Abstract
Age-related deficits in source memory have been attributed to alterations in prefrontal cortex (PFC) function, but little is known about the neural basis of such changes. The present study examined the time course of item and source memory retrieval by recording event-related potentials (ERPs) in patients with focal lesions in lateral PFC and in healthy older and young controls. Both normal aging and PFC lesions were associated with decrements in item and source memory. However, older controls showed a decrease in item hit rate with no change in false alarms, whereas patients showed the opposite pattern. Furthermore, ERPs revealed notable differences between the groups. The early positive-going old/new effect was prominent in the young but reduced in patients and older adults, who did not differ from each other. In contrast, older adults displayed a prominent left frontal negativity (600–1200 ms) not observed in the young. This left frontal effect was substantially smaller and delayed in the patients. The current results provide novel insights into the effects of aging on source memory and the role of the lateral PFC in these processes. Older controls appeared to adopt alternate memory strategies and to recruit compensatory mechanisms in left PFC to support task performance. In contrast, the lateral frontal patients were unable to use these mechanisms, thus exhibiting difficulties with strategic memory and monitoring processes.
Keywords: Prefrontal cortex, Episodic memory, Source memory, Aging, Frontal, Event-related potentials
1. Introduction
Many aspects of human memory have been functionally and neuroanatomically dissociated. Episodic memory has been defined as “a neurocognitive (brain/mind) system, uniquely different from other memory systems, that enables human beings to remember past experiences” (Tulving, 2002, p. 1). Episodic memory (memory for personally experienced events) was originally distinguished from semantic memory (memory for general facts) by the different types of information held in store (Tulving, 1972, 2002). Furthermore, episodic memory for the content of a conversation, for example, may be more persistent than the memory of who made the comments. Prefrontal cortex (PFC) has been implicated in source memory or memory for the spatio-temporal context in which items or facts were learned, while playing a less essential role in memory for the items themselves (Shimamura, 1995).
Although item memory declines with age, source memory is disproportionately impaired (Fabiani and Friedman, 1997; Henkel et al., 1998; Schacter et al., 1991; Senkfor and Van Petten, 1996; Simons et al., 2004; Spencer and Raz, 1994). These source difficulties in the elderly have been correlated with neuropsychological measures of frontal lobe function, such as verbal fluency and the Wisconsin Card Sorting Test (Craik et al., 1990). Glisky et al. (1995) divided older adults into groups based on neuropsychological measures of frontal and temporal lobe function. They found a double dissociation between source and item memory: subjects high in temporal lobe function performed better on item memory than those who scored low on temporal tests, while subjects high in frontal function were more accurate at source memory than those who scored low on frontal tests. Performing an orienting task that encouraged integration of item and source attributes alleviated the source memory deficit in the “low-frontal” older adults (Glisky et al., 2001).
Patients with damage in the PFC show deficits in source memory (Janowsky et al., 1989b) and memory for temporal order (Shimamura et al., 1990; McAndrews and Milner, 1991) despite relatively preserved item memory. In addition to source memory, the frontal lobes have been implicated in free recall (Incisa della Rocchetta and Milner, 1993; Janowsky et al., 1989a). An unresolved question is whether these memory impairments are a primary effect of frontal lobe damage or secondary to deficits in some other function (Milner, 1964; Schacter, 1987; Shimamura, 1995). Frontal patients also show deficits in attention and in the gating of irrelevant stimuli (Knight et al., 1999), which could contribute to inefficient use of encoding and retrieval strategies (Gershberg and Shimamura, 1995; Mangels, 1997; Stuss et al., 1994). This frontal participation in strategy application and planning (Shallice, 1982) may be essential for the encoding and retrieval of source information, which is typically outside the central focus of attention during encoding as compared to item information.
In contrast to source memory, item recognition memory is often spared in PFC lesioned patients. Wheeler and Stuss (2003) tested recognition using the remember/know procedure (R/K) to assess recollection and familiarity, respectively. No impairments in overall recognition were noted in frontal patients. Damage to dorsolateral PFC did not impair either R or K performance, but lesions in the frontal poles resulted in worse scores on recollection (Wheeler and Stuss, 2003). Likewise, overall recognition performance was intact in a group of 25 non-amnesic frontal patients (Verfaellie et al., 2004), and there was no increase in false positive responses to semantically related lures. However, in three of these patients (two of whom had left dorsolateral PFC damage), false alarm responses to new words were elevated while hit rates were normal, similar to other results in left lateral PFC patients (Alexander et al., 2003; Swick and Knight, 1999). In a continuous recognition test, we observed that frontal patients were not less accurate at identifying old stimuli that repeated at different delays (Swick and Knight, 1999). However, they did commit more false alarms to new stimuli. The lack of a disproportionate decline in accuracy with increasing delay suggested that this deficit may be a strategic or attentional one rather than purely mnemonic in nature.
Patients with frontal damage and healthy older adults show deficits in tests of source memory that are disproportionate to their deficits in simple old/new recognition tests, as compared to healthy young adults. Together with other sorts of data suggesting that cognitive declines with advancing age are most pronounced in tasks that strongly rely on prefrontal cortex, this parallel has formed one strand of a broader “frontal theory of aging” (Spencer and Raz, 1994; West, 1996, 2000). In its simplest form, this hypothesis suggests that prefrontal cortex is particularly vulnerable to normal aging, so that – crudely speaking – older adults may fall on a continuum between young adults and those with frank damage to this region. The fact that prefrontal gray matter volumes show greater age-related decrement than do other cortical regions (Raz et al., 1997; Van Petten et al., 2004) is sometimes cited as support for this view, but we have argued elsewhere that this result may have little bearing on the issue because gray matter volumes begin to decrease early in life (by age 9 to 15) and do not always show a consistent relationship to cognitive abilities across the lifespan (Van Petten, 2004, Van Petten et al., 2004). The simplest version of the frontal theory of aging also faces a challenge from reports that older adults sometimes show greater rather than lesser prefrontal activity than young adults performing the same tasks (Cabeza et al., 2004; Logan et al., 2002; Park et al., 2003). An alternative view is that cumulative small declines in mnemonic and perceptual processes served by more posterior cortical regions place increased burdens on prefrontal executive functions with advancing age and that increased prefrontal activity can – in some circumstances – help compensate for the declining efficiency of other brain regions. These views need be orthogonal, in that performance deficits could arise from declines in basic memory processes, coupled with the attempt to compensate for these declines via a system that is itself somewhat compromised.
Overall, there remain many unanswered questions about the roles of prefrontal cortex in episodic memory and how these change with advancing age. Surprisingly, there have been no direct comparisons of young adults, older adults, and frontal patients performing memory tasks accompanied by measures of neural activity that can identify prefrontal contributions. The current study was designed to start to fill this gap, using a source memory paradigm. Below, we briefly review hemodynamic and event-related potential studies showing prefrontal engagement in source memory tests.
Functional imaging studies opened a new avenue of research into frontal lobe involvement in memory processes (Buckner and Tulving, 1995). Based on early PET activation studies, Tulving and colleagues (1994) made the influential proposal that left PFC is preferentially involved in the encoding of new items into episodic memory, whereas right PFC is specifically implicated in episodic memory retrieval. More recent experiments have emphasized the importance of left PFC (relative to right PFC) during conditions in which retrieval is made more difficult, such as requiring recollection of contextual detail or a greater degree of controlled processing (Dobbins et al., 2002; Kahn et al., 2004; Nolde et al., 1998; Ranganath et al., 2000; Wheeler and Buckner, 2003).
Event-related potentials (ERPs) have been used to examine the time course of memory retrieval processes. In young control subjects, the attempt to retrieve contextual information (e.g., voice, location, temporal order, action) about studied words or pictures has been linked with a sustained positive potential at prefrontal scalp sites (Johansson et al., 2002; Kuo and Van Petten, in press; Senkfor, 2002; Senkfor and Van Petten, 1998; Senkfor et al., 2002; Trott et al., 1999; Van Petten et al., 2000; Wilding and Rugg, 1996). This effect begins at approximately 700 ms post-stimulus and continues for at least another 500 ms. Item recognition is associated with an earlier positive component observed in both simple old/new recognition tests and source memory tests. The early old/new effect is widespread across the scalp and reduced or eliminated in amnesia due to medial temporal or diencephalic damage (Olichney et al., 2000). In older control subjects, the amplitude of the prefrontal source ERP effect is typically reduced to greater extent than the item ERP effect (Senkfor and Van Petten, 1996; Trott et al., 1997), although one study reported an intact source effect in the elderly (Mark and Rugg, 1998). These latter authors suggested that the poor source accuracy of older participants in the study of Trott et al. (1997) contributed to their failure to observe the prefrontal source effect.
In an effort to improve the performance of older adults, Wegesin and colleagues (2002) modified the study phase procedures used by Trott et al. (1997, 1999), in which subjects learned nouns presented in sentences and made subsequent source (List 1/List 2) judgments. The participants in Wegesin et al.'s experiment studied shorter lists for an unlimited amount of time, performed an elaborative encoding task, received two presentations of each list, and were instructed on the use of strategies to improve their memory performance. As a result of these changes, source accuracy in the elderly improved to 67% (as compared to 55% in Trott et al., 1997). However, older adults were still significantly impaired compared to the young, who improved to 95% correct source accuracy (as compared to 67% in Trott et al., 1997). Importantly, the ERPs for correct source trials were qualitatively different in young and older adults. The young showed the typical prefrontal source effect, larger over right hemisphere electrodes, whereas the elderly showed a sustained, negative-going wave with a broad scalp distribution (but maximal over central electrodes). This result was interpreted as evidence that young and older adults use different cortical networks during source retrieval (Wegesin et al., 2002).
The present study examined the effects of aging and PFC damage on source memory retrieval processes as measured by ERPs. Neurological patients with unilateral PFC lesions, age-matched older controls, and young control participants studied lists of words spoken in either a male voice or a female voice. In an earlier experiment with these materials (Senkfor and Van Petten, 1998), the encoding task was a semantic decision based on the words alone, and the retrieval task was a decision about whether the word was old and spoken in the same voice as at study, old but spoken in the other voice, or new. However, because preliminary results (Senkfor and Van Petten, 1996) and extensive piloting (Swick, Machado, Senkfor, and Van Petten, unpublished observations) yielded poor performance in older subjects, the design was modified in several ways. To improve source memory accuracy in older controls, the encoding task focused subjects' attention on the relevant source information (male or female voice) and the lists were presented twice. Words in the retrieval phase were presented visually to avoid the potential benefit of context re-instatement for old words in the original voice or conflict from an old word in a different voice (Dodson and Shimamura, 2000). Subjects made old/new discriminations first followed by a male/female decision for words called old (similar to the design used by Wilding and Rugg, 1996; Mark and Rugg, 1998).
Frontal patients were selected for single focal lesion visible on CT or MRI scans. Lesions were centered in the posterior portion of Brodmann areas 9 and 46, but damage extended inferiorly and posteriorly to areas 6, 8, 44, and 45 in some individuals. The patients are described in more detail in the Experimental procedures section and in Table 1 and Fig. 1.
Table 1. Description of frontal patients.
| Patient | Age | Hem | Ed | Onset | Etiology | Aphasia |
|---|---|---|---|---|---|---|
| WA | 77 | L | 14 | 12 | Stroke | Anomic |
| RC | 51 | L | 12 | 17 | Ruptured AVM | None |
| JC | 74 | L | 16 | 11 | Stroke | Anomic/apraxia of speech |
| WE | 69 | L | 12 | 4 | Stroke | Anomic |
| MF | 65 | L | 12 | 2 | Stroke | Anomic (mild) |
| JM | 55 | L | 11 | 2 | Stroke | Anomic |
| NT | 59 | L | 12 | 3 | Stroke | None |
| EB | 80 | R | 12 | 15 | Stroke | None |
| SR | 78 | R | 12 | 4 | Stroke | None |
Note: Hem, hemisphere of lesion; Ed, years of formal education; onset, years since onset of damage. Aphasia category based on scores from the Western Aphasia Battery.
Fig. 1.
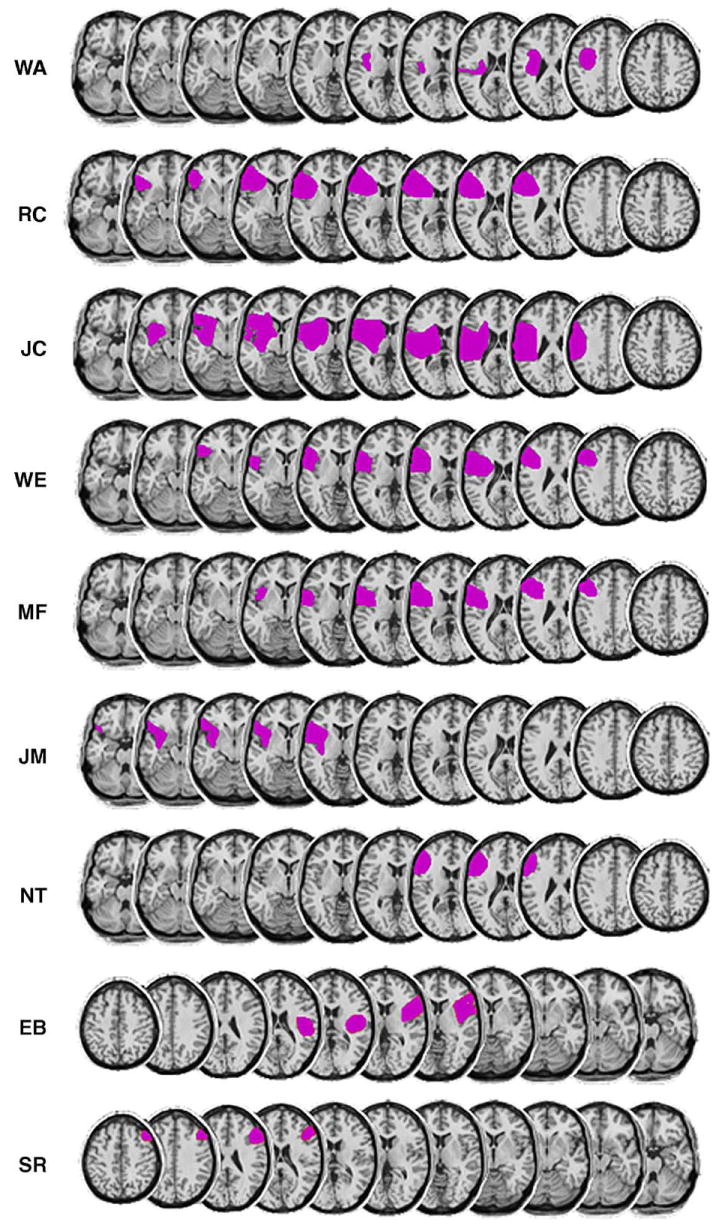
Lesion reconstructions for the lateral frontal patients (7 with left, 2 with right hemisphere damage). Lesions were estimated from MRI or CT scans and transcribed onto sequential axial templates. Left hemisphere lesions are shown from ventral to dorsal (left to right), and right hemisphere lesions from dorsal to ventral (left to right).
We predicted that elderly controls would show impairments in accuracy and reductions in the prefrontal ERP effect related to source retrieval attempts. Furthermore, if the neural generators of the prefrontal source effect are indeed localized to PFC, then the lesioned patients should show further reduction or elimination of this late positive prefrontal ERP, in addition to worse performance than the age-matched controls. We predicted that any item memory impairments in the present task would be surpassed by difficulties retrieving contextual (source) information.
2. Results
2.1. Behavioral performance
Accuracies and reaction times (RTs) are shown in Tables 2 and 3, respectively. The ability to discriminate studied and unstudied words was initially calculated as Pr (hit rate minus false alarm rate); hits were defined as a response of “old” to an old word, independent of the accuracy of the source decision. A main effect of group was obtained [F(2,24) = 17.25, P < .0001]. Young subjects were superior to older controls [F(1,16) = 19.39, P < .001], who were in turn better than the frontal patients [F(1,16) = 7.06, P < .02]. Response bias was calculated as Br (FA rate / (1 – Pr), according to Snodgrass and Corwin (1988). Although frontal patients tended to have more liberal biases than younger or older controls, there were no significant group differences. However, for consistency with prior studies, we also performed separate comparisons of hit and FA rates for the patients and their age-matched controls. The patients' reduced hit rate did not reach significance [F(1,16) = 1.16], whereas their FA rate was significantly higher [F(1,16) = 5.34, P < .05; 18.4% for patients, 4.8% for older controls].
Table 2. Accuracy measures (±SEM).
| Group | Pr (item) | Br (item) | Item overall | Source overall |
|---|---|---|---|---|
| Young | 93.8 ± 1.5 | 0.21 ± 0.07 | 96.9 ± 0.8 | 97.2 ± 1.5 |
| Older | 76.6 ± 3.6 | 0.23 ± 0.07 | 88.3 ± 1.8 | 88.1 ± 2.7 |
| Frontal | 56.1 ± 6.8 | 0.39 ± 0.08 | 78.0 ± 3.4 | 73.9 ± 1.9 |
Note: Pr = hit rate minus false alarm rate; Br = false alarm rate divided by (1 − Pr); overall item accuracy = hits plus correct rejections divided by total trials; overall source accuracy = correct source trials divided by trials with correct old responses. All numbers in percents, except Br which is a ratio.
Table 3. Reaction times (ms ± SEM).
| Group | CR | H/H | H/M | FA | Miss |
|---|---|---|---|---|---|
| Young | 1081 ± 36 | 1105 ± 50 | 1490 ± 167 | 1762 ± 147 | 1323 ± 99 |
| Older | 1079 ± 56 | 1188 ± 67 | 1344 ± 70 | 1626 ± 123 | 1277 ± 85 |
| Frontal | 1631 ± 102 | 1513 ± 80 | 1623 ± 102 | 1815 ± 114 | 1721 ± 102 |
Note: CR, correct rejection; H/H, correctly identified as old and source decision correct; H/M, correctly identified as old but source decision incorrect; FA, new word incorrectly judged as old; Miss, old word incorrectly judged as new.
Source accuracy was computed as the percentage of recognized old words correctly categorized as to the voice heard during the study phase (chance = 50%). A main effect of group on source accuracy was observed [F(2,24) = 32.47, P < .0001]. The young group showed ceiling-level performance and was superior to the older group (P < .005), which was in turn more accurate than the frontal patient group (P < .001).
The relationship between item and source accuracy was also examined across groups. These analyses used measures of item and source accuracy that are analogous in having the same chance level, 50%. Item accuracy was defined as hits plus correct rejections, divided by the total number of trials, and source accuracy as above. Fig. 2 shows that item and source accuracy were generally correlated [r = .89 across all 27 participants, P < .0001]. However, Fig. 2 also suggests that frontal patients were more likely than other participants to perform worse on source than item decisions. In ANOVAs with accuracy measure (item vs. source) and group status as factors, patients were disproportionately worse on the source measure as compared to young adults (measure × group interaction [F(1,16) = 4.29, P = .05]), but not when compared to older controls [F(1,16) = 2.00]. Although the older controls had lower accuracy than young adults overall, their decrement in source accuracy was proportional to their decrement in item accuracy [F < 1].
Fig. 2.
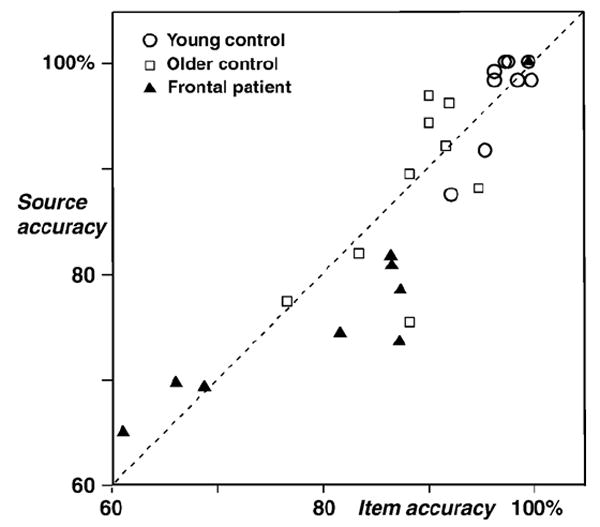
Item accuracy (hits and correct rejections divided by the total number of trials, chance performance 50%) compared to source accuracy (correct source decisions divided by the total number of correct “old” decisions, chance performance 50%).
It appears that lateral PFC damage impaired source memory to a greater degree than item recognition, but future studies are needed in which source and item memory are tested separately. To further investigate this issue in the current dataset, source accuracy was examined in PFC patients with high item accuracy. A subset of four patients was matched with older controls on the overall item recognition measure (86.9% for patients, 88.3% for controls; P > .6). Source performance was worse in these patients (78.7%) than in controls (88.1%; P < .05).
RTs were measured for both decisions. The initial decision was nominally about the studied/unstudied status of the word only but may reflect source memory as well. Comparisons were thus restricted to the categories of correctly recognized new words (correct rejections, CR) and old words followed by correct source decisions (hit/hits, H/H). Given their generally high accuracy levels, both young and old controls had too few errors to examine the other categories (false alarms, hit/misses, and misses). An ANOVA with factors of group and condition (CR vs. H/H) yielded a main effect of group [F(2,24) = 18.80, P < .001] and a condition × group interaction [F(2,24) = 3.52, P < .05]. The patients were much slower than controls, and they were slower for CR than for H/H stimuli (both control groups showed the opposite pattern). The young and old controls showed identical RTs for CR; the young were faster than their elders for H/H trials, but not significantly so (P > .3).
For items called old, a subsequent decision was made on the voice in which they were spoken. Voice decision times for H/H trials showed a significant effect of group [F(2,24) = 14.34, P < .001] due to the fact that the patients (716 ms) were much slower than young (418 ms) and older (404 ms) controls, who did not differ from each other.
2.2. ERPs
The average ERP waveforms elicited during the CR (new) and hit/hit (H/H) conditions are shown in Figs. 3–5 for young controls, older controls, and frontal patients, respectively. Visual inspection of the waveforms revealed pronounced differences between the groups. In young controls, the dominant effect was a broadly distributed positive potential for hit/hit trials as compared to new words, from approximately 400 to 800 ms. This was followed by a small late negative wave from 1000 to 1400 ms that was larger at midline and right hemisphere electrodes. In contrast, the elderly controls showed a substantially diminished positivitity to H/H words from 400 to 800 ms, which was only discernable at right frontocentral and temporal electrodes. Overlapping in this time frame was the most prominent feature in the ERPs for the elderly control group: a large and sustained negativity for the hit/hit trials from approximately 600 to 1200 ms, largest at prefrontal to central sites and much larger over the left than right hemisphere. The frontal patients showed a small early old/new effect in the 400–800 ms latency range, which, interestingly, was visible primarily over the side ipsilateral to the lesion. The ERPs of frontal patients also showed a late negative wave for hit/hits relative to new words, but this was much smaller in amplitude and delayed in latency relative to their age-matched control group.
Fig. 3.
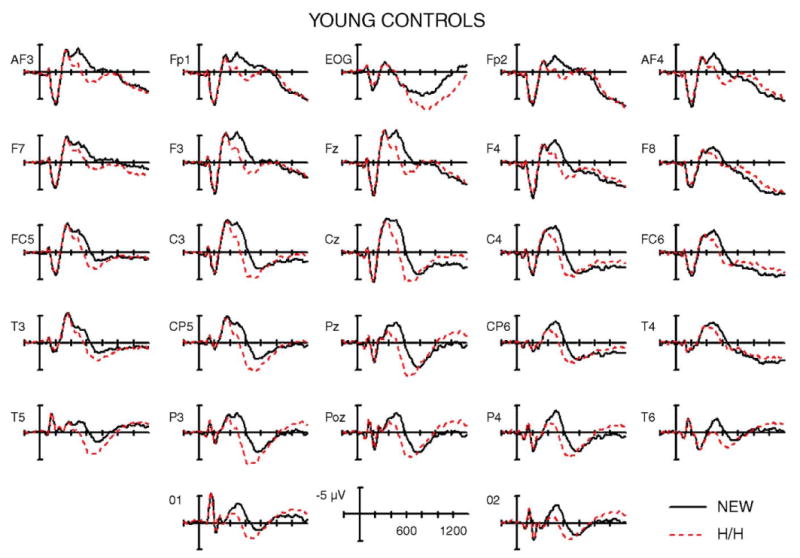
Grand average ERPs from young control subjects for correctly rejected new words (NEW) and correctly recognized old words with accurate source judgments (H/H). Unlike the older controls, note the absence of the anterior negativity and the widespread scalp distribution of the positivity to correctly remembered old words (400–800 ms).
Fig. 5.
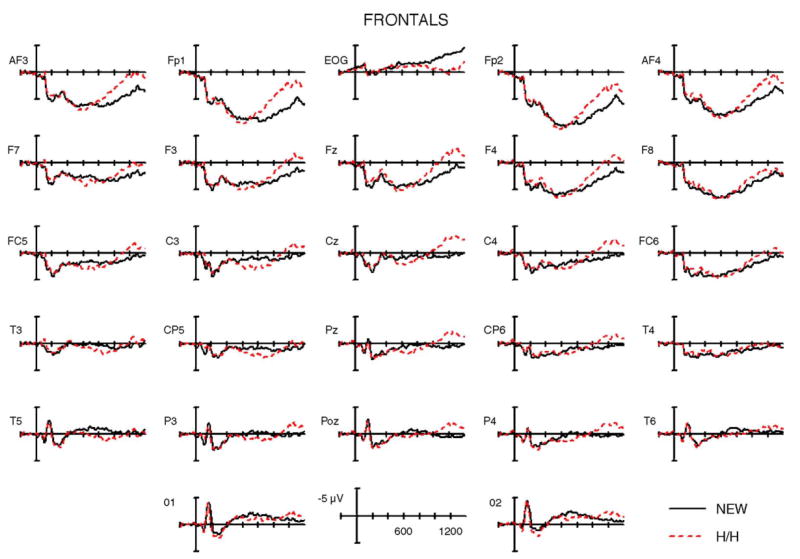
Grand average ERPs from patients with damage to lateral PFC. The lateral ERPs from the 2 right frontal patients were flipped so that, for all patients, the left side of the head is the ipsilesional side. The frontal negativity is significantly reduced, particularly at left posterior frontal scalp sites (e.g., F7, FC5) over lesioned cortex. The item ERP old/new effect is actually larger over left centro-parietal and temporal electrodes (e.g., at CP5, T5) than in older controls, due to reduction of the overlapping anterior negative component.
ERP data were initially quantified by measuring mean amplitudes within two latency windows to capture these positive (400–800 ms) and negative (600–1200 ms) components. The data were analyzed using repeated measures ANOVAs with factors of group (young, old, frontal), condition (CR, H/H), and electrode (n = 27). Only two levels of condition were included because young controls had very few trials for H/M, FA, and Miss. Relatively high source accuracy in the older adults precluded statistical analysis of H/M trials (mean number of trials was 13, range 4–32).
2.3. Early latency window
For the early (400–800 ms) interval, significant interactions between condition and group [F(2,24) = 7.25, P < .005] and condition, electrode, and group [F(52,625) = 3.19, P < .01] were obtained. For young controls, there was a significant main effect of condition [F(1,8) = 24.91 P = .001], with more positive amplitudes for H/H (0.8 μV) than for CR (−1.2 μV). This effect was widely distributed over the scalp as seen in Figs. 6 and 7; the condition × electrode interaction was not significant (P > .15). For the older controls, the condition × electrode interaction [F(26,208) = 3.98, P < .05] reflected the restricted distribution of the early old/new effect, which was visible at only right frontocentral electrodes. A follow-up analysis examined 100 ms latency windows for a set of six sites on the right and left sides (F4, F8, C4, FC6, CP6, T4 versus the homologous sites on the left). The early positive old/new effect was significant in only the 500–600 ms latency window on the right side [F(1,8) = 5.45, P < .05]. For the frontal group, a similar analysis showed a larger positivity for hit/hits on the side ipsilateral to the lesion, extending from 500 to 800 ms [500–600 ms, F(1,8) = 3.88, P = .08; 600–700 ms, F(1,8) = 6.00, P < .05; 700–800 ms, F(1,8) = 6.49, P < .05]. Overall, the early old/new effect was much diminished in both the frontal patients and their age-matched controls but visible over a restricted region of the scalp in both groups and not notably smaller in the patients as compared to healthy older adults (see Fig. 6).
Fig. 6.
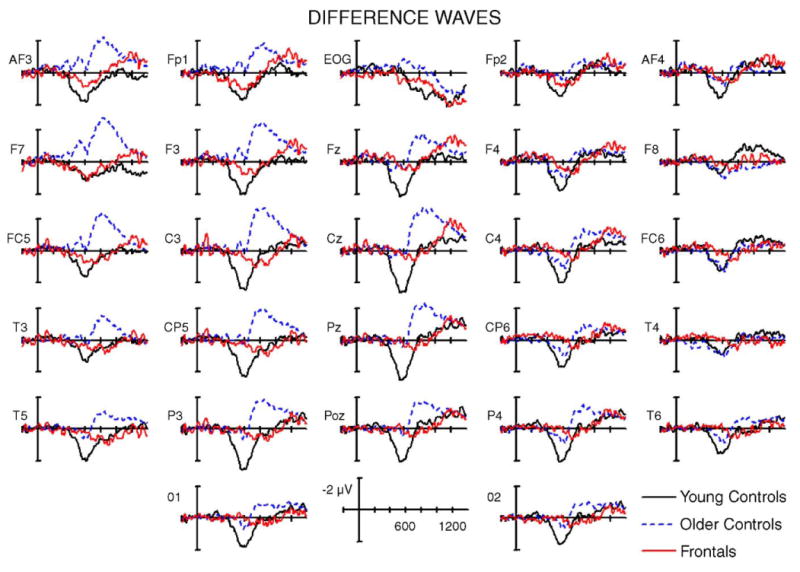
Difference waveforms (hit/hit minus correct rejection) for the three groups.
Fig. 7.
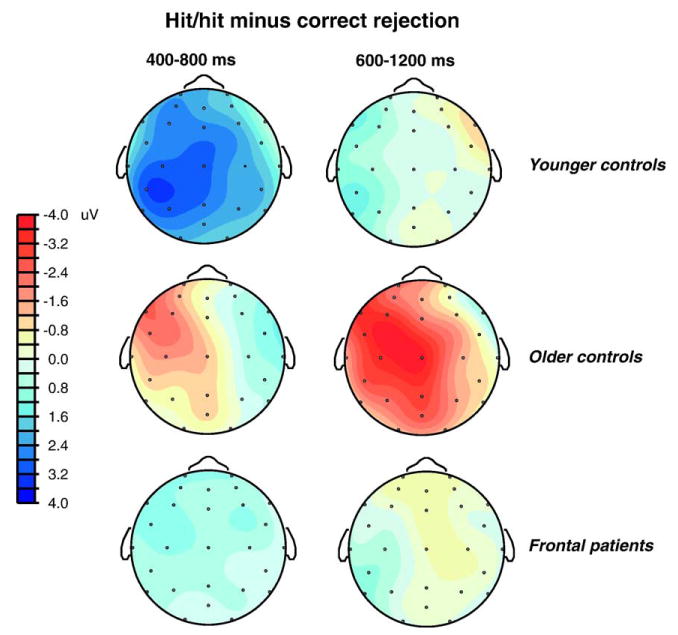
Topographic maps showing the spatial distribution of the difference between correct old (hit/hit) and correct new trials.
2.4. Late latency window
For the 600–1200 ms window, a condition by group interaction [F(2,24) = 4.56, P < .05] reflected qualitative differences in the memory effects among the three groups. Fig. 3 shows that the early old/new effect extended to about 800 ms in the young subjects before the ERPs for hit/hits and correct rejections grew to resemble each other. For the broad 600–1200 ms latency window, the young group thus showed no significant difference between conditions. In general, the young adults generated greater positivity to H/H relative to new words in the earlier part of this interval, although there were no significant differences when measured for the entire 600 ms window. Conversely, the late negative wave for hit/hits generated by the older adults (Fig. 4) produced a main effect of condition in this group [F(1,8) = 6.41, P < .05]. The condition × electrode interaction approached significance [F (26,208) = 3.03, P < .07]. Finally, the frontal patients did not show any significant difference between the two conditions during the 600–1200 ms interval.
Fig. 4.
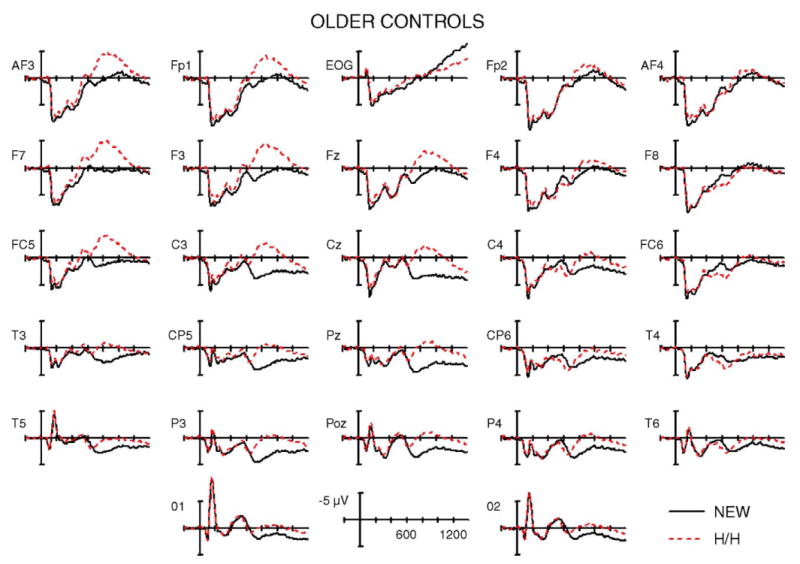
Grand average ERPs from age-matched controls for correctly rejected new words (NEW) and correctly recognized old words with accurate source judgments (H/H). The most prominent feature is a left lateralized frontal negativity (600–1200 ms) to old words that obscures the typical ERP old/new positivity (400–800 ms) at left temporal and parietal electrodes (compare T4 to T3).
To more closely examine the scalp distribution of the late negative wave in older controls, a follow-up ANOVA included the factors of condition (new, hit/hit), hemisphere (left, right), and anterior–posterior location (anterior—Fp1/2, F3/4, F7/8, FC5/6, C3/4; posterior—T 3/4, CP5/6, T5/6, P3/4, O1/2). The three-way interaction between these factors approached significance [F(1,8) = 4.28, P = .07]. ANOVAs at anterior electrode locations showed that the main effect of condition was significant over the left hemisphere [F(1,8) = 13.81, P < .005], but not over the right (P = .6), verifying the left anterior scalp distribution.
In the patients, more fine-grained amplitude measurements were taken in 100 ms windows (from 900 to 1400 ms) to capture the late negativity to H/H words, which was more limited in duration than in age-matched controls. From 1100 to 1200 ms, the ERPs to H/H items were more negative than to new items [F(1,8) = 10.32, P < .05], as was the case for the 1200–1300 ms interval [F(1,8) = 6.93, P < .05]. The condition × electrode interaction was significant for this latter window [F(26,208) = 3.25, P < .05], but this was not due to a left-lateralized frontal distribution as in older controls (see Fig. 6). Follow-up ANOVAs for these latency two windows included the factors of condition (new, hit/hit), hemisphere (ipsilesional, contralesional), and anterior–posterior location (as in older controls, above). The interaction between anterior–posterior location and condition was significant for 1200–1300 ms [F(1,8) = 6.87, P < .05] and showed a trend for 1100–1200 ms [F(1,8) = 4.03, P = .077]. Separate ANOVAs for the anterior and posterior regions showed that the main effect of condition was significant over anterior sites (P < .05), but not over posterior sites (P > .2) in the 1200–1300 ms window. No interaction with hemisphere was observed for any of these analyses, indicating that the late anterior negativity in frontal patients did not differ over the ipsilesional and contralesional sides.
3. Discussion
The present study demonstrated differential effects of normal aging and frontal lobe damage on behavioral performance and ERPs recorded during a recognition memory task that required retrieval of source information. Compared to the accuracy of young controls, which was close to ceiling, normal aging was associated with decrements in memory for studied items and for the voice in which they were spoken. Likewise, patients with lesions centered in posterior lateral PFC regions showed less accurate performance for both item and source memory judgments relative to their age-matched control group. In contrast to the graded decreases in item accuracy across groups, the ERPs showed qualitative differences in memory effects (differences between studied and unstudied words), which we attribute to the requirement to make a source memory judgment. In comparison to correctly identified new words, accurate retrieval of studied words and their sources was accompanied by a positive-going wave from approximately 300 to 800 ms in young controls. In contrast, attempted source memory retrieval in older adults was associated with a prominent left frontocentral ERP effect (600–1200 ms post-stimulus) not observed in the young. This left frontocentral negativity was substantially diminished in amplitude and delayed in latency in the lateral PFC patients. The current results provide novel insights into the effects of aging on source memory and the role of the lateral PFC in these processes. Older controls may recruit compensatory mechanisms (alternate retrieval strategies or other attention-related mechanisms) in the left PFC to boost performance in the task, albeit not to the level achieved by young controls. In contrast, the lateral frontal patients were unable to use these mechanisms, thus exhibiting difficulties with strategic memory and monitoring processes. The results will be discussed with regard to the literatures on how normal aging and frontal damage affect item and source memory.
3.1. Item recognition
Older adults and frontal patients performed worse than the young adults in discriminating studied from unstudied words. The age effect on accuracy in the control groups was expected given the general decline in episodic memory across normal aging (Park et al., 2002; Verhaeghen and Salthouse, 1997). The ERP effect associated with item recognition – more positive potentials in the 300–800 ms latency range1 – showed a substantial reduction in the older control group as compared to the young adults. Large age-related reductions in this early old/new effect have been reported in most comparisons of young and old healthy adults, even when the older adults discriminate studied from unstudied items with a relatively high level of accuracy (Joyce et al., 1998; Senkfor and Van Petten, 1996; Swick and Knight, 1997; Trott et al., 1997, 1999; Wegesin et al., 2002; cf. Mark and Rugg, 1998 for different results). However, the apparent magnitude of the ERP item recognition effect in the older adults is likely to have suffered some degree of temporal overlap and cancellation from the very large negativity elicited by correctly recognized items in the current experiment. The observation of an early old/new effect over only the right scalp in older adults (Figs. 6 and 7) is likely due to cancellation of this effect on the left side by the temporally overlapping negative old/new effect. Although the current results confirm other observations of a substantial reduction in this effect in older adults as compared to younger, it is difficult to estimate the exact magnitude of the age difference in the current data.
The patients had lower accuracy than their age-matched controls due to both a mildly reduced hit rate and a substantially inflated false alarm rate. The early old/new effect in the ERPs of the frontal patients was much smaller than in the young ERPs, but no smaller than in the ERPs of the older control subjects. Across studies, different degrees and patterns of impairment on recognition accuracy have been observed in frontal patients. Some have found that recognition memory is spared altogether (e.g., Janowsky et al., 1989a; Thaiss and Pertides, 2003; Wheeler and Stuss, 2003). In others, frontal damage results in an elevated FA rate with little impact on hit rate (Swick and Knight, 1999; Rapcsak et al., 1999; nonamnesic patients in Verfaellie et al., 2004), which is distinct from the profile of medial temporal or diencephalic amnesia (Swick and Knight, 1999; Verfaellie et al., 2002). We have previously argued that elevated FA rates combined with relative preservation of hit rates and early old/new ERP effects are indicative of a deficit in monitoring or evaluation rather than a raw memory deficit (Swick and Knight, 1999).
In contrast, one recent study shows both a reduced hit rate and an increased FA rate (Baldo et al., 2002), and another shows a deficit in forced-choice recognition where response bias is not relevant (Lee et al., 2002). Some of the discrepancies across studies may reflect differences in the material tested, particularly the similarity between studied items and lures, or the presentation of other material between the study and test phases, given that frontal patients are more sensitive to interference (Shimamura et al., 1995). Both recent reports of impaired recognition in frontal patients included lures that were similar to studied words and/or intervening material (Baldo et al., 2002; Lee et al., 2002). The studied and new words used here were all concrete nouns, but otherwise were not especially confusable. Another contribution to the variability across studies may arise from differences in patient selection, particularly the degree of language impairment when verbal materials are used (note that about half of the frontal patients here had some degree of word-finding difficulty). In contrast to the variable (but never devastating) degree of simple recognition impairment across studies, there has been greater consensus that source memory and memory for temporal order are impaired in patients with PFC lesions (Butters et al., 1994; Janowsky et al., 1989b; Johnson et al., 1997; McAndrews and Milner, 1991; Mangels, 1997; Shimamura et al., 1990).
3.2. Source memory
Young adults attained a very high (ceiling) level of accuracy in the source judgment of recalling which voice originally spoke the words that had been recognized as old. In contrast to a large number of previous reports, ERPs of the young subjects also lacked any late old/new effects. In particular, a late-onset (∼800 ms) prefrontal positivity for hits as compared to correct rejections has characterized source memory tests in several laboratories but was strikingly absent in the present paradigm (Johansson et al., 2002; Senkfor and Van Petten, 1998; Trott et al., 1997, 1999; Van Petten et al., 2000; Wegesin et al., 2002; Wilding et al., 1995; Wilding and Rugg, 1996). We assume that the high accuracy and absence of the late prefrontal effect in young adults are both consequences of the procedures implemented here in a deliberate attempt to boost the performance of older controls and patients: directing attention to the source attribute (voice) during the study phase, repeating words during the study phase, and presenting test words visually to eliminate any possible interference from misleading cues during the retrieval phase. In our previous word-and-voice experiments using an encoding task that focused attention on the semantic content of the word, one study phase presentation, and auditory test words (Senkfor and Van Petten, 1996, 1998), source accuracies for young and older adults were 78% and 60% (respectively), as compared to 97% and 88% (respectively) here. We have argued that the late positive prefrontal ERP effect reflects instigation of a secondary memory search for the difficult-to-recover link between two stimulus attributes (e.g., word and voice) after they have been independently recognized (Senkfor and Van Petten, 1998). Under the encoding conditions used here, the young adults may have formed a sufficiently tight link between the two attributes that they were retrieved as a single unit, making this secondary memory search unnecessary. From behavioral results in older adults with high and low scores on neuropsychological tests thought to tap prefrontal integrity, Glisky and colleagues (2001) have argued that appropriate encoding conditions may make source memory tests less frontally dependent (see Thaiss and Pertides, 2003; Kuo and Van Petten, in press for related suggestions).
Source accuracy of the older control group was significantly reduced relative to the young, but – in contrast to some prior studies – we observed little suggestion of a selective source memory deficit in this older group (see Johnson et al., 1993; Spencer and Raz, 1994 for reviews). The magnitude of the age effect in source accuracy was equivalent to that in item accuracy (9.1% vs. 8.6%, respectively).2 In absolute terms, the source accuracy of the older adults was also quite good (88%) and substantially better than in most previous ERP studies of older adults performing source memory tasks (Mark and Rugg, 1998; Senkfor and Van Petten, 1996; Trott et al., 1997, 1999; Wegesin et al., 2002; but see Li et al., 2004 for a contrast between easier and harder source tests).
Their ERPs indicate that the older adults engaged qualitatively different neural processes than the young adults to support this high level of accuracy. Beginning ∼600 ms after stimulus onset, correctly recognized old words elicited a large negative potential that was strongly lateralized to the left, with a frontal scalp distribution. The left frontal negativity was dramatically reduced in patients with lateral PFC regions, indicating that this component is dependent on the frontal regions that were damaged in the patients. This late left frontal effect was completely absent in the ERPs of the young. The qualitative age difference in brain electrical activity suggests that the older adults recruited frontal brain regions to perform a task that did not require extensive frontal activity in the young adults. Given that the earlier signature of successful memory retrieval was greatly reduced in the older group, yet accuracy was only modestly impaired, the most parsimonious interpretation is that this frontal recruitment was effective in maintaining performance in the face of declines in basic memory processes served by more posterior brain regions. Relative to these older controls, two performance deficits were observed in the frontal patients: elevated false alarm rates (for the patient group as a whole) and lower source accuracy (for those matched on item accuracy). These behavioral deficits in the patients further suggest that an intact lateral PFC was needed for optimal performance and that the left frontal negativity was functionally significant in the older controls. We return to the topic of prefrontal compensation in aging below but first discuss the relationship between the specific ERP effect observed here and those in other studies.
The late frontal negative potential that dominated the older adults' ERPs is distinct from our prior observations in young adults performing source memory tasks (Senkfor and Van Petten, 1998; Senkfor et al., 2002; Van Petten et al., 2000). It was also not evident in a prior source memory test in older adults judging words and voices (with much lower accuracy levels than those here, Senkfor and Van Petten, 1996). In one other aging experiment, studied items elicited frontal positivities, but this experiment did not include new trials during the test phase for comparison (Senkfor, submitted). Moreover, we observed no sign of a negative-going old/new effect in older adults during the encoding phase of this experiment, when the words were incidentally repeated during a gender decision task. Instead, the ERP repetition effects were restricted to the more typical finding of an enhanced positive potential with a broad posterior scalp distribution (Larsen and Swick, 2000).
Long-latency negative components – larger for studied than new items – have been reported in a few ERP studies of source memory. In some studies, these have had a posterior scalp distribution and may thus be unrelated to the observations here (Cycowicz et al., 2001; Friedman et al., 2005).3 However, three recent studies describe late negative potentials with a frontocentral spatial distribution and temporal properties that are also like those observed here (beginning ∼600 ms after stimulus onset). All three compared younger and older adults performing source memory tasks and observed a frontocentral negative potential for studied items only in the older adults (Duarte et al., 2006; Li et al., 2004; Wegesin et al., 2002). Moreover, the age-related effect was strongly left-lateralized in the Li et al. (2004) experiment and appeared to also be left-lateralized in Duarte et al. (2006); these effects thus seem to be the same as that observed here.
The strong age dependence of the late negative potential in three previous studies and the current results suggests that older adults can use qualitatively different strategies than young adults during source memory tests. The nature of this qualitative difference is currently a matter of speculation that requires further research (see also Wilding and Sharpe, 2004). Two potential explanations are differences in memory search or task scheduling processes. Cycowicz, Friedman, and colleagues have suggested that late negativities in source memory tests – observed over posterior cortex – reflect a directed search for source information in memory (Cycowicz et al., 2001; Friedman et al., 2005). In the current results, the late negativity elicited by old items in elderly adults has a distinct frontocentral scalp distribution and may be related to the two-step decision sequence required by the source memory task: first determining if the word is old or new then judging the link between word and voice. In contrast, new items require only a single decision. For old items, scheduling and differentiating two judgments may place a heavier burden on frontal mechanisms in older than younger adults, and the frontal negativity elicited by old items may reflect a “task scheduling” process. Such a process may be specific to episodic memory tests or may be more generally required in tasks that require sequential decisions about a single stimulus. What can be concluded at present is that this late negative potential is dependent on the integrity of PFC, although it remains possible that some portion of the activity is generated in other regions of cortex that receive input from lateral PFC.
3.3. Reconciling neuroimaging, neuropsychological, and ERP results
Shallice (2003) has pointed out the difficulties inherent in reconciling the results of neuropsychological studies with those from functional neuroimaging, particularly for cognitive processes that are poorly understood, such as control processes involved in episodic memory retrieval. Without an explicit processing model of how a task is performed, it is impossible to predict the effects of a lesion (Shallice, 2003). Another caveat is that the degree of lateralization in neuroimaging studies is often relative and not absolute. Thus, a unilateral lesion may not produce a deficit predicted by the neuroimaging data (e.g., Swick and Knight, 1996) if the spared hemisphere can compensate. A different problem is the issue of temporal resolution. Some of the PFC activations reported in neuroimaging studies of episodic memory are likely due to neural activity that occurs after subjects have made a response, so it is difficult to determine if pre-retrieval or post-retrieval processes are implicated in a given experiment. Nevertheless, it is informative to compare results across these different methodologies.
In a PET study, Cabeza and colleagues (2002) demonstrated that source memory retrieval was associated with right-lateralized PFC activity in young adults and in older adults who performed poorly in the task. In contrast, older adults who performed well on the source memory task (and on standardized memory tests) showed activation of bilateral PFC regions. The authors suggested that this bilateral recruitment reflected a compensatory mechanism that enabled the high-performing elders to do as well as the young group. Although the specifics of this finding differed from those observed in the present study, the concept of recruiting compensatory brain networks in aging (see also Reuter-Lorenz, 2002), or performing a memory task using different cognitive strategies, can be applied to our results. In young controls, an early ERP retrieval effect was bilaterally distributed and did not have a frontal focus, probably because the task was so easy for these subjects. In contrast, a left frontal ERP effect was uniquely generated in the elderly during correct item recognition, beginning at 600 ms and continuing for another 600 ms. The observation of left-hemisphere-dominant PFC activity has been reported in fMRI studies of source memory retrieval in young adults (Ranganath et al., 2000; Dobbins et al., 2002), and the extension of similar experimental designs to elderly control subjects will be an important next step.
In conclusion, our study demonstrates that, under appropriate conditions, older adults can adopt alternate memory strategies and recruit compensatory mechanisms to improve performance. Frontal patients were not able to recruit these regions in lateral PFC, thus exhibiting difficulties with the strategic memory and monitoring processes required to effectively encode and retrieve “who said what”.
4. Experimental procedures
4.1. Participants
Participants were 9 patients with unilateral damage to the lateral prefrontal cortex (mean age 67.6 years), 9 age-matched controls (mean 69.7 years, range 63–82 years), and 9 young controls (21.3 years, range 18–27). Detailed characteristics of individual patients are given in Table 1. Frontal patients were selected for single focal lesion visible on CT or MRI scans, primarily caused by infarction in the precentral branch of the middle cerebral artery. Patients with lacunar infarcts or white matter hyperintensities were excluded. Lesions were centered in the posterior portion of Brodmann areas 9 and 46, but damage extended inferiorly and posteriorly to areas 6, 8, 44, and 45 in some individuals. Patients with significant medical complications, psychiatric disturbances, substance abuse, multiple neurological events or dementia were excluded. Lesions were transcribed onto corresponding axial templates by an independent rater and then projected onto a lateral view of the brain by computer software (Fig. 1). All subjects were right handed and were matched approximately for education level (frontals 12.6 years; elderly 14.7 years; young 14.9). English was the primary language for all participants. The subjects were paid for their participation, and signed informed consent statements approved by the Institutional Review Boards of the Martinez Department of Veterans Affairs and the University of California, Davis.
4.2. Stimuli
Stimuli consisted of 480 concrete nouns that were recorded in both a male and female voice (a subset of those from Senkfor and Van Petten, 1998). Mean duration was 621 ms (range = 276–1326 ms), and mean frequency of usage was 17 per million based on Frances and Kucera (1982). These were divided into two halves, presented for study or new at test, matched for mean frequency of usage. The words were initially recorded onto analog tapes (ElectroVoice RE16 microphone, Sony TC-WR87ES tape deck) then low-pass-filtered at 9 kHz (Butter-worth 6-pole) and digitized at a sampling rate of 20 kHz by an analog-digital card (DT 2821) under the control of a personal computer. Each sound file was edited to ensure that the beginning of the file corresponded to the beginning of the word so that the ERPs would be well-synchronized to stimulus onset. Amplitudes were scaled to equate the maximum peak-to-peak values across words. During the actual experiment, the stimuli were played back through the same type of A–D card and were filtered (Grass Auditory Stimulus Control Model). Volume levels were set to a comfortable listening level for each participant.
4.3. Study phase
The experimental session was divided into 12 study/test cycles, preceded by a practice study and test list. Participants were thus forewarned about the nature of the memory test, so that the encoding phases were intentional. Each study list consisted of 20 words, 10 in the male voice and 10 in the female voice. The order of these lists was randomized for each subject. Within each block, the 20-word study list was presented twice in a different random order. Stimuli never repeated across study/test cycles.
Participants were seated in a dim, sound-attenuated booth and performed a gender decision task for each study word. Stimulus words were presented aurally at a rate of one every 3500 ms. The cue words “male” and “female” were displayed in the lower left and right hand corners of the computer monitor; subjects were asked to press the left button on a joystick if the voice of the speaker was male and the right button if the speaker was female. Subjects were told that the gender decision task would help to facilitate later memory for the voice in which each item was spoken.4 After a 90-second retention interval, during which time the experimenter engaged in conversation with the participant and reviewed the task instructions, the memory test phase was presented.
4.4. Test phase
Test lists were visual, and each included 20 new words, 10 studied in a male voice and 10 studied in a female voice. Each test word was displayed in the center of the screen, along with labels designating “old” and “new” responses in the lower left or right corner of the screen. Participants made recognition decisions by pushing one button if the word was old and the other button if the word was new. The stimulus display remained on the screen until a response was given or a maximum of 3300 ms. Hand use was counterbalanced across participants, except for four patients with motor deficits (hemiparesis), who used two fingers of the left hand to make their decisions. Two older control subjects were likewise instructed to use only their left hand for button presses. In the case of words called “old,” subjects then had to decide if the visually presented word had been spoken initially in the male or female voice. A second display was presented 2000 ms after the response, consisting of 5 centrally presented question marks, along with labels in the lower left and right sides of the screen that instructed subjects to push the left button if the word had been presented in the male voice or the right button if the word had been presented in the female voice.
4.5. ERP recording
Electrophysiological signals were recorded from 27 electrode sites using an Electro-Cap, with electrode placements (according to the 10–20 International System) at Fp1, Fp2, Fz, F3, F4, F7, F8, Cz, C3, C4, Pz, P3, P4, T3, T4, T5, T6, O1, and O2, with additional placements at AF3/4, FC5/6, CP5/6, and POz. A linked mastoid reference was used. EOG was monitored by electrodes placed below and lateral to the left eye, also referred to linked mastoids. Signals were amplified (×50,000) and filtered (0.1–100 Hz) via a Grass Neurodata acquisition system. EEG was continuously digitized at 160 Hz per channel and stored for off-line analysis. The recording epoch was 1600 ms.
4.6. Data analysis
Reaction time (RT) and accuracy data were sorted into five conditions: correct rejections (CR), new words correctly identified as new; false alarms (FA), new words erroneously identified as old; hit/hit (H/H), correctly identified old words with correct source classification; hit/miss (H/M), correctly identified old words with incorrect source classification; and misses (Miss), old words erroneously identified as new.
Trials of EEG data contaminated by eye movements, muscle artifact, excessive peak-to-peak deflection (over 100 μV), and amplifier blocking were automatically rejected from the averaged data. Trials with correctable blinks (free of other artifacts) were corrected using an adaptive filtering algorithm developed by Dale (1994). After artifact rejection, ERP averages for each condition were computed for individual subjects; grand averages were computed across subjects separately for the three groups. For the frontal group average, the lateral electrodes were classified as ipsilateral or contralateral to the lesion site because left (or right) scalp sites overlay damaged hemispheres for some patients (n = 7) and intact hemispheres for others (n = 2). For convenience, lateral ERP data from the 2 right frontal patients were flipped so that the left side of the head corresponds to the ipsilesional side. Thus, for all the patients, the left side of the head is the ipsilesional side, and for controls, ERPs on the left were recorded from the left side of the head (i.e., ipsi- and contralesional do not apply to controls). Difference waveforms were derived by subtracting ERPs to new stimuli from ERPs to old stimuli. The data were quantified by computing mean amplitudes in defined latency windows (400–800 and 600–1200 ms windows) relative to a 200 ms pre-stimulus baseline. Follow-up analyses measured mean amplitudes in 100 ms latency windows to quantify effects that were more restricted in latency.
Statistical analyses were carried out on PC and Macintosh computer systems using repeated measures analyses of variance (ANOVAs). Greenhouse–Geisser corrections for multiple comparisons were employed when appropriate; the corrected P values and the uncorrected degrees of freedom are reported. ERP amplitude measures were analyzed by Condition, Group, and Electrode.
Acknowledgments
This work was supported by grants DC03424 from the National Institute on Deafness and Communication Disorders, 98-47 CNS-QUA.05 from the James S. McDonnell Foundation and AG14792 from the National Institute on Aging. Thanks to Bob Knight and Bob Rafal for patient referrals, Lydia Peters for assistance in collecting the data, and Liana Machado for helpful discussions in the early stages of the project.
Footnotes
In work from our laboratories, the difference between hits and correct rejections begins ∼300 ms for printed words, somewhat later (∼400 ms) for spoken words, and somewhat earlier (∼200 ms) for drawings or photos.
The age effect for both item and source accuracy was consistent across subjects so that, in contrast to our strategy of examining source accuracy in subsets of older adults and frontal patients matched on item accuracy, there was no subset of older adults that could be matched to the young for item accuracy.
The late negative potential observed by Cycowicz et al. (2001) and Friedman et al., (2005) was maximal over parietal and occipital scalp, in contrast to the frontal topography of the late negative difference between studied and unstudied items observed here. However, detailed comparisons of scalp topography are hindered by the use of differential recordings between scalp sites and the mastoids here versus scalp sites and the nose tip in these prior studies.
Preliminary results from the encoding task were presented in Larsen and Swick (2000).
References
- Alexander MP, Stuss DT, Fansabedian N. California Verbal Learning Test: performance by patients with focal frontal and non-frontal lesions. Brain. 2003;126:1493–1503. doi: 10.1093/brain/awg128. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Tulving E. Neuroimaging studies of memory: theory and recent PET results. In: Grafman J, editor. Handbook of Neuropsychology. Vol. 10. Elsevier; Amsterdam: 1995. pp. 439–466. [Google Scholar]
- Butters MA, Kaszniak AW, Glisky EL, Eslinger PJ, Schacter DL. Recency discrimination deficits in frontal lobe patients. Neuropsychology. 1994;8:343–353. [Google Scholar]
- Baldo JV, Delis D, Kramer J, Shimamura AP. Memory performance on the California Verbal Learning Test—II: findings from patients with focal frontal lesions. J Int Neuropsychol Soc. 2002;8:539–546. doi: 10.1017/s135561770281428x. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Price SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Morris LW, Morris RG, Loewen ER. Relations between source amnesia and frontal lobe functioning in older adults. Psychol Aging. 1990;5:148–151. doi: 10.1037/0882-7974.5.1.148. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D, Snodgrass JG. Remembering the color of objects: an ERP investigation of source memory. Cereb Cortex. 2001;11:322–334. doi: 10.1093/cercor/11.4.322. [DOI] [PubMed] [Google Scholar]
- Dale AM. Ph D Dissertation. University of California; San Diego: 1994. Source Localization and Spatial Discriminant Analysis of Event-Related Potentials: Linear Approaches. [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dodson CS, Shimamura AP. Differential effects of cue dependency on item and source memory. J Exper Psychol, Learn, Mem, Cogn. 2000;26:1023–1044. doi: 10.1037//0278-7393.26.4.1023. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, Knight RT. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. J Cogn Neurosci. 2006;18:33–47. doi: 10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D. Dissociations between memory for temporal order and recognition memory in aging. Neuropsychologia. 1997;35:129–141. doi: 10.1016/s0028-3932(96)00073-5. [DOI] [PubMed] [Google Scholar]
- Frances WN, Kucera H. Frequency Analysis of English Usage: Lexicon and Grammar. Houghton Mifflin; Boston: 1982. [Google Scholar]
- Friedman D, Cycowicz YM, Bersick M. The late negative episodic memory effect: the effect of recapitulating study details at test. Cogni Brain Res. 2005;23:185–198. doi: 10.1016/j.cogbrainres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Gershberg FB, Shimamura AP. Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychol. 1995;33:1305–1333. doi: 10.1016/0028-3932(95)00103-a. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Polster MR, Routhieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–235. [Google Scholar]
- Glisky EL, Rubin SR, Davidson PS. Source memory in older adults: an encoding or retrieval problem. J Exper Psychol, Learn, Mem, Cogn. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Henkel LA, Johnson MK, De Leonardis DM. Aging and source monitoring: cognitive processes and neuropsychological correlates. J Exp Psychol Gen. 1998;127:251–268. doi: 10.1037//0096-3445.127.3.251. [DOI] [PubMed] [Google Scholar]
- Incisa della Rocchetta A, Milner B. Strategic search and retrieval inhibition: the role of the frontal lobes. Neuropsychologia. 1993;31:503–524. doi: 10.1016/0028-3932(93)90049-6. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Kritchevsky M, Squire LR. Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behav Neurosci. 1989a;103:548–560. doi: 10.1037//0735-7044.103.3.548. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989b;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Johansson M, Stenberg G, Lindgren M, Rosen I. Memory for perceived and imagined pictures: an event-related potential study. Neuropsychologia. 2002;40:986–1002. doi: 10.1016/s0028-3932(01)00148-8. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay SD. Source monitoring. Psychol Bull. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Johnson MK, O'Connor M, Cantor J. Confabulation, memory deficits, and frontal dysfunction. Brain Cogn. 1997;34:189–206. doi: 10.1006/brcg.1997.0873. [DOI] [PubMed] [Google Scholar]
- Joyce CA, Paller KA, McIsaac HK, Kutas M. Memory changes with normal aging: behavioral and electrophysiological measures. Psychophysiology. 1998;35:559–678. [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional–neuroanatomic correlates of recollection: implications for models of recognition memory. J Neurosci. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol. 1999;101:159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- Kuo TY, Van Petten C. Prefrontal engagement during source memory retrieval depends on the prior encoding task. J Cog Neurosci. doi: 10.1162/jocn.2006.18.7.1133. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J, Swick D. Left frontal lesions eliminate the ERP repetition effect during repeated encoding of item and source information. Abstr-Soc Neurosci. 2000;26:973. [Google Scholar]
- Lee AC, Robbins TW, Smith S, Calvert GA, Tracey I, Matthews P, Owen AM. Evidence for asymmetric frontal-lobe involvement in episodic memory from functional magnetic resonance imaging and patients with unilateral frontal-lobe excisions. Neuropsychologia. 2002;40:2420–2437. doi: 10.1016/s0028-3932(02)00081-7. [DOI] [PubMed] [Google Scholar]
- Li J, Morcom AM, Rugg MD. The effects of age on the neural correlates of successful episodic retrieval: an ERP study. Cogn Affect Behav Neurosci. 2004;4:279–293. doi: 10.3758/cabn.4.3.279. [DOI] [PubMed] [Google Scholar]
- Logan J, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Mangels JA. Strategic processing and memory for temporal order in patients with frontal lobe lesions. Neuropsychology. 1997;11:207–221. doi: 10.1037//0894-4105.11.2.207. [DOI] [PubMed] [Google Scholar]
- Mark RE, Rugg MD. Age effects on brain activity associated with episodic memory retrieval. An electrophysiological study. Brain. 1998;121:861–873. doi: 10.1093/brain/121.5.861. [DOI] [PubMed] [Google Scholar]
- McAndrews MP, Milner B. The frontal cortex and memory for temporal order. Neuropsychologia. 1991;29:849–859. doi: 10.1016/0028-3932(91)90051-9. [DOI] [PubMed] [Google Scholar]
- Milner B. Some effects of frontal lobectomy in man. In: Warren JM, Akert K, editors. The Frontal Granular Cortex and Behavior. McGraw Hill; New York: 1964. pp. 313–334. [Google Scholar]
- Nolde SF, Johnson MK, D'Esposito M. Left prefrontal activation during episodic remembering: an event-related fMRI study. NeuroReport. 1998;26:3509–3514. doi: 10.1097/00001756-199810260-00032. [DOI] [PubMed] [Google Scholar]
- Olichney J, Van Petten C, Paller K, Salmon D, Iragui V, Kutas M. Word repetition in amnesia: electrophysiological evidence of spared and impaired memory. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Welsh RC, Marshuetz C, Gutchess AH, Mikels J, Polk TA, Noll DC, Taylor SF. Working memory for complex scenes: age differences in frontal and hippocampal activations. J Cogn Neurosci. 2003;15:1122–1134. doi: 10.1162/089892903322598094. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D'Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. J Neurosci. 2000;5:RC108. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapcsak SZ, Reminger SL, Glisky EL, Kaszniak AW, Comer JF. Neuropsychological mechanisms of false facial recognition following frontal lobe damage. Cogn Neuropsychol. 1999;16:267–292. [Google Scholar]
- Raz N, Gunning FM, Head D, Dupois JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P. New visions of the aging mind and brain. Trends Cogn Sci. 2002;6:394–400. doi: 10.1016/s1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Memory, amnesia, and frontal lobe dysfunction. Psychobiol. 1987;15:21–36. [Google Scholar]
- Schacter DL, Kaszniak A, Kihlstrom JF, Valdiserri M. The relation between source memory and aging. Psychol Aging. 1991;6:559–568. doi: 10.1037//0882-7974.6.4.559. [DOI] [PubMed] [Google Scholar]
- Senkfor AJ. Episodic action memory: characterization of the time course and neural circuitry. In: Stamenov M, Gallese V, editors. Mirror Neurons and the Evolution of the Brain. John Benjamins Publishing Company; Amsterdam: 2002. pp. 87–99. [Google Scholar]
- Senkfor AJ, Van Petten C. ERP measures of source and item memory in young and elderly subjects. Psychophysiology. 1996;33:S77. [Google Scholar]
- Senkfor AJ, Van Petten C. Who said what: an event-related potential investigation of source and item memory. J Exper Psychol, Learn, Mem, Cogn. 1998;24:1005–1025. doi: 10.1037//0278-7393.24.4.1005. [DOI] [PubMed] [Google Scholar]
- Senkfor AJ, Van Petten C, Kutas M. Episodic action memory for real objects: an ERP investigation with perform, watch, and imagine action encoding tasks versus a non-action encoding task. J Cogn Neurosci. 2002;14:402–419. doi: 10.1162/089892902317361921. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shallice T. Functional imaging and neuropsychology findings: how can they be linked? NeuroImage. 2003;20:S146–S154. doi: 10.1016/j.neuroimage.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. Memory and the frontal lobe. In: Gazzaniga MS, editor. The Cognitive Neurosciences. MIT Press; Cambridge, MA: 1995. pp. 803–813. [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–814. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Jurica PJ, Mangels JA, Gershberg FB, Knight RT. Susceptibility to memory interference effects following frontal lobe damage: findings from tests of paired-associate learning. J Cogn Neurosci. 1995;7:144–152. doi: 10.1162/jocn.1995.7.2.144. [DOI] [PubMed] [Google Scholar]
- Simons JS, Dodson CS, Bell D, Schacter DL. Specific- and partial-source memory: effects of aging. Psychol Aging. 2004;19:689–694. doi: 10.1037/0882-7974.19.4.689. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Memory for facts, source and context: can frontal lobe dysfunction explain age-related differences? Psychol Aging. 1994;9:149–159. doi: 10.1037//0882-7974.9.1.149. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Palumbo CL, Buckle L, Sayer L, Pogue J. Organizational strategies of patients with unilateral or bilateral frontal lobe injury in word learning tasks. Neuropsychology. 1994;8:355–373. [Google Scholar]
- Swick D, Knight RT. Is prefrontal cortex involved in cued recall? A neuropsychological test of PET findings. Neuropsychologia. 1996;34:1019–1028. doi: 10.1016/0028-3932(96)00011-5. [DOI] [PubMed] [Google Scholar]
- Swick D, Knight RT. Event-related potentials differentiate the effects of aging on word and nonword repetition in explicit and implicit memory tasks. J Exper Psychol, Learn, Mem, Cogn. 1997;23:123–142. doi: 10.1037//0278-7393.23.1.123. [DOI] [PubMed] [Google Scholar]
- Swick D, Knight RT. Contributions of prefrontal cortex to recognition memory: electrophysiological and behavioral evidence. Neuropsychology. 1999;13:155–170. doi: 10.1037//0894-4105.13.2.155. [DOI] [PubMed] [Google Scholar]
- Thaiss L, Petrides M. Source versus content memory in patients with a unilateral frontal cortex or a temporal lobe excision. Brain. 2003;126:1112–1126. doi: 10.1093/brain/awg112. [DOI] [PubMed] [Google Scholar]
- Trott CT, Friedman D, Ritter W, Fabiani M. Item memory and source memory: differential age effects revealed by event-related potentials. NeuroReport. 1997;8:3373–3378. doi: 10.1097/00001756-199710200-00036. [DOI] [PubMed] [Google Scholar]
- Trott CT, Friedman D, Ritter W, Fabiani M, Snodgrass JG. Episodic priming and memory for temporal source: event-related potentials reveal age-related differences in prefrontal functioning. Psychol Aging. 1999;14:390–413. doi: 10.1037//0882-7974.14.3.390. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of Memory. Academic Press; New York: 1972. pp. 381–403. [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Senkfor AJ, Newberg WM. Memory for drawings in locations: spatial source memory and event-related potentials. Psychophysiology. 2000;37:551–564. [PubMed] [Google Scholar]
- Van Petten C, Plante E, Davidson PSR, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42:1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Schacter DL, Cook SP. The effect of retrieval instructions on false recognition: exploring the nature of the gist memory impairment in amnesia. Neuropsychologia. 2002;40:2360–2368. doi: 10.1016/s0028-3932(02)00074-x. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Rapcsak SZ, Keane MM, Alexander MP. Elevated false recognition in patients with frontal lobe damage is neither a general nor a unitary phenomenon. Neuropsychology. 2004;18:94–103. doi: 10.1037/0894-4105.18.1.94. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood. Estimates of linear and non-linear age effects and structural models. Psychol Bull. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Wegesin DJ, Friedman D, Varughese N, Stern Y. Age-related changes in source memory retrieval: an ERP replication and extension. Cog Brain Res. 2002;13:323–338. doi: 10.1016/s0926-6410(01)00126-4. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- West R. A defense of the frontal lobe hypothesis of cognitive aging. J Int Neuropsychol Soc. 2000;6:727–729. doi: 10.1017/s1355617700666109. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J Neurosci. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT. Remembering and knowing in patients with frontal lobe injuries. Cortex. 2003;39:827–846. doi: 10.1016/s0010-9452(08)70866-9. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Sharpe H. The influence of response-time demands on electrophysiological correlates of successful episodic retrieval. Cogn Brain Res. 2004;18:185–195. doi: 10.1016/j.cogbrainres.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Doyle MC, Rugg MD. Recognition memory with and without retrieval of context: an event-related potential study. Neuropsychologia. 1995;33:743–767. doi: 10.1016/0028-3932(95)00017-w. [DOI] [PubMed] [Google Scholar]


