Abstract
Background
In recent studies, subjects who had achieved suppression of the human immunodeficiency virus (HIV) RNA level while receiving an initial 3-drug antiretroviral regimen successfully maintained suppression while receiving treatment with a “boosted” protease inhibitor (PI) alone. We projected the long-term outcomes of this treatment simplification strategy to inform the design of a proposed multicenter, randomized clinical trial.
Methods
We used published studies to estimate the efficacy, adverse effects, and cost of a sequence of HIV drug regimens for the simplification strategy, compared with those outcomes for the current standard-of-care (SOC) strategy. Using a published simulation model of HIV disease, we projected life expectancy, discounted quality-adjusted life expectancy (QALE), and discounted lifetime medical costs for each strategy.
Results
Subjects who have not developed PI-resistant HIV infection at the time of failure of the simplification regimen have a greater life expectancy (27.9 vs. 27.1 years) and QALE (14.9 vs. 14.7 years), compared with SOC subjects, because they receive an additional line of therapy without negative consequences for future treatment options. The QALE for the simplification strategy remains higher than that for the SOC, unless a large proportion of patients experiencing virologic failure while receiving the simplification regimen develop PI resistance. Depending on the probability of simplification regimen failure, the advantage is maintained even if HIV develops PI resistance in 42%–70% of subjects. Projected lifetime costs are $26,500–$72,400 per person lower for the simplification strategy than for the SOC strategy.
Conclusions
An HIV treatment simplification strategy involving use of a boosted PI alone may lead to longer survival overall at lower cost, compared with the SOC combination therapy, because the simplification strategy potentially adds an additional line of therapy. The risk of emergence of PI resistance during treatment with a simplified regimen is a critical determinant of the viability of this strategy.
The challenge currently facing HIV researchers and clinicians in developed countries is to determine the optimal use of available therapies, in order to further increase the duration of survival and minimize adverse effects at an acceptable cost. Causes of virologic failure, which are not mutually exclusive, include insufficient adherence to treatment, drug toxicity, and viral resistance [1]. Several clinical studies have examined a novel treatment strategy that involves use of a ritonavir-“boosted” protease inhibitor (PI) alone [2–11]. The proposed rationale behind this strategy has been to reduce nucleoside reverse-transcriptase inhibitor (NRTI)–related toxicities and costs.
Boosted PI regimen simplification has been tested both as initial therapy [5, 6] and as part of an “induction maintenance” strategy, in which patients with virologic suppression who are receiving combination therapy switch regimens to a boosted PI alone [2–4, 7–12]. In the latter type of study, patients who experienced virologic rebound while receiving a boosted-PI alone have been able to reattain virologic suppression by resuming treatment with NRTIs, generally without the development of PI resistance [2–4, 7–12]. However, the full benefits of the strategy can only be evaluated by taking into account long-term outcomes on subsequent regimens that would not be observed during a clinical trial.
AIDS Clinical Trials Group protocol A5237 is a proposed randomized, comparative study of continued use of 3 drugs versus simplification of the regimen to atazanavir-ritonavir alone. Our objective was to simulate the impact of this treatment simplification strategy on long-term outcomes for patients who have successfully responded to their initial antiretroviral regimen. From these results, we draw conclusions about the expected benefits of the strategy from a population perspective, and we identify implications for trial design.
METHODS
Analysis overview
We used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model (Appendix A; online only) [13], a simulation state-transition model of HIV disease, to project life expectancy, quality-adjusted life expectancy (QALE), and direct medical costs for the simplification strategy, compared with those for the current standard of care. As inputs to the model, we used published studies on the efficacy, adverse effects, and cost of a sequence of HIV drug regimens appropriate for previously treatment-naive subjects who have achieved suppression of the HIV RNA level while receiving treatment based on (1) a continued standard-of-care strategy or (2) a simplification strategy of atazanavir-ritonavir alone. Because currently available data do not indicate the proportion of subjects undergoing the simplification strategy who have virus that will develop atazanavir resistance, we also calculated the “break-even” proportion of subjects with genotypic or phenotypic resistant HIV (referred to as “PI resistance”) under the simplification strategy that would result in a QALE equivalent to that for the standard of care.
Results are reported as undiscounted life expectancies, QALEs discounted to present value at an annual rate of 3%, undiscounted annual costs, and lifetime direct medical costs discounted at an annual rate of 3% [14]. All costs are reported in 2005 US dollars.
Target population
The mean age (±SD) at the time of presentation for care was 39 ± 10 years, and 75% of subjects were male [15]. The distribution of HIV RNA levels was as follows: 26% of subjects had a level > 30,000 copies/mL, 25% of subjects had a level of 10,001–30,000 copies/mL, 25% of subjects had a level of 3001–10,000 copies/mL, 16% of subjects had a level of 501–3000 copies/mL, and 8% of subjects had a level ≤500 copies/mL [16]. We used published clinical trial data to derive the CD4 cell count distribution (mean CD4 cell count ±SD, 525 ±278 cells/μL), which is varied in sensitivity analyses [17].
Antiretroviral therapy sequencing and efficacy
We used published clinical trial data to estimate the virologic efficacy of each line of antiretroviral therapy in the standard-of-care and simplification strategies (table 1) [10, 17–21]. All subjects were assumed to have maintained suppression of the HIV RNA level for 96 weeks while receiving an antiretroviral regimen consisting of 2 NRTIs and a nonnucleoside reverse-transcriptase inhibitor (NNRTI) before the start of this analysis [17]. In the standard-of-care strategy, subjects receive 5 sequential regimens of combination antiretroviral therapy: the initial NNRTI-based regimen; 2 ritonavir-boosted, PI-based regimens; 1 regimen containing ritonavir-boosted darunavir, with or without enfuvirtide; and a final, minimally effective salvage regimen that does not include enfuvirtide. Regimens are sequentially less effective, reflecting the poorer outcomes reported in treatment-experienced patients [22]. Because long-term follow-up data are limited, particularly for ritonavir-boosted PI regimens [23], we conservatively assumed that subjects could continue to receive any regimen and experience suppression of the HIV RNA level for a maximum of 10 years.
Table 1.
Efficacy and cost of antiretroviral regimens.
| Line of therapy | NNRTIs, PIs, and other agents | NRTIs and other agents | Patients with an HIV RNA level < 400 copies/mL,% (weeks after treatment initiation) | CD4 cell count increase, cells/μL (weeks after treatment initiation) | Cost per month,2005 US$ | Reference |
|---|---|---|---|---|---|---|
| Standard-of-care strategy | ||||||
| Continue initial regimena | Efavirenz | Tenofovir-emtricitabine | 93 (48)b | 47 (48)c | 1120 | [17] |
| Second line | Atazanavir-ritonavir | Zidovudine-lamivudine, abacavir | 70 (48)d | 110 (48) | 2050 | [19] |
| Third line | Lopinavir-ritonavir | Tenofovir, lamivudine, stavudine | 58 (48)e | 121 (48) | 1650 | [19] |
| Fourth line | Darunavir-ritonavir | OBR (includes didanosine), with or without enfuvirtide | 59 (24); 37 (48) | 75 (24) | 2770f | [21] |
| Fifth line | OBR | OBR | 12 (48) | 45 (48) | 1960 | [18] |
| Simplification strategy with PI resistance | ||||||
| Simplification regimena | Atazanavir-ritonavir | None | 91 (24); 83 (48) | 16 (24) | 1020 | [10] |
| Second line | Efavirenz | Tenofovir-emtricitabine | 93 (48) | 47 (48) | 1120 | [17] |
| Third line | Lopinavir-ritonavir | Zidovudine-lamivudine, abacavir | 58 (48) | 121 (48) | 1640 | [19] |
| Fourth line | Darunavir-ritonavir | OBR (includes stavudine), with or without enfuvirtide | 59 (24); 37 (48) | 75 (24) | 2840 | [21] |
| Fifth line | OBR | OBR | 12 (48) | 45 (48) | 1960 | [18] |
| Simplification strategy with no PI resistance | ||||||
| Simplification regimena | Atazanavir-ritonavir | None | 91 (24); 83 (48) | 16 (24) | 1020 | [10] |
| Second line | Atazanavir-ritonavir | Tenofovir-emtricitabine | 70 (48) | 110 (48) | 1720 | [19] |
| Third line | Efavirenz | Zidovudine-lamivudine, abacavir | 60 (48)g | 94 (48) | 1450 | [20] |
| Fourth line | Lopinavir-ritonavir | Tenofovir, lamivudine, stavudine | 58 (48) | 121 (48) | 1650 | [19] |
| Fifth line | Darunavir-ritonavir | OBR (includes didanosine), with or without enfuvirtide | 59 (24); 37 (48) | 75 (24) | 2770 | [21] |
| Sixth line | OBR | OBR | 12 (48) | 45 (48) | 1960 | [18] |
| Hypothetical integrase inhibitor regimen (used in certain sensitivity analyses)h | Integrase inhibitor | OBR | 77 (16); 51 (48) | 86 (16) | 1960 | [36] |
NOTE. Subjects could maintain suppression of the HIV RNA level for a maximum of 10 years while receiving any individual regimen. NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; OBR, optimized background therapy; PI, protease inhibitor.
All subjects were assumed to have maintained suppression of the HIV RNA level for 96 weeks while receiving an antiretroviral regimen consisting of 2 NRTIs and 1 NNRTI before the beginning of this analysis.
This suppression rate was derived from the tenofovir DF arm of the Gilead 903 study; this rate is equal to the probability of having suppression at week 144 in the study given suppression at week 96 [17].
This CD4 cell count increase is equal to one-half of the difference between the CD4 cell count increase at week 48 and that at week 144 [17] in the Gilead 903 study.
This is the suppression rate for subjects with < 4 PI mutations in the BMS 045 study [19].
This is the suppression rate for all subjects (with and without mutations) in the BMS 045 study [19].
Forty-seven percent of subjects were assumed to be receiving enfuvirtide [21].
Sixty percent of subjects had an HIV RNA level < 500 copies/mL [20].
Administered immediately before darunavir-ritonavir.
In the simplification strategy, all subjects in the analysis are initially switched from their current suppressive therapy to a simplified maintenance regimen consisting of atazanavir-ritonavir alone. In the base case, we projected that 83% of recipients would attain an HIV RNA level < 400 copies/mL at 48 weeks, on the basis of the 24-week results reported for the pilot AIDS Clinical Trials Group trial [10]. After virologic failure occurs with this regimen, subjects are assigned to different sequences of treatment regimens, depending on whether they have developed PI resistance. On the basis of data reported in previous studies [3, 10, 24, 25], subjects who do not develop PI resistance are able to attain resuppression of the HIV RNA level after initial atazanavir-ritonavir treatment failure by adding back NRTIs. Therefore, these subjects receive a total of 6 sequential regimens, starting with the initiation of the simplification regimen: (1) atazanavir-ritonavir alone; (2) the resuppressive atazanavir-ritonavir regimen, which includes NRTIs; (3) an NNRTI-based regimen; (4) ritonavir-boosted lopinavir with NRTIs; (5) ritonavir-boosted darunavir with NRTIs, with or without enfuvirtide; and (6) a final, minimally effective salvage regimen.
Subjects who develop PI resistance while receiving atazanavir-ritonavir alone are assumed to be unable to attain re-suppression of the HIV RNA level while receiving this regimen; therefore, they receive only 5 regimens: (1) atazanavir-ritonavir alone, followed by (2) an NNRTI-based regimen; (3) ritonavir-boosted lopinavir with NRTIs; (4) ritonavir-boosted darunavir with NRTIs, with or without enfuvirtide; and (5) a final, minimally effective salvage regimen.
Quality-of-life benefit for avoidance of or delay in NRTI-associated toxicities
The incidence and quality-of-life impacts of regimen-specific NRTI-associated toxicities were calculated for the specific NRTIs assumed for each regimen on the basis of the literature (table 2) [1, 26–35]. Nephrotoxicity (for tenofovir), anemia (for zidovudine), and hypersensitivity reaction (for abacavir) are assumed to lead to a change in the antiretroviral therapy regimen. In the first regimen, abacavir is substituted for tenofovir, and zidovudine is substituted for abacavir; in subsequent regimens, either stavudine or didanosine is substituted, depending on prior NRTI exposure.
Table 2.
Nucleoside reverse-transcriptase inhibitor (NRTI)–associated toxicities.
| NRTI,a toxicity | Probability, %b | Treatment change | Quality-of-life reduction, % | Reference(s) |
|---|---|---|---|---|
| Tenofovir, nephrotoxicity | 7–14 | Switch to abacavir | … | [32] |
| Abacavir, hypersensitivity reaction | 8 | Switch to zidovudine | … | [1] |
| Zidovudinec | ||||
| Anemia | 3 | Switch to stavudine | … | [1] |
| Lipoatrophy | 15 | … | 13 | [29, 33, 35] |
| Stavudinec | ||||
| Lipoatrophy | 45 | … | 13 | [29, 33, 35] |
| Neuropathy | 26–44 | … | 6 | [26, 31, 34] |
| Didanosine,c neuropathy | 18 | … | 6 | [26, 31, 34] |
All NRTI regimens include a 0.3%–1% probability of severe lactic acidosis, with a 49% probability of death due to severe lactic acidosis [27].
Data are the probability that the toxicity will ever occur while the patient is receiving a regimen containing the referenced drug.
Sensitivity analyses
In sensitivity analyses, we varied baseline assumptions to determine the point at which the simplification strategy would provide an outcome equivalent to that for the standard of care. The proportion of subjects with HIV RNA levels < 400 copies/mL at week 48 of treatment with atazanavir-ritonavir alone was varied from 90% to 75% (compared with 83% in the base case), the mean CD4 cell count at entry was varied by ± 50 cells/μL (compared with 525 cells/μL in the base case), and the efficacy of the atazanavir-ritonavir regimen with NRTIs was improved to be equal to the efficacy of continuation of the NNRTI regimen in the standard-of-care strategy.
We also performed sensitivity analyses on the quality-of-life assumptions relating to NRTI-associated toxicities, to evaluate their impact on the overall benefit of the simplification strategy; these included assuming that lipoatrophy and neuropathy toxicities continue to affect the quality of life while the patient receives subsequent regimens, increasing and decreasing all toxicity effects by 50%, and ignoring toxicity effects altogether. Additional sensitivity analyses included reducing the cost of atazanavir-ritonavir treatment ($1020 per month) to be equivalent to the cost of lopinavir-ritonavir treatment ($615 per month) and including the cost of monthly HIV RNA testing (compared with every 3 months in the base case).
To evaluate the potential impact of new drug classes, we constructed a hypothetical regimen containing an integrase inhibitor, with a projected efficacy based on 16-week results reported for the integrase inhibitor MK-0518 (table 1) [36]. In sensitivity analyses, this regimen was included in all strategies immediately before the ritonavir-boosted darunavir regimen.
RESULTS
Compared with the current standard of care, a strategy of regimen simplification with boosted PI therapy is associated with an increased duration of survival, as long as PI resistance does not develop. Simplification regimen recipients who do not develop PI resistance have an undiscounted life expectancy of 27.9 years, compared with 27.1 years for standard-of-care subjects (table 3). Discounted QALE for the simplification regimen subjects is 14.9 years, compared with 14.7 years for patients receiving the standard-of-care regimen. However, simplification strategy subjects who have developed PI resistance at the time of simplification regimen failure have an undiscounted life expectancy of 26.5 years and a QALE of 14.5 years. These values are shorter than the values for subjects receiving the standard of care. For an entire population, the expected QALE of the simplification strategy remains higher than that for the standard-of-care strategy, even when a large proportion of simplification strategy subjects develop PI resistance at the time of virologic failure. In the base case (in which 83% of subjects have a suppressed HIV RNA level at 48 weeks while receiving the simplification regimen), 56% of those who experience virologic failure can develop PI resistance before the QALE of the simplification strategy becomes less than that for the standard-of-care strategy.
Table 3.
Life expectancy and lifetime costs.
| Simplification strategy | ||||
|---|---|---|---|---|
| Base case | Standard of care | Without PI-resistant HIV | With PI-resistant HIV | Subjects with PI-resistant HIV, break-even % |
| Undiscounted life expectancy, years | 27.1 | 27.9 | 26.5 | 57.2 |
| Discounted life expectancy, years | 17.3 | 17.5 | 17.0 | 40.4 |
| Discounted QALE, years | 14.7 | 14.9 | 14.5 | 56.4 |
| Undiscounted lifetime cost, 2005 US$ | 760,300 | 731,200 | 646,300 | … |
| Undiscounted annual cost, 2005 US$ | 28,100 | 26,200 | 24,400 | … |
| Discounted lifetime cost, 2005 US$ | 456,700 | 430,200 | 384,300 | … |
| Sensitivity analyses | ||||
| Discounted QALE, years | ||||
| Decrease toxicity QOL effects by 50% | 14.8 | 14.9 | 14.6 | 38.2 |
| No NRTI toxicity benefit | 14.9 | 15.1 | 14.6 | 26.0 |
| Increase toxicity QOL effects by 50% | 14.5 | 14.8 | 14.4 | 71.2 |
| QOL effects of lipoatrophy and neuropathy continue for life | 14.4 | 14.7 | 14.4 | 85.9 |
| Discounted lifetime cost | ||||
| Monthly HIV RNA testing for first 6 months of simplification regimen | 456,700 | 430,600 | 384,800 | … |
| Monthly HIV RNA testing for duration of simplification regimen | 456,700 | 436,700 | 390,900 | … |
| Set cost of atazanavir-ritonavir equal to that of lopinavir-ritonavir | 440,500 | 379,400 | 350,700 | … |
NOTE. NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; QALE, quality-adjusted life expectancy; QOL, quality of life.
Simplification strategy subjects who do not develop PI resistance at the time of virologic failure are projected to live longer than subjects receiving the standard of care, because they receive an additional line of therapy without compromising future treatment options. Standard-of-care strategy subjects spend a mean of 6.7 discounted quality-adjusted life years (QA-LYs) receiving their first line of therapy (i.e., an NNRTI-based regimen) and 2.8 QALYs receiving their second line of therapy (a boosted PI plus NRTIs), for a total of 9.5 QALYs (figure 1). This represents a mean undiscounted total time receiving these regimens of 14.2 years. Simplification strategy subjects who do not develop PI resistance spend an average of 10.7 QALYs receiving 3 similarly effective regimens: atazanavir-ritonavir alone (5.8 QALYs), atazanavir-ritonavir with NRTIs (3.0 QALYs), and an NNRTI-based regimen (1.8 QALYs). This represents a mean undiscounted total time receiving these regimens of 16.7 years.
Figure 1.
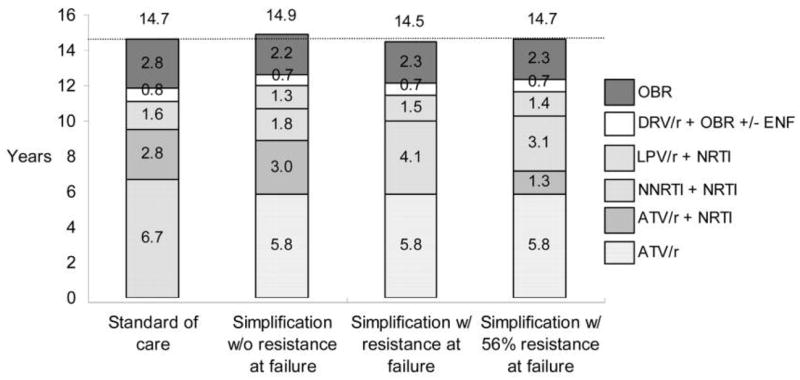
Discounted quality-adjusted life expectancy, by treatment strategy. ATV/r, atazanivir-ritonavir; DRV/r, darunavir-ritonavir; ENF, enfuvirtide; LPV/r, lopinavir-ritonavir; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; OBR, optimized background regimen; ±, with or without.
We conducted sensitivity analyses that changed both the efficacy of the atazanavir-ritonavir regimen and the CD4 cell count distribution at entry into the analysis (figure 2). In the worst-case scenario, with the lowest virologic suppression rate and mean baseline CD4 cell count (i.e., 75% of patients have an HIV RNA level < 400 copies/mL at 48 weeks and with a mean CD4 cell count of 475 cells/μL at cohort entry), the QALE of subjects receiving the simplification strategy is 14.3 QALYs for those who have not developed PI resistance at the time of failure of the first regimen and 13.9 QALYs for those who have developed PI resistance, compared with 14.2 QALYs for subjects receiving the standard of care. The break-even percentage of subjects receiving the simplification strategy who can develop PI resistance decreases to 33%. In contrast, with a 90% virologic suppression rate at 48 weeks and a mean baseline CD4 cell count of 575 cells/μL, 92% of simplification strategy subjects would need to develop PI resistance before QALE would be lower for the simplification group. In this scenario, subjects undergoing the standard of care spend more time receiving regimens with lipoatrophy and neuropathy, resulting in greater quality-of-life decrements of treatment. Their slightly longer life expectancy, compared with simplification regimen subjects who develop resistance, is offset by greater reductions in quality of life associated with treatment-related adverse effects. When the efficacy of the atazanavir-ritonavir regimen with NRTIs is improved, the break-even percentages are 28% in the base case, 22% with a mean CD4 cell count of 475 cells/μL at cohort entry, and 38% with a mean CD4 cell count of 575 cells/μL at cohort entry.
Figure 2.
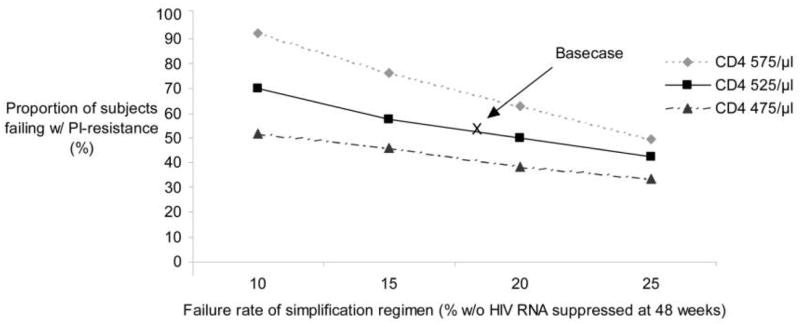
“Break-even” proportion of subjects with HIV with protease inhibitor (PI) resistance after failure of a simplification regimen that would result in a quality-adjusted life expectancy equivalent to that of the standard of care (see Results).
We conducted several sensitivity analyses that varied the toxicity benefit of the simplification strategy (table 3). When toxicities are decreased by 50%, the QALE for subjects receiving the simplification strategy becomes 14.9 years for those who do not develop resistance and 14.6 years for those who develop resistance, compared with 14.8 years for subjects receiving the standard of care; the break-even threshold becomes 38%. When we ignore the toxicity benefit altogether, the break-even threshold becomes 26%; if the rate of toxicities is increased by 50%, the break-even threshold becomes 71%. If we assume the quality-of-life effects of lipoatrophy and neuropathy continue for life, the break-even percentage becomes 86%.
Average discounted lifetime costs for all patients receiving the simplification strategy, including those who develop PI resistance, are lower than for patients receiving the standard of care. The average discounted lifetime cost for simplification strategy subjects is $430,200 for those who do not develop PI resistance and $384,300 for those who develop PI resistance, compared with $456,700 for standard-of-care strategy subjects (table 3). The average undiscounted annual cost for simplification strategy subjects without resistance ($26,200) or with resistance ($24,400) is lower than for standard-of-care subjects ($28,100), and this occurs consistently (figure 3).
Figure 3.
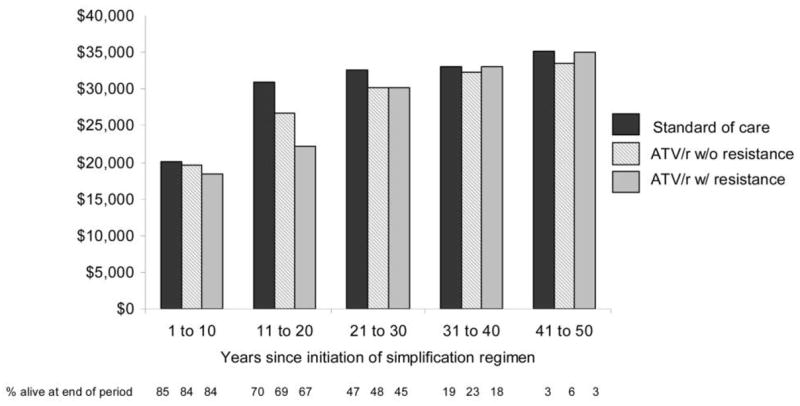
Average undiscounted annual cost per patient, by treatment strategy, in 2005 US dollars. ATV/r, atazanavir-ritonavir.
If HIV RNA testing is conducted monthly (instead of every 3 months) during the first 6 months of simplified maintenance therapy, the discounted lifetime cost is $430,600 for subjects who do not develop resistance and $384,800 for subjects who do develop resistance, compared with $456,700 for subjects receiving the standard of care. If monthly HIV RNA testing continues for the duration of the simplification regimen, the cost of the simplification strategy remains lower for subjects without resistance ($436,700) and subjects with resistance ($390,900). When the cost of atazanavir-ritonavir is lowered to equal that of lopinavir-ritonavir, the discounted lifetime cost is $379,400 for subjects who do not develop resistance and $350,700 for subjects who develop resistance, compared with $440,500 for subjects receiving the standard of care.
In the scenario in which a hypothetical regimen containing an integrase inhibitor is added to each strategy, undiscounted life expectancy increases by 1.0–1.3 years. The break-even threshold is 39%, and the average discounted lifetime costs for simplification strategy subjects who do or do not develop PI resistance remain lower than the cost for standard-of-care strategy subjects.
DISCUSSION
We used the CEPAC model to project life expectancy, QALE, and direct medical costs for simplified antiretroviral maintenance therapy. We found that, from a population perspective, the average patient will have a longer projected life expectancy and QALE with the simplification strategy than with the current standard-of-care strategy. Moreover, the simplification strategy has a lower projected lifetime cost than the standard-of-care strategy. When we conducted a sensitivity analysis that included the cost of more frequent viral load testing, the cost advantage for the simplification strategy remained (although we did not take into account the inconvenience to patients of more frequent viral load testing).
This analysis has several important implications for the development of treatment simplification trials [37]. First, the study highlights the tension between population benefits and the preferences and potential outcomes of individual subjects. Although a population of patients treated with the simplification strategy will experience an overall net increase in survival and quality-adjusted survival, compared with recipients of the standard of care, these specific outcomes may be worse for individuals who develop PI resistance. Although we project the risk of PI resistance to be low on the basis of current data, some individuals considering enrolling in the trial might wish to avoid even a low risk of suboptimal outcomes. The selection of an antiretroviral regimen always entails making trade-offs among regimen attributes, including convenience, cost, potential adverse effects, and the risk of developing resistance. Nevertheless, clinicians may also be reluctant to refer potential subjects to a trial even if the long-term expected outcomes are beneficial, because these benefits may not be directly observable at the conclusion of the trial and may not accrue to the individual enrolled patient.
Second, this analysis emphasizes the importance of clearly defining—and of measuring as an end point—the proportion of subjects with PI resistance among those who experience simplification regimen failure. This proportion should also be taken into account when defining stopping rules for the trial. In contrast, quality-of-life and medical costs can be modeled, and results of this analysis are less sensitive to the aforementioned variables, so they do not need to be collected directly in the clinical trial. Finally, this study highlights the need for the trial to include a sufficiently long follow-up period to describe subsequent treatment choices and outcomes for subjects who experience treatment failure with the simplification strategy, both with and without PI resistance.
This study has several limitations. Much of the benefit attributable to the simplification strategy is from adding an additional line of therapy, with most patients not experiencing a penalty from virologic failure. We base this finding on the frequent absence of genotypic or phenotypic PI resistance when viral rebound occurs and on the ability of most patients in simplification studies to regain virologic suppression by resuming NRTI therapy. However, low levels of HIV RNA (< 20 copies/mL) have been detected in some patients who receive a simplification regimen [12]. In addition, it remains unknown whether the efficacy of boosted PI-based therapy plus NRTIs for these individuals is unaffected by a period during which the patient receives a boosted PI alone. On the other hand, we did not take into account the evidence that atazanavir is associated with a unique resistance profile characterized by an absence of cross-resistance to other PIs [38].
In addition, we project that the simplification strategy will defer further into the future regimens that are less effective and more toxic. When we project future regimens, we consider only drugs currently available and do not take into account the likely introduction of new drugs or drug classes. To simplify the modeling, the quality-of-life effects of drug toxicities are considered only for NRTIs, the drug class that is “spared” as a result of the simplification strategy, rather than for all drug classes.
Simulation modeling can be a valuable tool in planning clinical trials [39]. For the AIDS Clinical Trials Group A5237 protocol team, this study identified key trial end points and highlighted differences in perspective between individual clinical trial subjects and population benefits when considering a treatment simplification strategy. The probabilities of developing PI resistance that are acceptable for the population may be higher than those that are acceptable to subjects enrolling in the trial. The risks and benefits of clinical trial participation need to be clearly understood and evaluated by potential subjects, with assistance from the subjects’ primary caregivers and the trial investigators. The AIDS Clinical Trials Group decided not to go forward with the study, but members of the protocol team are now investigating the possibility of conducting the study with alternative sponsorship. If the outcomes of such a trial are consistent with currently available pilot data, the results could support a treatment simplification approach that has the potential to further improve life expectancy, reduce the burden of treatment-related adverse effects, and lower costs for patients who are successfully controlling HIV infection while receiving their initial regimens.
Acknowledgments
Financial support. National Institute of Allergy and Infectious Diseases (K23 AI055038, K23 AI55038, K24 AI062476, K25 AI50436, P30 AI060354, R37 AI042006, U01 AI27661, U01 AI68636, U01 AI69419, U01 AI069472, and U01 AI46383), National Institute on Drug Abuse (K01 DA017179), National Center for Research Resources (KL2 RR024154), and the General Clinical Research Centers Program (M01-RR00047).
Potential conflicts of interest. S.S. has received research grants or contracts from or has served as a consultant for Abbott, Bristol-Myers Squibb, Novartis, and Pfizer. P.E.S. has been a consultant for Abbott, Bristol-Myers Squibb, Gilead, and GlaxoSmithKline; has received honoraria for teaching from Abbott, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, Tibotec, and Virco; and has received grant support from Pfizer, Merck, and GlaxoSmithKline. T.J.W. has received research support from Tibotec and is on the speaker’s bureau for Merck. J.E.M. has received research support from the BMS Foundation. All other authors: no conflicts.
References
- 1.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents—a working group of the Office of AIDS Research Advisory Council. [Accessed 19 January 2007];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 10 October 2006. Available at: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 2.Arribas J, Pulido F, Delgado R, et al. Lopinavir/ritonavir as single-drug maintenance therapy in patients with HIV-1 viral suppression: forty eight week results of a randomized, controlled, open label, clinical trial (OK04 Study) [abstract WEPE12.3C05]. Program and abstracts of the XVI International AIDS Conference; Toronto, Canada. Geneva: International AIDS Society; 2006. [Google Scholar]
- 3.Arribas JR, Pulido F, Delgado R, et al. Lopinavir/ritonavir as single-drug therapy for maintenance of HIV-1 viral suppression: 48-week results of a randomized, controlled, open-label, proof-of-concept pilot clinical trial (OK Study) J Acquir Immune Defic Syndr. 2005;40:280–7. doi: 10.1097/01.qai.0000180077.59159.f4. [DOI] [PubMed] [Google Scholar]
- 4.Cameron D, da Silva B, Arribas J, et al. A two-year randomized controlled clinical trial in antiretroviral-naive subjects using lopinavir/ritonavir (LPV/r) monotherapy after initial induction treatment compared to an efavirenz (EFV) 3-drug regimen (Study M03–613) [abstract THLB0201]. Program and abstracts of the XVI International AIDS Conference; Toronto, Canada. Geneva: International AIDS Society; 2006. [Google Scholar]
- 5.Delfraissy JF, Flandre P, Delaugerre C, et al. MONARK trial (MONotherapy AntiRetroviral Kaletra): 48-week analysis of lopinavir/ritonavir (LPV/r) monotherapy compared to LPV/r + zidovudine/lamivudine (AZT/3TC) in antiretroviral-naive patients [abstract THLB0202]. Program and abstracts of the XVI International AIDS Conference; Toronto, Canada. Geneva: International AIDS Society; 2006. [Google Scholar]
- 6.Gathe JC, Washington M, Piot D, Mayberry C. Preliminary pilot data on the safety and efficacy of Kaletra (LPV/r) dosed alone for the treatment of HIV in ARV-naive patients: greater than or equal 24 data [abstract H-845]. Program and abstracts of the 43rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL. Washington, DC: American Society for Microbiology; 2003. [Google Scholar]
- 7.Kahlert C, Hupfer M, Wagels T, et al. Ritonavir boosted indinavir treatment as a simplified maintenance “mono”-therapy for HIV infection. AIDS. 2004;18:955–7. doi: 10.1097/00002030-200404090-00017. [DOI] [PubMed] [Google Scholar]
- 8.Nunes EP, Oliveira MS, Almeida MMTB, et al. 48-Week efficacy and safety results of simplification to single agent lopinavir/ritonavir (LPV/r) regimen in patients suppressed below 80 copies/mL on HAART- the KalMo study [abstract TUAB0103]. Program and abstracts of the XVI International AIDS Conference; Toronto, Canada. Geneva: International AIDS Society; 2006. [Google Scholar]
- 9.Pierone G, Jr, Mieras J, Bulgin-Coleman D, et al. A pilot study of switch to lopinavir/ritonavir (LPV/r) monotherapy from nonnucleoside reverse transcriptase inhibitor-based therapy. HIV Clin Trials. 2006;7:237–45. doi: 10.1310/hct0705-237. [DOI] [PubMed] [Google Scholar]
- 10.Swindells S, DiRienzo AG, Wilkin T, et al. Regimen simplification to atazanavir-ritonavir alone as maintenance antiretroviral therapy after sustained virologic suppression. JAMA. 2006;296:806–14. doi: 10.1001/jama.296.7.806. [DOI] [PubMed] [Google Scholar]
- 11.Vernazza P, Daneel S, Schiffer V, et al. Simplified protease-inhibitor–only regimen with ritonavir-boosted atazanavir (ATARITMO-study) [abstract WEPE0073]. Program and abstracts of the XVI International AIDS Conference; Toronto, Canada. Geneva: International AIDS Society; 2006. [Google Scholar]
- 12.Karlstrom O, Josephson F, Sonnerborg A. Early virologic rebound in a pilot trial of ritonavir-boosted atazanavir as maintenance monotherapy. J Acquir Immune Defic Syndr. 2007;44:417–22. doi: 10.1097/QAI.0b013e31802e2940. [DOI] [PubMed] [Google Scholar]
- 13.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 14.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 15.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–7. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 16.Samet JH, Freedberg KA, Savetsky JB, Sullivan LM, Stein MD. Understanding delay to medical care for HIV infection: the long-term non-presenter. AIDS. 2001;15:77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- 17.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 18.Clotet B, Raffi F, Cooper D, et al. Clinical management of treatment-experienced, HIV-infected patients with the fusion inhibitor enfuvirtide: consensus recommendations. AIDS. 2004;18:1137–46. doi: 10.1097/00002030-200405210-00007. [DOI] [PubMed] [Google Scholar]
- 19.Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–94. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 20.Albrecht MA, Bosch RJ, Hammer SM, et al. Nelfinavir, efavirenz, or both after the failure of nucleoside treatment of HIV infection. N Engl J Med. 2001;345:398–407. doi: 10.1056/NEJM200108093450602. [DOI] [PubMed] [Google Scholar]
- 21.Katlama C, Berger D, Bellos N, et al. Efficacy of TMC114/r in 3-class experienced patients with limited treatment options: 24-week planned interim analysis of 2 96-week multinational dose-finding trials [abstract 164LB]. Program and abstracts of the 12th Conference on Retroviruses and Opportunistic Infections; Boston, MA. Alexandria, VA: Foundation for Retrovirology and Human Health; 2005. [Google Scholar]
- 22.Losina E, Islam R, Pollock AC, Sax PE, Freedberg KA, Walensky RP. Effectiveness of antiretroviral therapy after protease inhibitor failure: an analytic overview. Clin Infect Dis. 2004;38:1613–22. doi: 10.1086/420930. [DOI] [PubMed] [Google Scholar]
- 23.Landay A, da Silva BA, King MS, et al. Evidence of ongoing immune reconstitution in subjects with sustained viral suppression following 6 years of lopinavir-ritonavir treatment. Clin Infect Dis. 2007;44:749–54. doi: 10.1086/511681. [DOI] [PubMed] [Google Scholar]
- 24.Hackett JR, Jr, Holzmayer V, Marlowe N, et al. Selection of protease inhibitor (PI) resistance mutations during virological failure of lopinavir/ritonavir (LPV/r) monotherapy in an induction-maintenance study [abstract 75] Antivir Ther. 2006;11:S85. [Google Scholar]
- 25.Norton M, Delaugere C, Batot G, Delfraissy JF, Rouzioux C. Drug resistance outcomes in a trial comparing lopinavir/ritonavir (LPV/r) monotherapy to LPV/r + zidovudine/lamivudine (MONARK Trial) [abstract 74] Antivir Ther. 2006;11:S84. [Google Scholar]
- 26.Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14:217–30. doi: 10.1002/hec.910. [DOI] [PubMed] [Google Scholar]
- 27.Claessens YE, Chiche JD, Mira JP, Cariou A. Bench-to-bedside review: severe lactic acidosis in HIV patients treated with nucleoside analogue reverse transcriptase inhibitors. Crit Care. 2003;7:226–32. doi: 10.1186/cc2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo JJ, Jang R, Louder A, Cluxton RJ. Acute pancreatitis associated with different combination therapies in patients infected with human immunodeficiency virus. Pharmacotherapy. 2005;25:1044–54. doi: 10.1592/phco.2005.25.8.1044. [DOI] [PubMed] [Google Scholar]
- 29.Lenert LA, Feddersen M, Sturley A, Lee D. Adverse effects of medications and trade-offs between length of life and quality of life in human immunodeficiency virus infection. Am J Med. 2002;113:229–32. doi: 10.1016/s0002-9343(02)01156-7. [DOI] [PubMed] [Google Scholar]
- 30.Moore RD, Keruly JC, Chaisson RE. Incidence of pancreatitis in HIV-infected patients receiving nucleoside reverse transcriptase inhibitor drugs. AIDS. 2001;15:617–20. doi: 10.1097/00002030-200103300-00011. [DOI] [PubMed] [Google Scholar]
- 31.Moore RD, Wong WM, Keruly JC, McArthur JC. Incidence of neuropathy in HIV-infected patients on monotherapy versus those on combination therapy with didanosine, stavudine and hydroxyurea. AIDS. 2000;14:273–8. doi: 10.1097/00002030-200002180-00009. [DOI] [PubMed] [Google Scholar]
- 32.Nelson M, Cooper D, Schooley R, et al. The safety of tenofovir DF for the treatment of HIV infection: the first 4 years [poster 781]. Program and abstracts of the 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. Alexandria, VA: Foundation for Retrovirology and Human Health; 2006. [Google Scholar]
- 33.Nolan D, Mallal S. Complications associated with NRTI therapy: update on clinical features and possible pathogenic mechanisms. Antivir Ther. 2004;9:849–63. [PubMed] [Google Scholar]
- 34.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:2293–303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schackman BR, Freedberg KA, Weinstein MC, et al. Cost-effectiveness implications of the timing of antiretroviral therapy in HIV-infected adults. Arch Intern Med. 2002;162:2478–86. doi: 10.1001/archinte.162.21.2478. [DOI] [PubMed] [Google Scholar]
- 36.Steigbigel R, Kumar P, Eron J, et al. Results of BENCHMRK-2, a phase III study evaluating the efficacy and safety of MK-0518, a novel HIV-1 integrase inhibitor, in patients with triple-class resistance virus [abstract 105bLB]. Program and abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. Alexandria, VA: Foundation for Retrovirology and Human Health; 2007. [Google Scholar]
- 37.Study comparing efficacy and safety of darunavir boosted with ritonavir to HART with 2 NRTI and darunavir boosted with ritonavir in HIV-1 infected patients. [Accessed 14 February 2007]; Available at: http://clinicaltrials.gov/show/NCT00421551.
- 38.Colonno R, Rose R, McLaren C, Thiry A, Parkin N, Friborg J. Identification of I50L as the signature atazanavir (ATV)–resistance mutation in treatment-naive HIV-1–infected patients receiving ATV-containing regimens. J Infect Dis. 2004;189:1802–10. doi: 10.1086/386291. [DOI] [PubMed] [Google Scholar]
- 39.Paltiel AD, Goldie SJ, Losina E, et al. Preevaluation of clinical trial data: the case of preemptive cytomegalovirus therapy in patients with human immunodeficiency virus. Clin Infect Dis. 2001;32:783–93. doi: 10.1086/319223. [DOI] [PubMed] [Google Scholar]
- 40.Multicenter AIDS Cohort Study (MACS) public dataset: release PO4. Springfield, VA: National Technical Information Service; 1995. [Google Scholar]
- 41.Cole SR, Hernan MA, Robins JM, et al. Effect of highly active anti-retroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158:687–94. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 42.Masur H, Kaplan JE, Holmes KK. Guidelines for preventing opportunistic infections among HIV-infected persons—2002: recommendations of the US Public Health Service and the Infectious Diseases Society of America. Ann Intern Med. 2002;137:435–78. doi: 10.7326/0003-4819-137-5_part_2-200209031-00002. [DOI] [PubMed] [Google Scholar]
- 43.Red Book. Montvale, NJ: Thomson PDR; 2005. [Google Scholar]
- 44.Schackman BR, Goldie SJ, Freedberg KA, Losina E, Brazier J, Weinstein MC. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making. 2002;22:27–38. doi: 10.1177/0272989X0202200103. [DOI] [PubMed] [Google Scholar]
- 45.Paltiel AD, Scharfstein JA, Seage GR, 3rd, et al. A Monte Carlo simulation of advanced HIV disease: application to prevention of CMV infection. Med Decis Making. 1998;18:S93–105. doi: 10.1177/0272989X98018002S11. [DOI] [PubMed] [Google Scholar]
- 46.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–95. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 47.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in zthe United States. J Infect Dis. 2006;194:11–9. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]


