed doses. However, the treatment favoured the growth of new bacteria related to the alpha-proteobacterium Stappia sp. and beta-proteobacterium Janthinobacterium agaricidamnosus (DGGE bands bands 1, 6 and 7 in Figure 2a) and other alpha-proteobacteria that were previuolsy present in the ascidian tissues (bands 2 and 3). Finally, only bacteria related to Mesorhizobium sp. (bands 4 and 5 in Figure 2a) were directly sensitive to the antibiotic treatment. Since treated colonies had stable and high antitumor activity, those bacteria related to Mesorhizobium sp. (bands 4 and 5), Stappia sp. (bands 6 and 7) and Janthinobacterium agaricidamnosus (band 1) were most likely not involved in the synthesis of cytotoxic compounds.
Table 2.
16S rRNA sequence identities of bands exicised from DGGE gel (520 pb)
| DGGE bands | Accession no. (GenBank) | Closest taxon (% similarity BLASTn) | Division |
|---|---|---|---|
| 1 | EF028016 | 98% Janthinobacterium agaricidamnosus (AY167838) | Beta-proteobacteria |
| 2 | EF028017 | 99% Uncultured alpha-proteobacterium NJ1-1-1 (AY626827) | Alpha-proteobacteria |
| 3 | EF028018 | 97% Uncultured alpha-proteobacterium NJ1-1-1 (AY626827) | Alpha-proteobacteria |
| 4 | EF028019 | 98% Mesorhizobium sp. (AY690680) | Alpha-proteobacteria |
| 5 | EF028020 | 99% Mesorhizobium sp. (AY690680) | Alpha-proteobacteria |
| 6 | EF028021 | 94% Stappia sp. (AY307927) | Alpha-proteobacteria |
| 7 | EF028022 | 94% Stappia sp. (AY307927) | Alpha-proteobacteria |
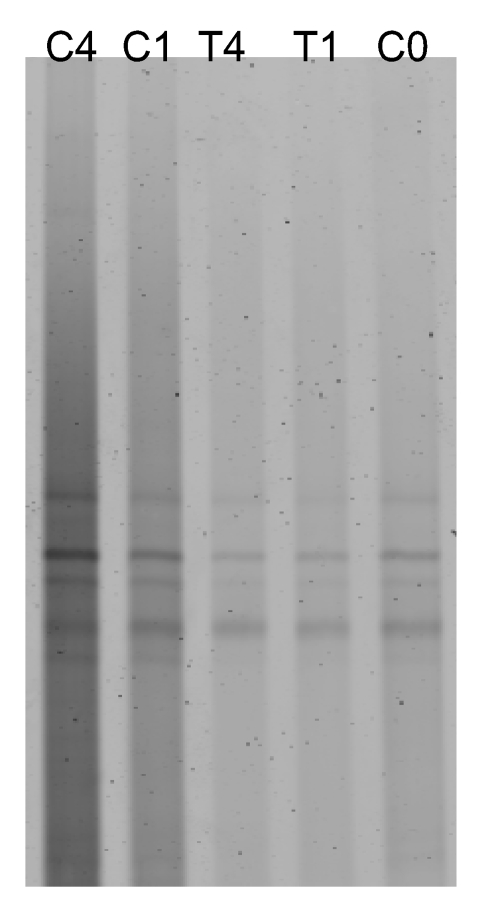
On the other hand, as expected, the antibiotic treatment did not affect to archaeal community associated to C. dellechiajei. As shown in Figure 3, treated and control samples displayed the same DGGE pattern.
Figure 3.
Antibiotic treatment of ascidian kept in aquaria. DGGE patterns representing the archaeal communities in different samples: control sample before starting the experiment (C0), sample treated for 1 week (T1), control sample without treatment cultured for 1 week (C1), sample treated for 4 weeks (T4) and control sample without treatment cultured for 4 weeks (C4).
2.3. Culture of ascidia-associated bacteria and in vitro antitumor activity of the isolates
A total of ninety-four isolates were obtained, and twenty-seven of them were randomly selected and analyzed by 16S rRNA gene PCR amplification and sequencing as described above. All the isolates were Gram negative. A predominant (85% of the selected colonies) colonial morphotype (number 1, see Table 3) was observed.
Table 3.
Identification of bacteria isolated from the ascidian C. dellechiajei
| Strain | aMorphotype | No. of isolates | bCulture media | Closest taxon (% similarity BLASTn) |
|---|---|---|---|---|
| Dell4 | 1 | 7 | 2,3 | 99.3% Alpha-proteobacterium NW001 (AF295099) |
| Dell5 | 1 | 6 | 2,3 | 99.3% Alpha-proteobacterium NW001 (AF295099) |
| Dell6 | 1 | 4 | 2,3 | 96.1% Alpha-proteobacterium NW001 (AF295099) |
| Dell7 | 1 | 5 | 2,3 | 91% Alpha-proteobacterium NW001 (AF295099) |
| Dell8 | 1 | 1 | 2,3 | 98.2% Alpha-proteobacterium Sb89 (AF218241) |
| Dell9 | 2 | 1 | 1 | 93.5% Erythrobacter luteolus (AY5739662) |
| Dell1 | 3 | 1 | 2 | 97% Gamma-proteobacterium C1 (AJ620879) |
| Dell2 | 4 | 1 | 3 | 97.6% Halomonas salina (X87217) |
| Dell3 | 5 | 1 | 3 | 98.9% Alteromonas macleodi (X82145) |
Colony morphotype according to pigmentation, size and shape
See Experimental
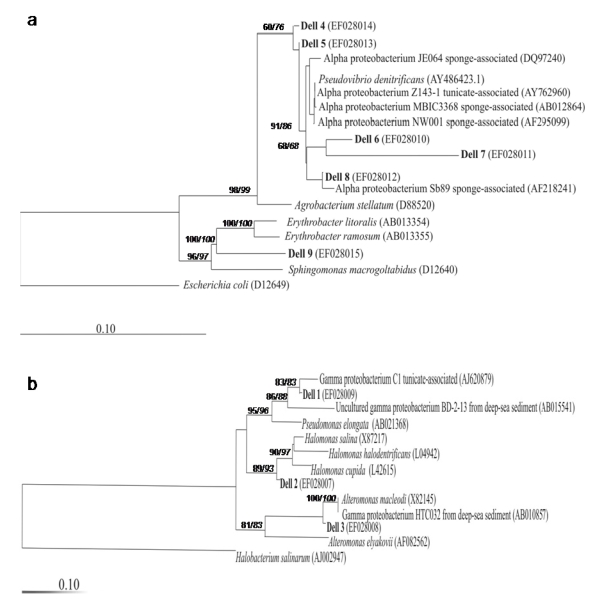
These bacteria formed irregular highly mucous colonies with white-yellow pigmentation, and were easily distinguishable from the other colonies. However, inside this morphotype, five different ARDRA patterns were found. 16S rRNA gene sequences analysis revealed that the isolates Dell4, Dell5, Dell6, Dell7, and Dell8 (representatives of each ARDRA pattern) were related to alpha-proteobacteria associated with invertebrate marine organisms (Figure 4a), like strain NW001 associated with the sponge Rhopaloeides odorabile [47], common throughout the Great Barrier Reef, strain SB89 isolated from the Mediterranean sponge Aplisina aerophoba [48], strains MBIC3368 and JE064 isolated from sponges [49, 50] and strain Z143-1 isolated from a Philippine tunicate [51].
Figure 4.
Phylogenetic relationships among the bacteria isolates from the ascidian C. dellechiajei and their relatives. (a) Neighbour-joining tree using Jukes-Cantor correction based on 16S rRNA gene partial sequences (430 bp) from strains Dell4-Dell8 and complete sequence (1435 bp) from strain Dell9 related to alpha-proteobacteria. Numbers at the branch nodes are quartet puzzling support values (normal face) and bootstrap values (1000 replicates) (italic face). The bacterium Escherichia coli was used as the outgroup. The horizontal scale bar represents 0.1 substitution per nucleotide position. (b) Neighbour-joining tree using Jukes-Cantor correction based on partial sequences (430 bp) of 16S rRNA gene from strains Dell1–Dell3 related to gamma-proteobacteria. Numbers at the branch nodes are quartet puzzling support values (normal face) and bootstrap values (1000 replicates) (italic face). The archaeon Halobacterium salinarum was used as the outgroup. The horizontal scale bar represents 0.1 substitution per nucleotide position.
Strains Dell 4, Dell 5 and Dell 8 displayed >98.2% partial rRNA gene sequence similarity to their closest cultured relatives, and Dell 6 and Dell 7 showed 96.1 and 91% respectively. The second morphotype was represented by Dell 9, which formed irregular colonies without pigmentation and was related to Erythrobacter litoralis (93.5% complete 16S rRNA sequence similarity), an aerobic anoxygenic phototroph, previously cultured from open surface waters [52]. The rest of the three strains isolated, Dell 1, Dell 2 and Dell 3, were related to gamma-proteobacteria (Figure 4b). Strain Dell 1, which showed pleomorphic forms in pure culture as well as cocci and rods, was related to the gamma-proteobacterium strain C1, isolated from C. dellechiajei [53, 54]. Strain Dell 2 was clustered with Halomonas sp. group (97.6% similarity with the closest specie, H. salina). Dell 2 colonies were circular, convex and sligthly opaque with smooth edges. Strain Dell 3, which formed circular, smooth, raised and cream-colored colonies, was related to Alteromonas macleodi (98.9% similarity). 16S rRNA gene clone sequences related to strains Dell1 (97.3% similarity) and Dell9 (99% similarity) have been found in the bacterial community associated with tunic tissues of C. dellechiajei using a culture independent approach [33].
All the ninety-four isolates were screened for antitumor compounds. None of the strains showed in vitro antitumor activity against breast SKBR3, colorectal H-116, pancreas PSN-1, glioblastoma T98G or lung A-549 cancer cell lines. Interestingly, phylogenetic analysis (Figure 4) showed that the isolates Dell 4, 5, 6, and 8 were closely related to the strains alpha-proteobacteria SB89 and Z143-1 associated with sponges and tunicates that produce secondary metabolites with antimicrobial activity against Staphylococcus aureus and Gram negative and positive reference strains [51]. Although antimicrobial activities were not analysed, since this was out of the scope of this study, others authors described cytotoxic compounds with antimicrobial properties extracted from C. dellechiajei [36, 55]. According to the close relationship between isolates Dell 4, 5, 6 and 8 with alpha-proteobacteria that produce antimicrobial products, these antibacterial activities detected in the ascidian extracts could be due to secondary metabolites produced by the associated bacteria.
2.4. Is the microbial community involved in the synthesis of cytotoxic compounds?
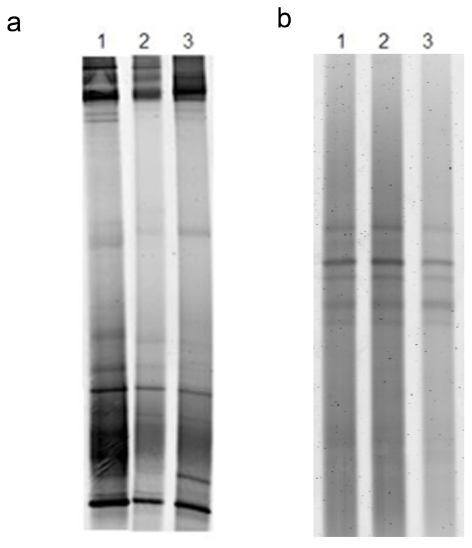
Turon et al. [21] and Rottmayr et al. [20] showed that in C. dellechiajei cytotoxic pyridoacridine alkaloids involved in chemical defense were stored in pigmented cells of the tunic, with maximum abundance in upper tunic zones. In addition, Turon et al. [21], detected signals corresponding to the pyridoacridine alkaloids shermilamine B and kuanoniamine D and their deacetylated from the compartments of the ascidian tissues, mainly pigmented cells, but no signals from the associated bacteria, by X-ray spectra. Therefore, these authors pointed out that the host produces these cytotoxic compounds, and not the associated bacteria. In order to clarify this point, we compared the microbial communities associated to the blue and green colonies by DGGE analysis in order to study the differences in the composition of the communities and correlate these data with antiproliferative results against tumor cells obtained from the two colour morphs extracts. As shown in figure 5, the DGGE patterns between the blue and green colonies was very similar. However, the green colonies did not show antiproliferative activity against tumor cells (see Table 1). Since the microbial communities associated to green and blue colonies are very similar and only the blue colonies show cytotoxicity, it does not seem probably that the associated bacteria are the source of the cytotoxic compounds nor they have an essential role in the production. These data, together with the lack of the activity in the isolated bacteria extracts and the results obtained from the antibiotic treatments of the ascidian, and data from Rottmayr et al. [20] and Turon et al. [21] do not indicate a microbial origin for the compounds.
Figure 5.
DGGE patterns representing the bacterial (a) and archaeal (b) communities associated to two blue colonies, sample C0 used in the antibiotic treatment (lane 1), one sample harvested in April 2005 (lane 2), and one green colony harvested in the same date (lane 3).
3. Conclusions
In summary, the current data provided from this and other studies [20, 21, 33, 46] that used complementary techniques, suggest an ascidian origin for the cytotoxic compounds, although it cannot be excluded that symbionts associated with C. dellechiajei synthesize cytotoxic compounds. It would be possible that the compounds were produced by the symbionts, immediately exuded to the ascidian tunic and finally stored in pigmented cells, as was the case with the ascidian L. patella and its symbiotic Prochloron sp. Cytotoxic patellamides A–C were isolated from the tunicate [56, 57], and were found distributed throughout the ascidian tunic. Salomon et al. [58] proposed that the location of these compounds indicated that they were synthesized by the tunicate. Lately, two independent studies [16, 17] identified the biosynthetic pathway of patellamides in the cyanobacteria Prochloron sp. by using a metagenomic approach and confirmed their function by heterologous expression in Escherichia coli. This work constitutes the first approach to study the involvement of the microbial community associated with the ascidian C. dellechiajei in the production of cytotoxic compounds, and could be used as a starting point to study in depth the source of cytotoxic metabolites in the tunicate by using a metagenomic approach [59].
4. Experimental Section
4.1. Sample collection
Specimens of the two varieties, blue and green, of the ascidian C. dellechiajei were collected by scuba diving to depths of 2–5 m in the Mediterranean sea (Cape Palos, Murcia, Spain). Samples for in vitro antitumor activity assays were collected in May 2002 and March 2003. Colonies for antibiotic treatment were harvested in October 2003. Colonies and seawater were transferred directly to a container with an autonomous aeration pump, and immediately brougth back to the laboratory. Samples were processed immediately for antiproliferative assays or cultured in aquarium for antibiotic treatment. Larvae were obtained from ripe colonies under aseptic conditions, rinsed three times in sterile seawater and processed for antiproliferative assays.
4.2. Maintenance of C. dellechiajei in aquarium and antibiotic treatment
Twenty four adult colonies were collected and maintained in a seawater aquarium for one month. Half of the colonies were treated every day with gentamicine sulfate (Sigma, 100 mg) and bacitracine (Sigma, 100 mg) per litre of seawater, and the other half were used as controls without antibiotic treatment. Antibiotics active against Bacteria that did not cause detrimental effects against eukaryotic (i.e. ascidian) cells were chosen, according to their action spectra [60]. Antibiotic concentration was adjusted according to Davidson et al. [18]. The aquarium water was changed every day. At the end of each week, triplicates of treated and control colonies were collected from the aquarium and used for antiproliferative activity measurements and DGGE analysis. Activity was measured for each of the three replicates while DGGE was carried out with only one of them. Prior to the experiment of the antibiotic treatment, five adult colonies were maintained in a seawater aquarium for seventy-five days, then collected and screened for antiproliferative activity in order to study the effect of the artificial maintenance of the colonies on the production of cytotoxic compounds.
4.3. DNA extraction, PCR and DGGE
Colonies from the antibiotic treatment experiment were dissected into tunic and zooids under aseptic conditions and rinsed three times in sterile seawater. Prior to DNA extraction, tunic tissues (1.3 g) were frozen and homogenized in liquid N2 with a sterile mortar. Pulverised tissue was suspended in 25 ml TE buffer (10 mM Tris-HCl and 1 mM EDTA, pH 8) with 0.5% SDS. DNA was purified by phenol-chloroform-isoamyl alcohol 25:24:1 (all Sigma) and ethanol precipitated essentially as described by Sambrook et al. [61]. The DNA pellet was air-dried at room temperature, resuspended in ultrapure sterile water (200 μL) and stored at −80 ºC. PCR amplification of partial 16s rRNA gene for Bacteria was performed using primers 341-GC and 907R [34], and 344-GC and 907R primers for Archaea [62]. The PCR conditions for Bacteria were as follows: 5 min at 94 ºC, 1 min at 65ºC, 3 min at 72ºC, 10 cycles of 1 min at 94ºC, 1 min at 64ºC, 3 min at 72ºC, 20 cycles of 1 min at 94ºC, 1 min at 55ºC, 3 min at 72ºC, with a final extension step of 72ºC for 10 min in a PTC-100 (MJ Instruments). The PCR conditions for Archaea were as follows: 94°C for 5 min (initial denaturation), and 30 cycles of 94°C for 30 s, 56 °C for 45 s, 72 °C for 2 min. The length of extension step was increased to 10 min in the last cycle.
Each 50 μL reaction sample contained 1.5 mM MgCl2, 10 mM Tris-HCl, 50 mM KCl, 200 μM of each dNTP, 1 U Taq I DNA polymerase (Invitrogen), 0.25 μM of each primer and 100 ng of DNA. The PCR products were quantified by comparison with a Low DNA Mass Ladder (Invitrogen) using agarose gel electrophoresis. DGGE analysis was carried out using a DCODE system™ (Universal mutation detection system, Bio-Rad) with a denaturing gradient of 45–60% for Bacteria and 40–55% for Archaea (100% was defined as 7 M urea and 40% deionized formamide) in a 0.75 mm-thick 6% polyacrylamide gel. Approximately 600–800 ng of PCR product were applied to each lane in the gel. Electrophoresis was performed at 5 V/cm at 60ºC for 16 h in 1X TAE buffer (40 mM Tris, 20 mM sodium acetate, 1 mM EDTA, pH 7.4). DGGE gels were stained with SYBR Gold (Molecular Probes), 100 μL/L in 0.5X TAE buffer, for 15 min, rinsed with 1X TAE buffer for 15 min, visualized and photographed with a Typhoon 9410 image analysis system (Amersham Biosciences). Selected DGGE bands were excised with a sterile blade and incubated overnight at 4ºC in ultrapure sterile water (20 μL). The eluent was used as template DNA for reamplification with the primers and conditions described above. PCR products were sequenced using primer 907R in a genetic analyzer ABI PRISM 310 (Applied Biosystem).
4.4. DGGE data analysis
A similarity dendrogram of the DGGE patterns from the antibiotic treatment experiment was computed using SPSS® 12.0 software (SPPS, Inc., Chicago, IL) with the Jaccard index (shared characters/total characters).
4.5. Bacterial isolation and 16S rRNA gene analysis
All culture media contained an ascidian organic extract (AOE) prepared as follows: a sample of the the colony (2 g) was homogenized using a mortar and boiled in filtered sterile seawater (35 mL) for 15 min. The homogenized colony was centrifuged at low-speed (2,000 rpm at room temperature, Labofuge 400R, Heraeus) for 1 min and the resulting supernatant constituted the AOE. Three different solid (2% agar) media were prepared with 12% v/v of AOE in filtered seawater collected at the sampling area. Medium 1 did not contain additional components, medium 2 was supplemented with 0.001% (p/v) yeast extract, and medium 3, with 0.001% (p/v) yeast extract and 1% (p/v) sucrose. The pH of the media was adjusted to 8 and they were sterilized at 121 ºC for 15 min. The plates were inoculated by either spreading a small piece of tunic (1–2 mm3) or homogenized tunic (100 μL, 2 g of tunic in 1 mL of filtered and sterile seawater). Cultures were incubated at room temperature under aerobic conditions for at least two weeks, and representatives of each bacterial colonial morphotype were serially streak-plated until pure cultures were obtained. Bacterial colonies were then suspended in TE buffer (400 μL, 10 mM Tris-HCl and 1 mM EDTA, pH 8) with 0.5 % SDS. DNA extraction and purification were carried out as described previously. PCR amplification of bacterial 16S rRNA genes was performed using primers 27f and 1492r [63]. Cycling conditions were as follows: 3 min at 94ºC, 30 cycles of 15 sec at 94ºC, 30 sec at 55ºC, 2 min at 72ºC and a final extension of 10 min at 72ºC. PCR products of bacterial 16S rRNA were analyzed using ARDRA [64] with the enzymes HinfI and MboI (Invitrogen) according to the manufacture's protocol. The digestion products were analyzed in 2% agarose gel (LE, FMC Bioproducts) in 0.5X Tris-boric acid-EDTA buffer. 16S rRNA genes from each restriction pattern were sequenced using primers B1055, 16S5r, 338f and 785f [65].
4.6. Phylogenetic analysis
16S rRNA gene sequences were initially compared with the reference sequences at NCBI (http://www.ncbi.nlm.nih.gov) using BLASTn [66]. The correlation between primary sequence and secondary structure was analyzed using the ARB sequence editor. Complete and partial sequences obtained in this study were added to an alignment of over 50.000 primary aligned gene structures available at http://db-central.arb-home.de/. Sequences not included in the ARB database were obtained from the GenBank database. Phylogenetic analysis was performed by using the three algorithms implemented in the ARB package: maximum-likelihood, neighbour joining using Jukes-Cantor correction and maximum parsimony. Phylogenetic trees calculated by neighbour-joining were evaluated after 1.000 bootstrap resampling of the data. In addition, the TREE-PUZZLE program of the ARB package was used to reconstruct phylogenetic trees by maximum-likelihood with quartet puzzling support values for each internal branch [67, 68].
4.7. Preparation of crude extracts from C. dellechiajei and microbial cultures for in vitro antitumor assays
For in vitro antitumor assays, samples of whole colonies (2 g), tunic tissue (1.8 g), zooids (100 mg) and larvae (50 mg) were homogenyzed separately in methanol-acetone (10 mL, 1:1 v/v) with a mortar and centrifuged at 4,500 rpm (Labofuge 400R, HERAEUS) for 1 min. The resulting supernatants were dried under vacuum with a rotary evaporator (Laborata 4000, Heidolph, France) at room temperature and resuspended in ultrapure sterile water at different final concentrations (50, 25, 10, 5 and 2.5 μg/mL).
Microbial cultures were grown in liquid media designed to favour the synthesis of the secondary metabolites (C. Acebal, personal communication). Media 1, 2 and 3 were supplemented with pharmamedia® (1.5% p/v), soya flour (0.8% p/v), corn step (0.8% p/v), yeast extract (0.2 % p/v), MgSO4 7H2O (5% p/v), and CaCO3 (0.3% p/v). pH was adjusted at 7.1. All media were incubated at 30ºC, 220 rpm for 4 days. Once grown, each culture (5 mL) was lyophilized, resuspended and homogenized in methanol-acetone (10 mL, 1:1 v/v) and centrifuged at 4,500 rpm (Labofuge 400R, HERAEUS) for 5 min. The resulting supernant was dried and resuspended at different final concentrations (50, 25, 10 and 5 μg/mL).
4.8. Tumor cell cultures
All the tumor cell lines were obtained from the American Type Culture Collection (ATCC). Human lung carcinoma A549, colon adenocarcinoma H116, breast carcinoma SKBR3 and pancreatic adenocarcinoma PSN1 were cultured in RPMI medium [69] containing 2 mM glutamine, 50 IU/ml penicillin, 50 μg/ml streptomycin. Media were supplemented with 5% fetal bovine serum (FBS) for A549 and H116 carcinomes, and 10% FBS for SKBR3 and PSN1 carcinomes. Caucasian glioblastoma T98G was maintained in RPMI 1640 medium [69] containing 2mM glutamine, 50 IU/mL penicillin, 50 μg/mL streptomycin, 1 mM pyruvate supplemented with 0.1 mM nonessential amino acids, and 10% FBS.
4.9. In vitro antiproliferative assays
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT, Sigma Chemical Co., St. Louis, MO) dye reduction assay in 96-well microplates was used, essentially as previously described [70]. Tumor cells (4x103 A-549 cells or 6x103 H-116, 6x103 PSN1, 6x103 SKBR3 and 6x103 T98G cells in a total volume of 200 μL of complete medium) were incubated in each well with different amounts of the ascidian and bacterial extracts (see above). After 2 days of incubation (37° C, 5% CO2 in a humid atmosphere), MTT (50 μL, 5 mg/mL in PBS) were added to each well and the plate was incubated for a further 2 h (37ºC). The resulting formazan was dissolved in DMSO (100 μL) and the absorbance read at 490 nm. All determinations were carried out in triplicate. IC50 was calculated as the concentration of drug yielding a 50% cell survival rate. The cytotoxicity assays were performed in two stages: first the extracts were tested for activity against human lung carcinoma A549 and colon adenocarcinoma H116. Only active extracts were further analyzed for activity against breast carcinoma SKBR3, pancreatic adenocarcinoma PSN1 and caucasian glioblastoma T98G.
Acknowledgements
This work was supported by grant BIO2000-005-P4-04 from the Spanish Ministry of Science and Education. M.M was a graduate fellowship from the Spanish “Ministerio de Educacion, Cultura y Deporte” (ref.2001-2038). We thank Nicolás Cuenca, Genma Navarrete, Fernando Santos, and Arantxa Peña, for technical assistance. Thanks Cristina Acebal for her effort in this study.
Footnotes
Sample Availability: Available from the authors.
References and Notes
- 1.Marinlit. Version september. A marine literature database produced and maintained by the Department of Chemistry, University of Canterbury, New Zealand, 2007; (www.chem.canterbury.ac.nz/marinlit/marinlit.shtml)
- 2.Faulkner DJ. Marine natural products. Nat Prod Rep. 2002;19:1–48. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]
- 3.Proksch P, Müller WEG. Frontiers in Marine Biotechnology. Horizon Bioscience; Norfolk, U.K: 2006. [Google Scholar]
- 4.Proksch P, Edrada RA, Ebel R. Drugs from the sea-current status and microbiological implications. Appl Microbiol Biotechnol. 2002;59:125–134. doi: 10.1007/s00253-002-1006-8. [DOI] [PubMed] [Google Scholar]
- 5.Harder T, Lau SCK, Dobretsov S, Fang TK, Qian PY. A distinctive epibiotic bacterial community on the soft coral Dendronephtya sp. and antibacterial activity of coral tissue extracts suggest a chemical mechanism against bacterial epibiosis. FEMS Microbiol Ecol. 2003;43:337–347. doi: 10.1111/j.1574-6941.2003.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 6.Harder T, Lau SCK, Tam WY, Qian PY. A bacterial culture-independient method to invesitigate chemically mediated control of bacterial epibiosis in marine invertebrates by TRFLP analysis and natural bacterial populations. FEMS Microbiol Ecol. 2004;47:93–99. doi: 10.1016/S0168-6496(03)00246-0. [DOI] [PubMed] [Google Scholar]
- 7.Mai-Pochnow A, Evans F, Dalisay-Saludes D, Stelzer S, Egan S, James S, Webb JS, Kjelleberg S. Biofilm development and cell death in the marine bacterium Pseudoalteromonas tunicata. Appl Environ Microbiol. 2004;70:3232–3238. doi: 10.1128/AEM.70.6.3232-3238.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YM, Yasuda M, Yamagishi A, Oshima A, Ohta S. Characterization of the endo-symbiont of a deep-sea bivalve, Calyptogena soyoae. Appl Environ Microbiol. 1995;61:823–827. doi: 10.1128/aem.61.2.823-827.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preston CM, Wu KY, Molinski TF, DeLong EF. A psycrophylic crenarchaeon inhabits a marine sponge: Cenarchaeum symbiosum gen. nov., sp. nov. Proc Natl Acad Sci USA. 1996;93:6241–6246. doi: 10.1073/pnas.93.13.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haygood MG, Davidson SK. Small subunit ribosomal RNA genes and in situ hybridation of bacterial symbionts in the larvae of the bryozoan Bugula neritina and proposal of “Candidatus Endobugula sertula”. Appl Env Microbiol. 1997;63:4612–4616. doi: 10.1128/aem.63.11.4612-4616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sipe AR, Wilbur AE, Cary SC. Bacterial symbiont transmission in the wood-boring shipworm Bankia setacea (Bivalvia: Teredinidae) Appl Environ Microbiol. 2000;66:1685–1691. doi: 10.1128/aem.66.4.1685-1691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubilier N, Mulders C, Ferdelman T, de Beer D, Pernthaler A, Klein M, Wagner M, Erseus C, Thiermann F, Krieger J, Giere O, Amann R. Endosymbiotic sulphate-reducing and sulphide-oxidizing bacteria in an oligochaete worm. Nature. 2001;411:298–302. doi: 10.1038/35077067. [DOI] [PubMed] [Google Scholar]
- 13.Kühl M, Larkum AW. The microenvironment and photosynthetic performance of Prochloron sp. in symbiosis with Didemnid ascidians. In: Seckbach J, editor. Symbiosis: Mechanisms and Models. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. pp. 273–290. [Google Scholar]
- 14.Moss C, Green DH, Pérez B, Velasco A, Henríquez R, McKenzie JD. Intracellular bacteria associated with the ascidian Ecteinascidia turbinata: phylogenetic and in situ hybridisation analysis. Mar Biol. 2003;143:99–110. [Google Scholar]
- 15.Munn CB. Symbiotic associations. In: Watts A, editor. Marine microbiology. Cromwell Press; Trowbridge, UK: 2004. pp. 167–181. [Google Scholar]
- 16.Long PF, Dunlap WC, Batershill CN, Jaspars M. Shotgun cloning and heterologous expression of the patellamide gene clusters as strategy to achieving sustained metabolite production. Chembiochem. 2005;6:1760–1765. doi: 10.1002/cbic.200500210. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt EW, Nelson JT, Rasko DA, Sudek S, Eisen JA, Haygood MG, Ravel J. Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemni, the cyanobacterial symbiont of Lissoclinum patella. Proc Natl Acad Sci USA. 2005;102:7315–7320. doi: 10.1073/pnas.0501424102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson SK, Allen SW, Lim GE, Anderson CM, Haygood MG. Evidence for the biosynthesis of bryostatins by the bacterial symbiont "Candidatus Endobugula sertula" of the bryozoan Bugula neritina. Appl Environ Microbiol. 2001;67:4531–4537. doi: 10.1128/AEM.67.10.4531-4537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hale KJ, Hummersone MG, Manaviazar S, Frigerio M. The chemistry and biology of the bryostatin antitumour macrolides. Nat Prod Rep. 2002;19:413–453. doi: 10.1039/b009211h. [DOI] [PubMed] [Google Scholar]
- 20.Rottmayr EM, Steffan B, Wanner G. Pigmentation and tunic cells in Cystodytes dellechiajei (Urochordata, Ascidiacea) Zoomorphology. 2001;120:159–170. [Google Scholar]
- 21.Turon X, López-Legentil S, Banaigs B. Cell types, microsymbionts, and pyridoacridine distribution in the tunic of three color morphs of the genus Cystodytes (Ascidiacea, Polycitoridae) Invertebr Biol. 2005;124:355–369. [Google Scholar]
- 22.Steffan B, Brix K, Pütz W. Biosynthesis of Shermilamine B. Tetrahedron Let. 1993;49:6223–6228. [Google Scholar]
- 23.Shimbo K, Tsuda M, Fukushi E, Kawabata J, Kobayashi J. Dytesinins A and B, new clerodane-type diterpenes with a cyclopropane ring from the tunicate Cystodytes sp. Tethraedron. 2000;56:7923–7926. [Google Scholar]
- 24.Loukaci A, Bultel-Poncé V, Longeon A, Guyot M. New lipids from the tunicate Cystodytes cf. dellechiajei:as PLA2 inhibitors. J Nat Prod. 2000;63:799–802. doi: 10.1021/np990443k. [DOI] [PubMed] [Google Scholar]
- 25.McDonald LA, Eldredge GS, Barrows LR, Ireland CM. Inhibition of topoisomerase II catalytic activity by pyridoacridine alkaloids from a Cystodytes sp. ascidian: A mechanism for the apparent intercalator-induced inhibition of topoisomerase II. J Med Chem. 1994;37:3819–3827. doi: 10.1021/jm00048a017. [DOI] [PubMed] [Google Scholar]
- 26.Ciufolini MA, Shen YC, Bishop MJ. A unified strategy for the synthesis of sulfur containing pyridoacridine alkaloids: antitumor agents of marine origin. J Am Chem Soc. 1995;117:12460–12469. [Google Scholar]
- 27.Dassonneville L, Wattez N, Baldeyrou B, Mahieu C, Lansiaux A, Banaigs B, Bonnard I, Bailly C. Inhibition of topoisomerase II by the marine alkaloid ascididemin and induction of apoptosis in leukemia cells. Biochem Pharmacol. 2000;60:527–537. doi: 10.1016/s0006-2952(00)00351-8. [DOI] [PubMed] [Google Scholar]
- 28.Torres YR, Bugni TS, Berlinck RG, Ireland CM, Magalhaes A, Ferreira AG, Rocha RM. Sebastianines A and B, novel biologically active pyridoacridine alkaloids from the Brazilian ascidian Cystodytes dellechiajei. J Org Chem. 2002;67:5429–5432. doi: 10.1021/jo011174h. [DOI] [PubMed] [Google Scholar]
- 29.Prado MP, Torres YR, Berlinck RGS, Desiderá C, Sanchez MA, Craveiro MV, Hadju H, da Rocha RM, Machado-Santelli GM. Effects of marine organisms extracts on microtubule integrity and cell cycle progression in cultured cells. J Exp Mar Biol Ecol. 2004;313:125–137. [Google Scholar]
- 30.Becerro MA, Turon X, Uriz MJ. Multiple functions for secondary metabolites in encrusting marine invertebrates. J Chem Ecol. 1997;23:1527–1547. [Google Scholar]
- 31.Tarjuelo I, López-Legentil S, Codina M, Turon X. Defence mechanisms of adults and larvae of colonial ascidians: patterns of palatability and toxicity. Mar Ecol Prog Ser. 2002;235:103–115. [Google Scholar]
- 32.López-Legentil S, Dieckmann R, Bontemps-Subielos N, Turon X, Banaigs B. Qualitative variation of alkaloids in colour morphs of Cystodytes (Ascidiacea) Bioch Syst Ecol. 2005;33:1107–1119. [Google Scholar]
- 33.Martínez-García M, Diaz-Valdés M, Wanner G, Ramos-Esplá A, Antón J. Microbial community associated with the colonial ascidian Cystodytes dellechiajei. Environ Microbiol. 2007;9:521–534. doi: 10.1111/j.1462-2920.2006.01170.x. [DOI] [PubMed] [Google Scholar]
- 34.Schäfer H, Bernard L, Courtis C, Lebaron P, Servais P, Pukall P, Stackebrandt E, Troussellier M, Guindulain T, Vives-Rego J, Muyzer G. Microbial community dynamics in Mediterranean nutrient-enriched seawater mesocosms: changes in the genetic diversity of bacterial populations. FEMS Microbiol Ecol. 2001;34:243–253. doi: 10.1111/j.1574-6941.2001.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 35.Bonnard I, Bontemps N, Lahmy S, Bonaigs B, Combaut G, Francisco C, Colson P, Houssie C, Waring MJ, Bailly C. Binding to DNA and cytotoxic evaluation of ascididemin, the major alkaloid from the Mediterranean ascidian Cystodytes dellechiajei. Anti Cancer Drug Des. 1995;10:333–346. [PubMed] [Google Scholar]
- 36.Lindsay B, Barrows L, Copp B. Structural requeriments for biological activity of the marine alkaloid ascididemin. Bioorg Med Chem Lett. 1995;5:739–742. [Google Scholar]
- 37.Lindquist N, Hay ME, Fenical W. Defense of ascidians and their conspicuous larvae: adult vs. larval chemical defenses. Ecol Monogr. 1992;62:547–568. [Google Scholar]
- 38.Lindquist N. Palatability of invertebrate larvae to corals and sea anemones. Mar Biol. 1996;126:745–755. [Google Scholar]
- 39.Lindquist N, Hay ME. Palatibility and chemical defense of marine invertebrate larvae. Ecol Monogr. 1996;66:431–450. [Google Scholar]
- 40.Hay ME. Marine chemical ecology: what’s known and what’s next? J Exp Mar Biol Ecol. 1996;200:103–134. [Google Scholar]
- 41.Uriz MJ, Turon X, Becerro MA, Galera J. Feeding deterrence in sponges. The role of toxicity, physical defenses, energetic contents, and life-history stage. J Exp Mar Biol Ecol. 1996;205:187–204. [Google Scholar]
- 42.Roughgarden J. The evolution of marine life cycles. In: Feldman MW, editor. Mathematical evolutionary theory. Princeton University Press; Princeton, NJ: 1989. pp. 270–300. [Google Scholar]
- 43.Kobayashi J, Cheng J, Wälchli MR, Nakamura H, Hirata Y, Sasaki T, Ohizumi Y. Cystodytins A, B, and C, novel tetracyclic aromatic alkaloids with potents antineoplasic activity from the okinawan tunicate Cystodytes dellechiajei. J Org Chem. 1988;53:1800–1804. [Google Scholar]
- 44.Carroll AR, Cooray NM, Pioner A, Scheuer PJ. A second shermilamine alkaloid from a tunicate Trididemnum spec. J Org Chem. 1989;54:4231–4232. [Google Scholar]
- 45.Kobayashi J, Tsuda M, Tanabe A, Ishibashi M. Cystodytins D-I, new cytotoxic tetracyclic aromatic alkaloids from the okinawan marine tunicate Cystodytes dellechiajei. J Nat Prod. 1991;54:1634–1638. doi: 10.1021/np50078a022. [DOI] [PubMed] [Google Scholar]
- 46.López-Legentil S, Bontemps-Subielos N, Turon X, Banaigs B. Temporal variation in the production of fours secondary metabolites in a colonial ascidian. J Chem Ecol. 2006;32:2079–2084. doi: 10.1007/s10886-006-9148-2. [DOI] [PubMed] [Google Scholar]
- 47.Webster NS, Hill RT. The culturable microbial community of the Great Barrier Reef sponge Rhopaloeides odorabile is dominated by an α-Proteobacterium. Mar Biol. 2001;138:843–851. [Google Scholar]
- 48.Hentschel U, Schmid M, Wagner M, Fieseler L, Gernert C, Hacker J. Isolation and phylogenetic analysis of bacteria with antimicrobial activities from the Mediterranean sponges Aplysina aerophoba and Aplysina cavernicola. FEMS Microbiol Ecol. 2001;35:305–312. doi: 10.1111/j.1574-6941.2001.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 49.Hamada T. Use of gyrB gene analysis to investigate phylogeny of marine bacteria. 1998 Unpublished. [Google Scholar]
- 50.Enticknap JJ, Kelly M, Peraud O, Hill RT. Characterization of a culturable alpha-proteobacterial symbiont common to many marine sponges and evidence for vertical transmission through the gametes. 2005 doi: 10.1128/AEM.72.5.3724-3732.2006. Unpublished. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guzman AS, Predicala RZ, Bernardo EB, Neilan B, Elardo SP, Mangalindan GC, Tasdemir D, Ireland CM, Barraquio WL, Concepcion GP. Marine alpha-proteobacterium from a Philippine tunicate and its anti-Staphylococcus aureus metabolite heptylprodigiosin. 2004 Unpublished. [Google Scholar]
- 52.Koblízek M, Béjà O, Bidigare RR, Christensen S, Benitez-Nelson B, Vetriani C, Kolber MK, Falkowski PG, Kolber ZS. Isolation and characterization of Erythrobacter sp. strains from the upper ocean. Arch Microbiol. 2003;180:327–338. doi: 10.1007/s00203-003-0596-6. [DOI] [PubMed] [Google Scholar]
- 53.Rottmayr EM. PhD thesis. Fakultät für Biologie der Ludwig-Maximilians-Universität; München, Germany: 2001. Licht- und elektronenmikroskopische Untersuchungen der Tunika von Cystodytes dellechiajei Della Valle (Urochordata, Ascidiacea) [Google Scholar]
- 54.Lehmann CM. Characterization of bacterium isolates C1 from Cystodytes dellechiajei. 2004 Unpublished. [Google Scholar]
- 55.Debard H, Banaigs B, Francisco F, Commeyras A. Use of ascididemin and derivates as antifouling agents. WO 98/21959. PCT Int Appl. 1998
- 56.In Y, Doi M, Inoue M, Ishida T, Hamada Y, Shioiri T. Patellamide A, a cytotoxic cyclic peptide from the ascidian Lissoclinum patella. Acta Crystalogr C. 1994;15:432–434. doi: 10.1107/s010827019300811x. [DOI] [PubMed] [Google Scholar]
- 57.Fu X, Do T, Schmitz FJ, Andrusevich V, Engel MH. New cyclic peptides from the ascidian Lissoclinum patella. J Nat Prod. 1998;61:1547–1551. doi: 10.1021/np9802872. [DOI] [PubMed] [Google Scholar]
- 58.Salomon CE, Faulkner DJ. Localization studies of bioactive cyclic peptides in the ascidian Lissoclinum patella. J Nat Prod. 2002;65:689–692. doi: 10.1021/np010556f. [DOI] [PubMed] [Google Scholar]
- 59.Clardy J. Using genomics to deliver natural products from symbiotic bacteria. Gen Biol. 2005;6:232. doi: 10.1186/gb-2005-6-9-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mojica FJM. Antiarchaeal antibiotics. In: Cienfuegos GA, Ruiz-Bravo A, Pablo MA, editors. New approaches in the use of antibiotics. Research Singpost; Kerala, India: 2003. pp. 83–108. [Google Scholar]
- 61.Sambrook E, Fritsch F, Maniatis T. Molecular cloning A laboratory manual. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 62.Muyzer G, de Waal EC, Uitterrlinden AG. Profiling in complex microbial population by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Micriobiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weisburg WE, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaneechoutte M, Rossau R, Vos PD, Gillis M, Janssens D, Paepe N, De Rouck, Fiers T, Claeys G, Kersters K. Rapid identification of bacteria of the Comamonadaceae with amplified ribosomal DNA-restriction analysis (ARDRA) FEMS Microbiol Lett. 1992;93:227–234. doi: 10.1111/j.1574-6968.1992.tb05102.x. [DOI] [PubMed] [Google Scholar]
- 65.Amann R, Ludwig W, Schleifer KH. Phylogenetic identification of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Strimmer K, von Haeseler A. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol Biol Evol. 1996;13:964–969. [Google Scholar]
- 68.Strimmer K, von Haeseler A. Likelihood-mapping: a simple method to visualize phylogenetic content of a sequence alignement. Proc Natl Acad Sci USA. 1997;94:6815–6819. doi: 10.1073/pnas.94.13.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moore GE, Woods LK. Culture media for human cells: RPMI 1630, RPMI 1634, RPMI 1640 and GEM 1717. T C A Man. 1976;3:503–508. [Google Scholar]
- 70.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]