Abstract
Lens epithelial cells are the parental cells responsible for growth and development of the transparent ocular lens. Many elegant investigations into their biology have focused on the factors that initiate and regulate lens epithelial cell differentiation. Because they serve key transport and cell maintenance functions throughout life, and are the primary source of metabolic activity in the lens, mechanisms to maintain lens epithelial cell integrity and survival are critical for lens transparency. The molecular chaperones α-crystallins are abundant proteins synthesized in the differentiated lens fiber cell cytoplasm. However, their expression in lens epithelial cells has only been appreciated very recently. Besides their important roles in the refractive and light focusing properties of the lens, α-crystallins have been implicated in a number of non-refractive pathways including those involving stress response, apoptosis and cell survival. The most convincing evidence for their importance in the lens epithelium has been shown by studies on the properties of lens epithelial cells from αA and αB-crystallin gene knockout mice. The effective combination of genetics, cell and molecular biology possible with lens epithelial cells is attracting an increasing number of researchers focused on understanding how lens epithelial cells coordinate survival, proliferation and differentiation.
Cell facts
Lens epithelial cells are responsible for the growth and development of the entire ocular lens
Lens development is controlled by cell cycle regulators, signaling molecules and growth factors such as FGF
Lens epithelial cells express members of the small heat shock protein family of molecular chaperones, αA and βB-crystallins, the first crystallins to be expressed during lens development
α-Crystallins likely protect lens epithelial cells from environmental stress throughout life, and thus may delay the onset of age-related cataract
Lens epithelial cell proliferation is deregulated in posterior capsule opacification leading to secondary cataract, a complication in up to 50% of cataract surgeries
1. Introduction
The ocular lens is an avascular and transparent tissue in which all the cells are derived by the proliferation and differentiation of lens epithelial cells. The anterior lens surface is lined by a single cell layer of cuboidal-shaped epithelial cells (Figure 1). The bulk of the lens is comprised of uniquely elongated fiber cells which can attain lengths of up to 1000 microns. The entire lens is encapsulated in a thickened basement membrane known as the capsule. The lens is the only tissue in the body that grows throughout life, yet lens cell division occurs on a very limited scale in a narrow band of epithelial cells, with a pattern of greater growth in the embryo than in adults. Because of its location along the optic axis of the eye, the lens is chronically exposed to environmental stress from solar radiation. The molecular chaperones expressed in lens epithelial cells include αA-crystallin, αB-crystallin, Hsp25/27, Hsp40, Hsc70, Hsp70 and TCP-1 (Andley et al., 1998; Bagchi et al., 2002; Nagineni and Bhat, 1992; Wang et al., 2004). While other reviews focus on antioxidant systems that protect lens epithelial cells from stress, this article considers the evidence that molecular chaperones αA and αB-crystallins are an important cell defense mechanism in lens epithelial cells.
Figure 1.
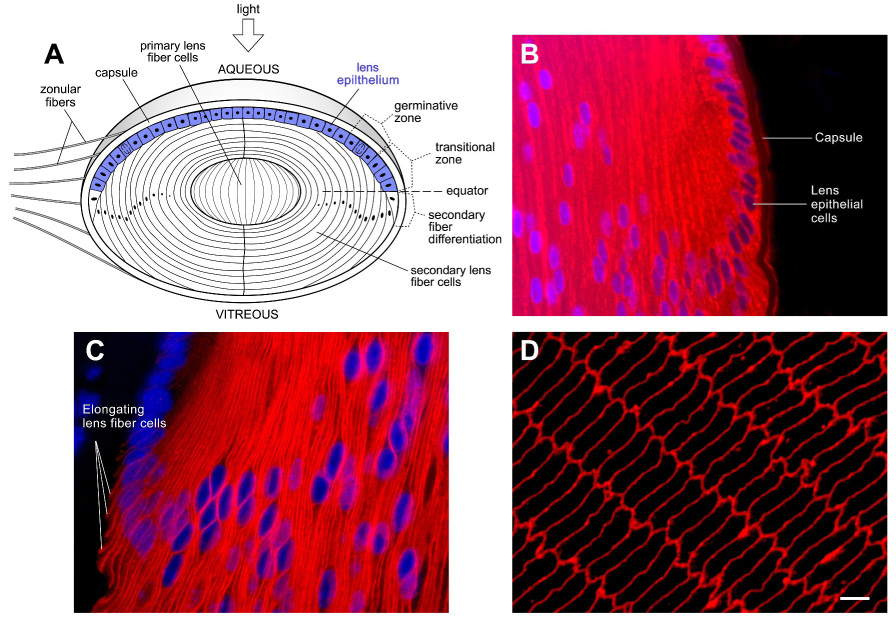
The lens has a unique cellular architecture consisting of a single layer of cuboidal epithelial cells which divide and differentiate into lens fiber cells. The tightly packed fiber cells have an elongated morphology and produce abundant levels of crystallins essential for transparency. (A) Schematic drawing of a lens sectioned along the optic axis to illustrate the location of the lens epithelial cells. Although all lens epithelial cells are capable of undergoing cell proliferation, in a mature lens, epithelial cell proliferation occurs mainly in a region above the lens equator called the germinative zone. Cells in the transitional zone have withdrawn from the cell cycle and are in transition to becoming secondary lens fiber cells. Formation of secondary lens fiber cells from epithelial cells occurs slightly posterior to the lens equator (horizontal dashed line). Throughout life, layers of newly formed fiber cells cover the older cells. As a result, older secondary fiber cells are located closer to the center of the lens. The primary fiber cells are formed during embryogenesis. Zonular fibers hold up the lens. (B–D) Immunofluorecence analysis of mouse lens sections with antibodies to αA-crystallin, the lens fiber cell-specific membrane intrinsic protein (MIP/AQP0) and Alexa568-labeled secondary antibodies. Nuclei were labeled with the DNA labeling fluorescent dye, DAPI. (B) αA-crystallin expression in the mid-sagittal section of a 5 day old mouse lens by immunofluorescence analysis. DNA is in blue. αA-crystallin is in red. Note that αA-crystallin immunostaining can be found in the cell cytoplasm of both lens epithelial and fiber cells. (C) In the equatorial region, the expression of lens fiber cell specific membrane protein MIP/AQP0 shown in red, marks the onset of differentiation, and elongation, of epithelial cells. (D) The bulk of the lens is composed of fiber cells which appear as hexagons in a cross-sectional slice of the lens. immunofluorescence staining for MIP is shown in red.
Developmentally, α-crystallin is the first crystallin synthesized in the mammalian lens where it is initially detected in the lens placode (Robinson and Overbeek, 1996). An intriguing hypothesis suggests that the α-crystallin molecular chaperones may act as survival proteins to prevent cell death during differentiation wherein an apoptosis like program is initiated to eliminate cellular organelles (Morozov and Wawrousek, 2006). Because differentiated lens fiber cells synthesize an abundance of crystallins, historically, most studies on crystallins have been performed in the context of their functions in lens fiber cells particularly in their modifications during age-related lens opacification (cataract). Consequently, much less known about α-crystallin expression and function in these cells. The expression and function of molecular chaperones in the lens epithelium is an emerging field focused on their desirable roles in protecting cells from unwanted environmental stresses to which the lens is exposed throughout life. Readers are directed to a recent comprehensive text for expert reviews on crystallins and the cell and developmental biology of the lens (Lovicu and Robinson, 2004).
2. Biological origins of lens epithelial cells
During embryogenesis, vertebrate lens epithelial cells begin development in the head ectoderm (Beebe and Coats, 2000; Lang, 2004; Lang and McAvoy, 2004; Robinson, 2006). The presumptive lens ectoderm (PLE) overlies the embryonic optic vesicle (OV) (Figure 2A). Association of these tissues soon leads to the growth of the PLE that thickens to form the lens placode (Figure 2B). The lens placode then begins to invaginate forming the lens pit which closes to form the lens vesicle (Figure 2C and D). The lens vesicle separates from the overlying surface ectoderm that will ultimately differentiate into the corneal epithelium (Figure 2E). Cells in the anterior segment of the lens vesicle differentiate to form the lens epithelium, while those in the posterior segment of the lens vesicle elongate towards the anterior, filling the lens vesicle lumen and are called primary lens fiber cells (Figure 2F). The primary lens fiber cells soon lose their ability to proliferate as fiber cell differentiation proceeds. The lens then grows by continued proliferation of epithelial cells and differentiation of fiber cells. Proliferation initially occurs throughout the epithelial compartment but during development becomes increasingly restricted to a small band of cells slightly above the lens equator, known as the germinative zone. Progeny of cells that shift below the equator enter a region called the transitional zone (Figure 1A). This zone denotes cells that are in transition to becoming differentiated fiber cells. Cells in the transitional zone withdraw from the cell cycle to give rise to secondary lens fiber cells, and begin to morphologically elongate and accumulate high concentrations of α, β and γ-crystallins, and several lens specific proteins including the most abundant lens fiber cell membrane protein MIP (AQP0) (Piatigorsky, 1981). These features of lens differentiation have been extensively investigated and are known to be the result of changes in gene expression of key cell cycle regulators and transcription factors.
Figure 2.
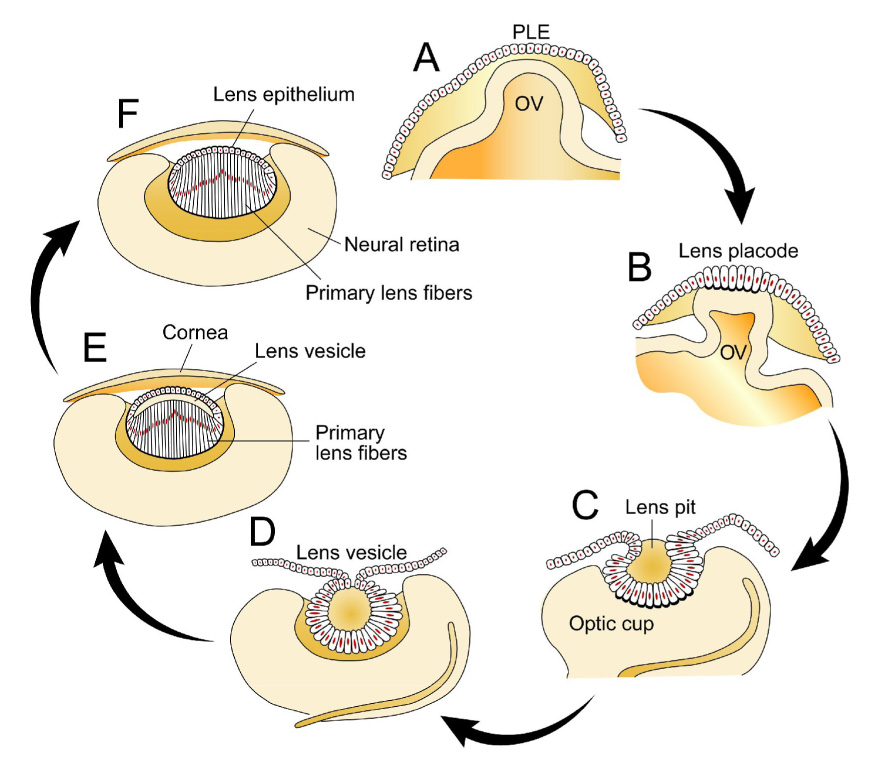
Embryonic development of the lens. (A) The vertebrate lens begins development as a sheet of surface ectoderm that is exposed to multiple inductive factors in the gastrulation stage of the embryo. Contact of the presumptive lens ectoderm (PLE) and the lens vesicle leads to a thickening of the PLE to form the lens placode. (B, C) Invagination of the lens placode forms the lens pit which then forms the lens vesicle. (D) Cells in the anterior of the lens vesicle proliferate and give rise to the lens epithelium while those in the posterior of the lens vesicle elongate into primary lens fiber cells. (E) Secondary fiber cells are formed by proliferation and differentiation of the lens epithelial cells near the equator. (F) The lens then grows throughout the life of a vertebrate by continued addition of lens fiber cells on top of the older fiber cells. There is no turnover in the lens cells and thus secondary fiber cells persist for life. (Adapted from Robinson, 2006; see text for further details).
The lens is a highly polarized tissue. The anterior of the lens is exposed to influences from the aqueous humor, which promote the maintenance and development of the epithelium, whereas the posterior environment promotes fiber cell differentiation (Figure 1A). Elegant experiments on lens inversion in the chicken eye first demonstrated that the polarity of the lens is dependent upon its position within the eye (reviewed in (Beebe and Coats, 2000; Robinson, 2006)). Inverting the lens so that the epithelium faced the posterior of the eye led to synthesis of a new lens epithelium on the anterior of the eye which differentiated into lens fiber cells.
Lens stem cells are thought to reside in the germinative zone, although recent work has shown that the central epithelial cells may be the site of lens stem cells (Griep, 2006; Zhou et al., 2006). Very recently, it has been hypothesized that lens stem cells may reside in the ciliary body (Remington and Meyer, 2007).
3. Functions of chaperones in lens epithelium
Molecular chaperones prevent undesired protein aggregation by binding non-native intermediates that may arise in response to cellular stress or during protein translation in vivo. Although the mechanism of α-crystallin’s chaperone action is not completely understood, it involves the formation of a complex between α-crystallin and substrate proteins. A binding site for substrate proteins has been identified in the sequences of both αA and αB-crystallin (reviewed in (Van Montfort et al., 2001)). Chaperones may be important in cell growth control of lens epithelial cells by preventing the aggregation of key proteins during periods of large cellular transitions during differentiation, and by targeting proteins involved in cell cycle progression to the proteasome. αA-crystallin has recently been shown to recognize epithelial sodium channels for some cells, and this may reflect a mechanism of regulation in lens epithelium (Kashlan et al., 2007).
Lens epithelial cells undergo cell growth, quiescence, irreversible withdrawal from the cell cycle and terminal differentiation into lens fiber cells. These processes are precisely regulated in a temporal and topographically distinct manner (Figure 1). How cells in the central epithelium are regulated differently from cells in the proliferative zone is not clearly understood. Does α-crystallin play a role in protecting the central epithelium in vivo?
3.1 Cell survival is correlated with expression of αA and αB in lens epithelium
At present considerable detail on the mechanism of cell survival by α-crystallin has been gleaned from in vitro cell culture studies. This body of information can guide future studies in determining how α-crystallin mediates its protective effect in the lens epithelium. In αA-crystallin-transfected cells, survival is promoted and apoptosis inhibited (Andley et al., 1998). Consistent with these results, knocking out the endogenous αA-crystallin gene in mice promotes cell death. This is correlated with reduction in cell proliferation and mitosis inhibition (Xi et al., 2003). For many cell types and stressors, αB-crystallin inhibits apoptosis by preventing the auto proteolytic maturation of caspase-3 (Arrigo et al., 2002; Kamradt et al., 2002). αA-crystallin may also enhance survival by interacting with anti-apoptotic factors Bax and Bcl-xs (Liu et al., 2004). In cultured cells, αB-crystallin was shown to inhibit the activation of ERK signaling pathway and attenuate apoptosis (Li et al., 2005).
3.2 Enhancing survival during differentiation
The dramatic changes in cell shape and length that characterize lens fiber cell differentiation rely on the cytoskeleton (Bassnett, 2002; Sue Menko, 2002). The stabilization of the microtubule, actin and intermediate filament cytoskeletons may require α-crystallin (Quinlan, 2002). In addition to its classical cytoplasmic localization, α-crystallin is associated with regions of plasma membrane in cells and in organelles such as the nucleus and golgi. The close association of membranes and α-crystallins suggests that the stabilization of the membranes may also require α-crystallin expression during lens epithelial cell differentiation. The coordinated expression of α-crystallin and integrin may reflect its cooperation with signaling molecules to induce cell cycle progression, withdrawal and survival as lens epithelial cells transition through the proliferation zone. These results suggest that α-crystallin function in epithelium involves its association with signaling complexes and classical cell signaling kinases. Indeed such an association has been shown in lens epithelial fractions (Menko, unpublished Results; (Menko et al., 2005)). Future studies to define a specific interaction of cell matrix and signaling proteins with α-crystallin may reveal the mechanism by which α-crystallin regulates different aspects of cell survival, proliferation and differentiation.
3.3 Ensuring the successful completion of mitosis and cytokinesis
αB-crystallin is known to be expressed in the lens epithelial cell cytoplasm. Primary cultures of αB knockout lens epithelial cells demonstrate genomic instability and undergo hyperproliferation at frequency 4 orders of magnitude greater than that predicted by spontaneous immortalization of rodent cells (Andley et al., 2001). Mechanistic studies demonstrate an upregulation of functionally impaired p53 protein (Bai et al., 2003). Consistent with defects in proliferation, mitotic αB knockout cells in culture display aberrant tubulin cytoskeleton during anaphase and cytokinesis. The expression of αA and αB-crystallin occurs in a cell cycle dependent manner, increasing in S phase prior to mitosis. Lack of both αA and αB-crystallin decreases the number of cells that enter the S phase during the cell cycle progression (Bai et al., 2004). Consistent with these studies, the association of αB-crystallin in a complex of FBX has been found to direct the ubiquitination of phosphorylated cyclin D1 during the cell cycle, signifying the importance of αB-crystallin in cell cycle regulation (Lin et al., 2006). Targeting key cell cycle regulatory molecules to the proteasome may be one of the functions of αB-crystallin in the lens epithelial growth control. The intracellular distribution of αA and αB-crystallin is predominantly in plasma membranes and fibrillar areas of the cytoplasm in lens epithelial fixed in situ in living animals (Wang et al., 2004). αB-crystallin expression in golgi compartment suggests its importance in golgi biogenesis (Gangalum et al., 2004). Which of these functions contribute to α-crystallin’s role as a chaperone in lens epithelial cells remains to be determined.
4. Pathologies
4.1 Posterior capsular opacification
The loss of transparency or cataract is a major cause of blindness. Surgical removal of cataract requires removal of the fiber cells while the lens capsule and epithelium remains in the eye, and the capsular bag is filled with an artificial lens. In nearly 50% of cataract surgeries, the uncontrolled proliferation, migration and aberrant differentiation of equatorial lens epithelial cells remaining after cataract surgery are the cause of posterior capsular opacification (PCO) or secondary cataract (Marcantonio and Vrensen, 1999). Specific therapies to target PCO are an intensive area of basic and clinical research. Inhibition of ERK signaling has been shown to block PCO in lens cultures (Walker et al., 2007). Using animal models to mimic cataract surgery may offer a way to better understand the mechanisms of abnormal lens epithelial cell growth and approaches to repress secondary cataract formation.
4.2 Age-related cataracts
Age-related cataracts are the leading cause of blindness and an intensive area of research. The lens epithelium serves key metabolic functions for the entire lens. Enzymes that protect the epithelium as well as key antioxidant systems reside in the epithelial cells. It has been suggested that lens epithelial cell death plays a key role in the development of human age-related cataract. Although there is controversy whether cell death is a mechanism for age-related cataracts, it is widely accepted that agents that induce cataract in animal models also induce lens epithelial cell apoptosis. Analysis of lens epithelial α-crystallin in human samples and in animal models may reveal how α-crystallin affects the development of age-related cataract. This is an important research area that needs further attention.
4.3 Hereditary cataracts
The study of hereditary cataracts associated with point mutations in α-crystallin genes is an emerging new area possible with recent combination of genetic and biochemical studies on the lens (Mackay et al., 2003). Mutations that lead to hereditary cataracts cause increased cell death in lens epithelial cells, and abnormal nuclear localization of αA-crystallin, but details of mechanisms that lead to hereditary cataracts are not known. Investigation into this theme of research is likely to provide information not only about hereditary cataract but also on the more common age-related cataracts.
5. Summary
The expanding knowledge of α-crystallin in lens epithelium is providing a view of its functions in which α-crystallin regulates and fine tunes aspects of cell survival, growth and proliferation. Recent progress in understanding mechanisms of chaperone function and newly created animal models for cataract caused by modulating chaperone function should lead to greater progress on the function of chaperones in lens epithelial cell biology. Novel experimental approaches are needed that address mechanisms by which the lens epithelium contributes to human cataract formation. Emphasis on the concept that αA and αB-crystallin are an integral component of the cell development and survival pathways available to the lens epithelium in order to survive for decades without renewal may lead to novel approaches to prevent or delay human cataract.
Acknowledgements
My sincere apologies go to those authors whose work could not be cited due to space constraints. A special thanks to Jing-hua Xi, Fang Bai and Belinda McMahan for their help in the course of this work. This work is supported by the National Institutes of Health (NIH) grants R01EY05681 and Core grant EY02687, and an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andley UP, Song Z, Wawrousek EF, Bassnett S. The molecular chaperone alphaA-crystallin enhances lens epithelial cell growth and resistance to UVA stress. J Biol Chem. 1998;273:31252–31261. doi: 10.1074/jbc.273.47.31252. [DOI] [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Brady JP, Bassnett S, Fleming TP. Lens epithelial cells derived from alphaB-crystallin knockout mice demonstrate hyperproliferation and genomic instability. Faseb J. 2001;15:221–229. doi: 10.1096/fj.00-0296com. [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Paul C, Ducasse C, Manero F, Kretz-Remy C, Virot S, Javouhey E, Mounier N, Diaz-Latoud C. Small stress proteins: novel negative modulators of apoptosis induced independently of reactive oxygen species. Prog Mol Subcell Biol. 2002;28:185–204. doi: 10.1007/978-3-642-56348-5_10. [DOI] [PubMed] [Google Scholar]
- Bagchi M, Katar M, Maisel H. Heat shock proteins of adult and embryonic human ocular lenses. J Cell Biochem. 2002;84:278–284. [PubMed] [Google Scholar]
- Bai F, Xi J, Higashikubo R, Andley UP. Cell kinetic status of mouse lens epithelial cells lacking alphaA- and alphaB-crystallin. Mol Cell Biochem. 2004;265:115–122. doi: 10.1023/b:mcbi.0000044365.48900.82. [DOI] [PubMed] [Google Scholar]
- Bai F, Xi JH, Wawrousek EF, Fleming TP, Andley UP. Hyperproliferation and p53 status of lens epithelial cells derived from alphaB-crystallin knockout mice. J Biol Chem. 2003;278:36876–36886. doi: 10.1074/jbc.M304010200. [DOI] [PubMed] [Google Scholar]
- Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74:1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Coats JM. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev Biol. 2000;220:424–431. doi: 10.1006/dbio.2000.9638. [DOI] [PubMed] [Google Scholar]
- Gangalum RK, Schibler MJ, Bhat SP. Small heat shock protein alphaB-crystallin is part of cell cycle-dependent Golgi reorganization. J Biol Chem. 2004;279:43374–43377. doi: 10.1074/jbc.C400371200. [DOI] [PubMed] [Google Scholar]
- Griep AE. Cell cycle regulation in the developing lens. Semin Cell Dev Biol. 2006;17:686–697. doi: 10.1016/j.semcdb.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, Sam S, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem. 2002;277:38731–38736. doi: 10.1074/jbc.M201770200. [DOI] [PubMed] [Google Scholar]
- Kashlan OB, Mueller GM, Qamar MZ, Poland PA, Ahner A, Rubenstein RC, Hughey RP, Brodsky JL, Kleyman TR. Small heat shock protein alpha A-crystallin regulates epithelial sodium channel expression. J Biol Chem. 2007 doi: 10.1074/jbc.M703409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RA. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48:783–791. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- Lang RA, McAvoy JW. Growth factors in lens development. In: Lovicu FJ, Robinson M, editors. Development of the ocular lens. Cambrigde University press; 2004. [Google Scholar]
- Li DW, Liu JP, Mao YW, Xiang H, Wang J, Ma WY, Dong Z, Pike HM, Brown RE, Reed JC. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Mol Biol Cell. 2005;16:4437–4453. doi: 10.1091/mbc.E05-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DI, Barbash O, Kumar KG, Weber JD, Harper JW, Klein-Szanto AJ, Rustgi A, Fuchs SY, Diehl JA. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol Cell. 2006;24:355–366. doi: 10.1016/j.molcel.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Lui L, Huang XQ, Liu Y, Li DW. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004;79:393–403. [PubMed] [Google Scholar]
- Lovicu FJ, Robinson ML. Development of the Ocular Lens. Cambridge University Press; 2004. [Google Scholar]
- Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alphaA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- Marcantonio JM, Vrensen GF. Cell biology of posterior capsular opacification. Eye. 1999;13(Pt 3b):484–488. doi: 10.1038/eye.1999.126. [DOI] [PubMed] [Google Scholar]
- Menko AS, Zhang L, Weber G, Orlova I, Bai F, Xi J, Andley UP. Regulation of signaling pathways by aA-crystallin: A possible role for aA-crystallin association with a6 integrin complexes. Invest Ophthalmol Vis Sci. 2005 Supplement Program number 3873. [Google Scholar]
- Morozov V, Wawrousek EF. Caspase-dependent secondary lens fiber cell disintegration in alphaA-/alphaB-crystallin double-knockout mice. Development. 2006;133:813–821. doi: 10.1242/dev.02262. [DOI] [PubMed] [Google Scholar]
- Nagineni CN, Bhat SP. Lens fiber cell differentiation and expression of crystallins in co-cultures of human fetal lens epithelial cells and fibroblasts. Exp Eye Res. 1992;54:193–200. doi: 10.1016/s0014-4835(05)80208-8. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Quinlan R. Cytoskeletal competence requires protein chaperones. Prog Mol Subcell Biol. 2002;28:219–233. doi: 10.1007/978-3-642-56348-5_12. [DOI] [PubMed] [Google Scholar]
- Remington SG, Meyer RA. Lens stem cells may reside outside the lens capsule: an hypothesis. Theor Biol Med Model. 2007;4:22. doi: 10.1186/1742-4682-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17:726–740. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA. Differential expression of alpha A-and alpha B-crystallin during murine ocular development. Invest Ophthalmol Vis Sci. 1996;37:2276–2284. [PubMed] [Google Scholar]
- Sue Menko A. Lens epithelial cell differentiation. Exp Eye Res. 2002;75:485–490. doi: 10.1006/exer.2002.2057. [DOI] [PubMed] [Google Scholar]
- Van Montfort R, Slingsby C, Vierling E. Structure and function of the small heat shock protein/alpha-crystallin family of molecular chaperones. Adv Protein Chem. 2001;59:105–156. doi: 10.1016/s0065-3233(01)59004-x. [DOI] [PubMed] [Google Scholar]
- Walker JL, Wolff IM, Zhang L, Menko AS. Activation of SRC kinases signals induction of posterior capsule opacification. Invest Ophthalmol Vis Sci. 2007;48:2214–2223. doi: 10.1167/iovs.06-1059. [DOI] [PubMed] [Google Scholar]
- Wang X, Garcia CM, Shui YB, Beebe DC. Expression and regulation of alpha-, beta-, and gamma-crystallins in mammalian lens epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:3608–3619. doi: 10.1167/iovs.04-0423. [DOI] [PubMed] [Google Scholar]
- Xi JH, Bai F, Andley UP. Reduced survival of lens epithelial cells in the alphaA-crystallin-knockout mouse. J Cell Sci. 2003;116:1073–1085. doi: 10.1242/jcs.00325. [DOI] [PubMed] [Google Scholar]
- Zhou M, Leiberman J, Xu J, Lavker RM. A hierarchy of proliferative cells exists in mouse lens epithelium: implications for lens maintenance. Invest Ophthalmol Vis Sci. 2006;47:2997–3003. doi: 10.1167/iovs.06-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]


