Abstract
CD4 lymphocyte count is an important surrogate marker of HIV disease progression, but it is often unavailable at the time of clinical events. We analysed data from the Cotrame cohort (1999–2004) and the Trivacan Structured Treatment Interruption trial (2002–2005) to estimate the incidence of opportunistic infections and death within specific CD4 strata in HIV-infected patients receiving highly active antiretroviral therapy (HAART) in sub-Saharan Africa. We used three methods of CD4 modelling: the first assumed that CD4 cell count remained constant until the next measurement; the second assumed that it changed immediately to the level of the subsequent measurement; and the third assumed that it followed a linear function between two consecutive CD4 measurements. The cohort used in this analysis consisted of 981 patients. The incidence rates of opportunistic infections were highest in the lower CD4 strata and decreased in the higher CD4 count strata. The incidence rates of mild opportunistic infections and severe bacterial infections, however, remained high in the highest CD4 stratum. Although all confidence intervals overlapped among the three methods, the incidence rate estimates showed differences of up to 74% in the lowest CD4 stratum. Different methods of estimating CD4 counts at the time of clinical events led to minor differences in incidence rates, except in the CD4 stratum <50 cells/mm3, where the follow-up time was shorter. All of the models indicate that the overall incidence of opportunistic infections under HAART in sub-Saharan Africa is high. This suggests that prophylaxis against opportunistic infections may be needed even for patients receiving HAART.
Keywords: CD4 cell count, Highly active antiretroviral therapy, Opportunistic infections, Sub-Saharan Africa, Time-dependent variable
Introduction
CD4 lymphocyte count is recognized as one of the most important predictors of HIV disease progression [1, 2]. It predicts opportunistic infections and death, and has been shown to be an important surrogate marker for determining the need for antiretroviral therapy as well as measuring the response to therapy [3]. As a result, it has been used to develop international guidelines for initiation of antiretroviral agents and opportunistic infection prophylaxis in both developed and developing countries [4].
Prophylaxis of opportunistic infections, particularly Pneumocystis jirovecii Pneumonia (PCP), as well as non-tuberculous mycobacterium (NTM), led to early improvements in the survival of HIV-infected patients in both the United States and Europe [5, 6]. Even in the era of effective antiretroviral therapy, these types of prophylaxis continue to be of use. In resource-limited settings, the incidence of opportunistic infections is higher than in the United States and Europe [7, 8]. The spectrum of common infections includes bacterial infections, malaria, fungal infections, and most importantly tuberculosis [7, 9]. In designing guidelines for the management of HIV-infected patients in these resource-limited countries, it is crucial to understand the incidence and impact of preventable opportunistic infections. It is also important to further clarify the impact of these infections on patients already receiving HAART. With detailed information about the incidence of opportunistic infections at each stage of illness, it will thus be possible to target patients at the highest risk for developing complications. However, CD4 cell counts are usually not available at the time of clinical events, especially in resource-limited settings where CD4 measurements are less likely to be performed.
Accordingly, our objective was to estimate the incidence of opportunistic infections and death by CD4 count in HIV-infected patients receiving effective antiretroviral therapy in Abidjan, Côte d’Ivoire and to examine the effect of three different methods for estimating CD4 cell count on these incidence rates.
Methods
Patients
Between 1996 and 2007, four ANRS (Agence Nationale de Recherches sur le Sida) funded studies enrolled HIV-infected patients in Côte d’Ivoire. From 1996 to 1998, the Cotrimo-CI-ANRS 059 trial assessed the efficacy of early cotrimoxazole chemoprophylaxis in adults [10] and the Ditrame-CI-ANRS 049 trial assessed the efficacy of a short course zidovudine regimen in reducing mother-to-child HIV transmission [11]. After both trials ended, a unique long-term cohort study, the Cotrame-ANRS 1203 cohort study, followed up on 723 participants through 2004 [7]. Starting in early 1999, Cotrame cohort participants were started on HAART once they reached the national criteria of inclusion in the Côte d’Ivoire/UNAIDS Drug Access Initiative [12]. Between 2002 and 2007, the Trivacan-ANRS 1269 trial assessed the benefits and risks of two structured treatment interruption strategies in adults in Côte d’Ivoire [13]. This trial comprised a pre-randomisation phase during which 840 untreated adults started HAART and were followed 6 to 18 months on continuous antiretroviral treatment before being randomised into the trial. In the Cotrame cohort and in the Trivacan trial pre-randomization phase, patients on HAART were prescribed cotrimoxazole and were followed using standardized procedures which have been described elsewhere [10, 13]. Our analysis includes all patients from the Cotrame cohort who were on HAART from April 28, 1999 to May 28, 2004, and all patients from the pre-randomization phase of Trivacan trial who started HAART in December 26, 2002 and continued treatment either up to the date of randomization, for randomized patients, or up to April 1st, 2005, for the rest.
Clinical events
Clinical events were characterized by the trial team using standardized definitions and were reviewed by an event documentation committee [7, 9]. Incident opportunistic infections were grouped together according to the causal microorganism (Appendix). Opportunistic infections were divided into severe or mild events. Severe opportunistic infections were defined as any WHO clinical stage 3 or 4 defining event or any other event requiring hospital admission or leading to death, including severe fungal infections, severe bacterial infections, severe malaria, tuberculosis, isospora, toxoplasmosis and Mycobacterium avium complex (MAC) bacteremia. Mild opportunistic infections were defined as all WHO clinical stage 2 defining events, mild fungal infections, mild bacterial infections and mild malaria which did not lead to hospital admission or death.
Statistical analysis
We estimated the overall incidence rates of death, any mild and severe opportunistic infections, bacterial, malaria and tuberculosis, as well as those incidence rates within specific CD4 cell count strata: <50 cells/mm3, 50–200 cells/mm3, 201–350 cells/mm3, and >350 cells/mm3. Only the first event of a specified opportunistic infection for each individual was analysed during the study period. The incidence rate of a given event within a specific CD4 stratum was defined as the number of patients having the first episode of that event in the given CD4 stratum divided by the total follow-up time at risk within the same CD4 cell count stratum, expressed per 100 person-years. Confidence intervals (95% CI) around incidence rates were calculated assuming a Poisson distribution. Median values related to patients’ characteristics were reported with the interquartile range (IQR). Analyses were done with SAS software version 8.02 (SAS Institute Inc., Cary, North Carolina, USA).
Estimating CD4 cell counts over time
Because CD4 cell counts were generally measured at pre-specified intervals, and not at the time of opportunistic infections, we had to estimate the value of CD4 cell counts at the time of an event. Three alternative methods were used (Fig. 1). The first assumed that CD4 cell count remained constant until the next measurement; the second assumed that CD4 cell count changed immediately to the level of the next measurement; and the third assumed linear interpolation between two consecutive CD4 measurements. The last CD4 measurement in follow-up was carried forward for all three methods. Figure 1 illustrates the case where an opportunistic event occurs at a follow-up time of 10 months. The estimated CD4 cell count was 220 cells/mm3 using the first method, 40 cells/mm3 using the second method, and 100 cells/mm3 using the third method.
Fig. 1.
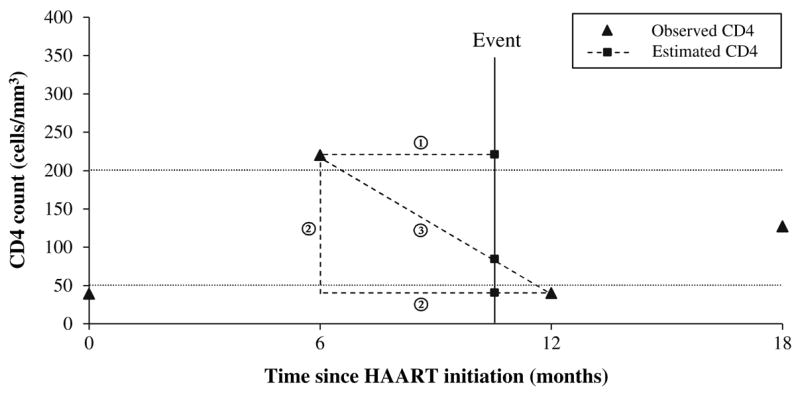
Illustration of the three different methods of modelling CD4 cell counts. In this example, we observed four CD4 cell counts: 39 cells/mm3 at time 0, 220 cells/mm3 at 6 months, 40 cells/mm3 at 12 months and 127 cells/mm3 at 18 months (solid triangles). An opportunistic infection which occurs at 10 months would have an estimated CD4 cell count (solid squares) of 220 cells/mm3 using the Constant Until Next Measurement method (①), 40 cells/mm3 using the Changing Value Immediately method (②), and 100 cells/mm3 using the Linear Interpolation method (③)
Results
One hundred forty one patients on HAART from the Cotrame cohort and 840 patients on HAART from the pre-randomization phase of the Trivacan trial were enrolled (Table 1). Patients were more likely to be women than men in both studies (62% and 76%, respectively; P < 0.001), and the median age at HAART initiation was 34 years (IQR 29–41). The median follow-up time on HAART was higher in Cotrame (32.8 vs. 8.0 months; P < 0.001), but not in cumulative follow-up time (366 versus 781 person-years). At study termination, 38 patients had died: the median time from HAART initiation until death was 6.8 months (IQR 2.3–13.0).
Table 1.
Characteristics of the patients starting HAART in Abidjan, Côte d’Ivoire, from April, 1999 through May, 2004 (Cotrame) and from December, 2003 through April, 2005 (Trivacan)
| Cotrame | Trivacan | Cotrame + Trivacan | |
|---|---|---|---|
| Baseline characteristics | |||
| Number in cohort | 141 | 840 | 981 |
| Women, number (%)* | 88 (62%) | 639 (76%) | 727 (74%) |
| Age, median (IQR), in years | 35 (29–41) | 34 (29–40) | 34 (29–41) |
| CD4 cell count/mm3, median (IQR)** | 131 (59–219) | 252 (188–322) | 236 (173–316) |
| Follow-up characteristics | |||
| Time of follow-up with HAART, median (IQR), in months* | 32.8 (21.2–40.9) | 8.0 (7.0–13.6) | 10.0 (7.0–17.1) |
| Delay between HAART initiation and death, median (IQR), in months | 7.7 (3.1–15.2) | 5.9 (2.1–11.9) | 6.8 (2.3–13.0) |
| Incidence rate of first eventa | |||
| Death, N = 38 (18 in Cotrame and 20 in Trivacan) | 5 (3–8) | 3 (2–4) | 3 (2–5) |
| Severe opportunistic infections, N = 324 (76 and 248) | 38 (30–47) | 41 (36–46) | 40 (36–44) |
| Mild opportunistic infections, N = 376 (100 and 276) | 70 (57–85) | 47 (41–52) | 51 (46–57) |
HAART, highly active antiretroviral therapy; IQR, interquartile range
P < 0.001,
P < 0.0001
Incidence rate per 100 person-years
The crude mortality rate was 3 (95% CI 2–5) per 100 person-years. The incidence rate of mild opportunistic infections was higher in Cotrame, 70 (95% CI 57–85) versus 47 (95% CI 41–52) per 100 person-years, and the incidence rate of severe opportunistic infections was similar in the two cohorts, 38 (95% CI 30–47) versus 41 (95% CI 36–46) per 100 person-years. The most frequent mild opportunistic infections were mild fungal and mild bacterial infections, and the most frequent severe opportunistic infections were severe bacterial infections (data not shown). All patients had a CD4 cell count measured before or at the time of HAART initiation; the median was higher in Trivacan (252 versus 131 cells/mm3; P < 0.001). One hundred twenty one patients (96%) in Cotrame and 824 (90%) in Trivacan had at least one CD4 cell count during follow-up after HAART initiation. The median number of CD4 cell counts per patient was 8 (IQR 4–14; range 1–23) in Cotrame and 3 (IQR 2–4; range 1–9) in Trivacan. The median time between the first episode of a specified event and the nearest CD4 measurement was 0.9 months (IQR 0.2–1.2, range 0–13.0). The median time between the last CD4 measurement and the end of the follow-up period was 1.8 months (IQR 0.7–4.0; range 0.0–17.8) in Cotrame and 1.0 month (IQR 1.0–1.1; range 0.4–2.5) in Trivacan.
We then estimated the mortality and incidence of the first episode of opportunistic infections within specific CD4 strata, according to the three different modelling methods. We first estimated the incidence of any severe opportunistic infection, as well as the incidence of the following specific severe opportunistic infections: tuberculosis, severe bacterial infections, severe fungal infections, and severe malaria (Table 2). We also estimated the incidence of any mild opportunistic infection, as well as the incidence of the following specific mild opportunistic infections: mild bacterial infections, mild fungal infections, and mild malaria (Table 3). Although all confidence intervals overlapped, results showed differences of up to 74% in incidence rates in the CD4 stratum <50 cells/mm3.
Table 2.
Incidence of death and severe opportunistic infections within specific CD4 strata according to the method of modelling
| CD4 stratum (cells/mm3) | Constant until next measurement
|
Changing value immediately
|
Linear interpolation
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Follow-up (years) | Number of events | IRa (95% CI) | Follow-up (years) | Number of events | IR (95% CI) | Follow-up (years) | Number of events | IR (95% CI) | |
| Death | |||||||||
| <50 | 15.24 | 7 | 46 (18–95) | 14.89 | 7 | 47 (19–97) | 13.16 | 7 | 53 (21–110) |
| 50–200 | 241.76 | 19 | 8 (5–12) | 187.38 | 19 | 10 (6–16) | 195.25 | 19 | 10 (6–15) |
| 201–350 | 496.44 | 11 | 2 (1–4) | 413.09 | 11 | 3 (1–5) | 471.81 | 11 | 2 (1–4) |
| >350 | 393.12 | 1 | 0 (0–1) | 531.20 | 1 | 0 (0–1) | 466.34 | 1 | 0 (0–1) |
| Any severe opportunistic infection | |||||||||
| <50 | 6.15 | 8 | 130 (56–256) | 4.01 | 10 | 249 (120–459) | 3.81 | 8 | 210 (91–414) |
| 50–200 | 170.06 | 79 | 46 (37–58) | 120.32 | 55 | 46 (34–59) | 127.33 | 60 | 47 (36–61) |
| 201–350 | 369.25 | 142 | 38 (32–45) | 306.19 | 111 | 36 (30–44) | 352.74 | 136 | 39 (32–46) |
| >350 | 268.24 | 95 | 35 (29–43) | 383.16 | 148 | 39 (33–45) | 329.81 | 120 | 36 (30–44) |
| Tuberculosis | |||||||||
| <50 | 13.07 | 1 | 8 (0–43) | 11.40 | 1 | 9 (0–49) | 10.38 | 1 | 10 (0–54) |
| 50–200 | 221.75 | 18 | 8 (5–13) | 169.02 | 20 | 12 (7–18) | 175.88 | 17 | 10 (6–15) |
| 201–350 | 483.04 | 11 | 2 (1–4) | 400.43 | 11 | 3 (1–5) | 458.72 | 13 | 3 (1–5) |
| >350 | 383.72 | 8 | 2 (1–4) | 520.74 | 6 | 1 (0–3) | 456.60 | 7 | 2 (1–3) |
| Severe fungal | |||||||||
| <50 | 14.91 | 0 | 0 (0–25) | 14.54 | 1 | 7 (0–38) | 12.88 | 0 | 0 (0–29) |
| 50–200 | 236.69 | 3 | 1 (0–4) | 182.80 | 2 | 1 (0–4) | 190.19 | 3 | 2 (0–5) |
| 201–350 | 492.75 | 0 | 0 (0–1) | 408.97 | 1 | 0 (0–1) | 467.99 | 1 | 0 (0–1) |
| >350 | 392.52 | 1 | 0 (0–1) | 530.55 | 0 | 0 (0–1) | 465.83 | 0 | 0 (0–1) |
| Severe bacterial | |||||||||
| <50 | 9.99 | 2 | 20 (2–72) | 7.77 | 6 | 77 (28–168) | 6.78 | 3 | 44 (9–129) |
| 50–200 | 208.78 | 24 | 11 (7–17) | 155.25 | 17 | 11 (6–18) | 162.86 | 21 | 13 (8–20) |
| 201–350 | 462.10 | 25 | 5 (3–8) | 381.34 | 22 | 6 (4–9) | 437.91 | 24 | 5 (3–8) |
| >350 | 355.68 | 21 | 6 (4–9) | 492.19 | 27 | 5 (4–8) | 429.00 | 24 | 6 (3–8) |
| Severe malaria | |||||||||
| <50 | 13.30 | 1 | 8 (0–42) | 12.52 | 2 | 16 (2–58) | 11.56 | 2 | 17 (2–62) |
| 50–200 | 232.45 | 9 | 4 (2–7) | 178.71 | 10 | 6 (3–10) | 185.55 | 8 | 4 (1–8) |
| 201–350 | 480.93 | 12 | 2 (1–4) | 398.61 | 9 | 2 (1–4) | 457.17 | 11 | 2 (1–4) |
| >350 | 384.55 | 11 | 3 (1–5) | 521.39 | 12 | 2 (1–4) | 456.96 | 12 | 3 (1–5) |
IR: Incidence rate per 100 person-years (see methods for details)
Table 3.
Incidence of mild opportunistic infections within specific CD4 strata according to the method of modelling
| CD4 stratum (cells/mm3) | Constant until next measurement
|
Changing value immediately
|
Linear interpolation
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Follow-up (years) | Number of events | IRa (95% CI) | Follow-up (years) | Number of events | IR (95% CI) | Follow-up (years) | Number of events | IR (95% CI) | |
| Any mild opportunistic infection | |||||||||
| <50 | 5.42 | 13 | 240 (128–410) | 4.26 | 7 | 164 (66–338) | 3.72 | 9 | 242 (111–459) |
| 50–200 | 144.91 | 96 | 66 (54–81) | 96.94 | 61 | 63 (48–81) | 107.07 | 77 | 72 (57–90) |
| 201–350 | 333.28 | 158 | 47 (44–55) | 266.31 | 133 | 50 (42–59) | 311.56 | 148 | 48 (40–56) |
| >350 | 250.86 | 109 | 43 (36–52) | 366.96 | 175 | 48 (41–55) | 312.14 | 142 | 45 (38–54) |
| Mild fungal | |||||||||
| <50 | 7.69 | 5 | 65 (21–152) | 6.06 | 6 | 99 (37–216) | 5.12 | 6 | 117 (43–255) |
| 50–200 | 185.62 | 64 | 34 (27–44) | 135.88 | 36 | 26 (19–37) | 145.54 | 46 | 32 (23–42) |
| 201–350 | 408.13 | 76 | 19 (15–23) | 333.08 | 67 | 20 (16–26) | 382.80 | 80 | 21 (17–26) |
| >350 | 309.34 | 52 | 17 (13–22) | 435.77 | 88 | 20 (16–25) | 377.33 | 65 | 17 (13–22) |
| Mild bacterial | |||||||||
| <50 | 11.00 | 3 | 27 (6–80) | 8.83 | 2 | 23 (3–82) | 8.68 | 2 | 23 (3–83) |
| 50–200 | 210.96 | 21 | 10 (6–15) | 157.82 | 19 | 12 (7–19) | 164.30 | 18 | 11 (6–17) |
| 201–350 | 457.59 | 35 | 8 (5–11) | 379.22 | 27 | 7 (5–10) | 435.13 | 35 | 8 (6–11) |
| >350 | 349.70 | 29 | 8 (6–12) | 483.39 | 40 | 8 (6–11) | 421.14 | 33 | 8 (5–11) |
| Mild malaria | |||||||||
| <50 | 14.81 | 0 | 0 (0–25) | 14.40 | 0 | 0 (0–26) | 12.67 | 0 | 0 (0–29) |
| 50–200 | 234.62 | 9 | 4 (2–7) | 179.87 | 8 | 4 (2–9) | 188.19 | 10 | 5 (3–10) |
| 201–350 | 480.34 | 19 | 4 (2–6) | 399.04 | 15 | 4 (2–6) | 456.75 | 13 | 3 (1–5) |
| >350 | 377.04 | 18 | 5 (3–8) | 513.49 | 23 | 4 (3–7) | 449.21 | 23 | 5 (3–8) |
IR: Incidence rate per 100 person-years (see methods for details)
For example, in the CD4 stratum <50 cells/mm3, the incidence rate of severe bacterial infections was estimated at 20 (95% CI 2–72) per 100 person-years using the Constant Until Next Measurement method, 77 (95% CI 28–168) per 100 person-years using the Changing Value Immediately method and 44 (95% CI 9–129) per 100 person-years using the Linear Interpolation method. However, combining the two lower CD4 strata resulted in similar incidence rates of severe bacterial infections among the three methods: 12 (95% CI 8–17), 14 (95% CI 9–21) and 14 (95% CI 9–21) per 100 person-years. As expected, the highest incidence rates occurred in the lowest CD4 stratum. All incidence rates decreased in the higher CD4 strata, except for mild malaria for which incidence rates were identical across CD4 strata. The incidence rates of mild opportunistic infection and severe opportunistic infections were still high in the CD4 stratum >350 cells/mm3, with incidence rates for the three methods of 43 (95% CI 36–52), 48 (95%CI 41–55) and 45 (95% CI 38–54) per 100 person-years for any mild opportunistic infection, and 35 (95% CI 29–43), 39 (95% CI 33–45) and 36 (95% CI 30–44) for severe opportunistic infections.
Discussion
In this analysis we estimated the incidence rates of opportunistic infections and death within specific CD4 strata in patients receiving HAART in sub-Saharan Africa. In these countries, CD4 measurements are relatively scarce because of a lack of laboratory facilities. Therefore, modelling CD4 cell counts between two consecutive measurements may be subject to a higher degree of variability than in regions of the world where CD4 tests are more common. Three different methods of modelling CD4 cell count between two consecutive measurements were examined. Based on the three methods used in this study, as in other studies in both sub-Saharan Africa [14, 15] and elsewhere [16, 17], the highest incidence rates occurred in the lowest CD4 cell count stratum. The incidence rates of opportunistic infections and death were lower in the higher CD4 cell count strata, except for mild malaria. However, the overall incidence of mild opportunistic infections and severe bacterial infections remained high, even in patients with CD4 counts >350 cells/mm3, as previously reported in sub-Saharan Africa [7, 18]. Moreover, our estimates of tuberculosis incidence rates are comparable to those reported by Badri et al. in South African patients receiving HAART [19].
Modelling of CD4 cell count differs depending on whether patients are untreated or treated with HAART. For untreated patients, complicated statistical methods have been used to estimate CD4 cell count at a given time, including fixed effects, random effects or mixed effects models [17, 20, 21]. These models can be used to assess risk factors for CD4 count decrease and then applied for prediction at the individual level. While patients are receiving HAART, the CD4 trajectory cannot be easily extrapolated, because patients may be either succeeding or failing therapy and CD4 cell counts are subject to major fluctuations. Therefore, we used three straightforward methods of modelling to derive opportunistic infection incidence rates within defined CD4 strata. The Constant Until Next Measurement method assumed that the CD4 cell count remained constant until a new CD4 cell count was observed. This method does not capture any increase in CD4 cells due to initiation of HAART, and thus underestimates the true CD4 cell count in the first interval for patients on HAART. By contrast, the Changing Value Immediately method captures the CD4 increase in the first interval compared with baseline but probably overestimates CD4 cell count in the first interval. Finally, the Linear Interpolation method also captures the initial rise and takes it into account in a progressive and linear way. We found that these methods may give different CD4 count estimates at the time of an event for individual patients, but provide similar estimates of opportunistic infection incidence rates at the population level, except in the lower CD4 stratum. Similar results were obtained after stratification by age and gender. However, we cannot conclude that one method is better than another and more detailed simulation studies are warranted to examine factors that alter the performance of these methods.
There are several limitations to this analysis. First, the analysis is based on data from a cohort of closely monitored HIV patients, where a higher number of CD4 cell count measurements were available compared to routine care. The results may not be generalizable to other cohorts in which CD4 counts are measured less frequently. For example, WHO guidelines recommend CD4 measurement every 6 months, unlike in this study, in which CD4 counts were measured on average every 3 months. Second, the duration of follow-up differed between the Cotrame cohort and the pre-randomization phase of Trivacan cohorts. However, we pooled data from these cohorts after stratifying follow-ups by CD4 cell count. Moreover, we only evaluated the incidence of primary opportunistic infections, not recurrences. Thus, we pooled follow-up periods during which the risk of opportunistic infection occurrence was quite homogenous. Third, the analysis included few subjects with CD4 cell counts <50 cells/mm3 and more important with short follow-up periods given that these patients were receiving HAART. This may explain some of the differences among the three methods and the very large 95% CI around the estimates of incidence rates in this CD4 stratum. Therefore, we cannot determine whether or not our estimates of incidence of opportunistic infections for subjects with CD4 cell counts <50 cells/mm3 obtained by the three different methods of CD4 cell count modelling are different. However, it is important to underline that when the two lower CD4 strata are combined, no differences are observed among the three methods.
In summary, we examined the incidence rates of opportunistic infections and death within specific CD4 strata in patients receiving HAART in sub-Saharan Africa. We used three methods to derive the opportunistic infection rates and to determine whether they led to different estimates. These methods may give different CD4 count estimates at the time of an individual opportunistic infection, but overall, they provide similar estimates of opportunistic infection incidence rates, except when applied in the CD4 strata <50 cells/mm3, where the follow-up time was short. The analyses demonstrated that in sub-Saharan Africa, the overall incidence of opportunistic infections, especially mild opportunistic infections, remains high in patients with high CD4 counts, even in those receiving HAART. These results suggest that in this setting, opportunistic infection prophylaxis may be needed even in patients with high CD4 counts receiving HAART. Moreover, they show the importance of implementing preventive measures other than prophylaxis in order to decrease the incidence of opportunistic infections such as malaria.
Acknowledgments
This study was supported by grants from the French Agence Nationale de Recherches sur le SIDA et les hépatites virales (ANRS 1286), the U.S. National Institute of Allergy and Infectious Diseases (NIAID AI058736, K23 AI0794, K24 AI062476, K25 AI50436 and CFAR P30 AI42851), and the U.S. Centers for Disease Control and Prevention (Cooperative Agreement U64/CCU 119525). We thank Lindsey L. Wolf and Caroline Sloan for administrative assistance.
Abbreviations
- ANRS
Agence Nationale de Recherches sur le SIDA et les hépatites virales
- CI
confidence intervals
- HAART
highly active antiretroviral therapy
- HIV
human immunodeficiency virus
- IQR
interquartile range
- NTM
non-tuberculous mycobacterium, PCP, pneumocystis jirovecii pneumonia
Appendix – Groups of clinical events
Severe opportunistic infections
Severe fungal infections
Cryptococcus
Esophageal candidiasis
Histoplasmosis
Severe bacterial infections
Pneumonia
Bacterial enteritis
Isolated bacteremia
Invasive urogenital infection
Other WHO clinical stage 4 defining bacterial events
Other WHO clinical stage 3 defining bacterial events
Severe malaria
Malaria requiring hospital admission or leading to death
Tuberculosis
Isospora
Toxoplasmosis
Mycobacterium avium complex (MAC) bacteremia
Other severe illnesses
Other WHO clinical stage 4 defining events
Other WHO clinical stage 3 defining events
Other events requiring hospital admission
Other events leading to death
Mild opportunistic infections
Mild fungal infections
Oral candidiasis
Vaginal candidiasis
Angular stomatitis
Onychomycosis
Dermatophytosis
Mild bacterial infections
Other WHO clinical stage 2 defining bacterial events
Unknown WHO clinical stage defining bacterial events
Mild malaria
Malaria not requiring hospital admission and not leading to death
Other mild illnesses
Other WHO clinical stage 2 defining events which do not lead to hospital admission or death
References
- 1.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien WA, Hartigan PM, Daar ES, Simberkoff MS, Hamilton JD. Changes in plasma HIV RNA levels and CD4+ lymphocyte counts predict both response to antiretroviral therapy and therapeutic failure. VA Cooperative Study Group on AIDS. Ann Intern Med. 1997;126:939–45. doi: 10.7326/0003-4819-126-12-199706150-00002. [DOI] [PubMed] [Google Scholar]
- 3.Gebo KA, Gallant JE, Keruly JC, Moore RD. Absolute CD4 vs. CD4 percentage for predicting the risk of opportunistic illness in HIV infection. J Acquir Immune Defic Syndr. 2004;36:1028–33. doi: 10.1097/00126334-200408150-00005. [DOI] [PubMed] [Google Scholar]
- 4.Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Recommendations of the Panel on Clinical Practices for Treatment of HIV. MMWR Recomm Rep. 2002;51:1–55. [PubMed] [Google Scholar]
- 5.2001 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. HIV Clin Trials. 2001;2:493–554. doi: 10.1310/AQML-UABK-5LLB-E615. [DOI] [PubMed] [Google Scholar]
- 6.1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Clin Infect Dis. 2000;30(Suppl 1):S29–65. doi: 10.1086/313848. [DOI] [PubMed] [Google Scholar]
- 7.Anglaret X, Messou E, Ouassa T, et al. Pattern of bacterial diseases in a cohort of HIV-1 infected adults receiving cotrimoxazole prophylaxis in Abidjan, Cote d’Ivoire. AIDS. 2003;17:575–84. doi: 10.1097/00002030-200303070-00013. [DOI] [PubMed] [Google Scholar]
- 8.Badri M, Bekker LG, Orrell C, Pitt J, Cilliers F, Wood R. Initiating highly active antiretroviral therapy in sub-Saharan Africa: an assessment of the revised World Health Organization scaling-up guidelines. AIDS. 2004;18:1159–68. doi: 10.1097/00002030-200405210-00009. [DOI] [PubMed] [Google Scholar]
- 9.Attia A, Huët C, Anglaret X, et al. HIV-1-related morbidity in adults, Abidjan, Côte d’Ivoire: A nidus for bacterial diseases. J Acquir Immune Defic Syndr. 2001;28:478–86. doi: 10.1097/00042560-200112150-00012. [DOI] [PubMed] [Google Scholar]
- 10.Anglaret X, Che ^ne G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d’Ivoire: a randomised trial. Lancet. 1999;353:1463–8. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 11.Dabis F, Msellati P, Meda N, et al. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Côte d’Ivoire and Burkina Faso: a double blind placebo-controlled multicentre trial. Lancet. 1999;353:786–92. doi: 10.1016/s0140-6736(98)11046-2. [DOI] [PubMed] [Google Scholar]
- 12.Seyler C, Anglaret X, Dakoury-Dogbo N, et al. Medium-term survival, morbidity and immunovirological evolution in HIV-infected adults receiving antiretroviral therapy, Abidjan, Cote d’Ivoire. Antivir Ther. 2003;8:385–93. [PubMed] [Google Scholar]
- 13.Danel C, Moh R, Minga A, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367:1981–9. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 14.Losina E, Anglaret X, Yazdanpanah Y, et al. Impact of opportunistic diseases on chronic mortality in HIV-infected adults in Cote d’Ivoire. S Afr Med J. 2006;96:526–9. [PubMed] [Google Scholar]
- 15.Mermin J, Lule J, Ekwaru JP, et al. Effect of cotrimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–34. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 16.Miller V, Sabin CA, Phillips AN, et al. The impact of protease inhibitor-containing highly active antiretroviral therapy on progression of HIV disease and its relationship to CD4 and viral load. AIDS. 2000;14:2129–36. doi: 10.1097/00002030-200009290-00009. [DOI] [PubMed] [Google Scholar]
- 17.Yazdanpanah Y, Che ^ne G, Losina E, et al. Incidence of primary opportunistic infections in two human immunodeficiency virus-infected French clinical cohorts. Int J Epidemiol. 2001;30:864–71. doi: 10.1093/ije/30.4.864. [DOI] [PubMed] [Google Scholar]
- 18.Anglaret X, Dakoury-Dogbo N, Bonard D, et al. Causes and empirical treatment of fever in HIV-infected adult outpatients, Abidjan, Cote d’Ivoire. AIDS. 2002;16:909–18. doi: 10.1097/00002030-200204120-00011. [DOI] [PubMed] [Google Scholar]
- 19.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–64. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 20.Seage GR, 3rd, Losina E, Goldie SJ, Paltiel AD, Kimmel AD, Freedberg KA. The relationship of preventable opportunistic infections, HIV-1 RNA, and CD4 Cell counts to chronic mortality. J Acquir Immune Defic Syndr. 2002;30:421–8. doi: 10.1097/00042560-200208010-00008. [DOI] [PubMed] [Google Scholar]
- 21.Yazdanpanah Y, Losina E, Anglaret X, et al. Clinical impact and cost-effectiveness of cotrimoxazole prophylaxis in patients living with HIV/AIDS in Côte d’Ivoire: a trial-based analysis. AIDS. 2005;19:1299–308. doi: 10.1097/01.aids.0000180101.80888.c6. [DOI] [PubMed] [Google Scholar]


