Abstract
HLA-A2-restricted CTL responses to immunodominant HIV-1 epitopes do not appear to be very effective in the control of viral replication in vivo. In this study, we studied human CD8+ T cell responses to the subdominant HLA-A2-restricted epitope TV9 (Gag p2419−27, TLNAWVKVV) to explore the possibility of increasing its immune recognition. We confirmed in a cohort of 313 patients, infected by clade B or clade C viruses, that TV9 is rarely recognized. Of interest, the functional sensitivity of the TV9 response can be relatively high. The potential T cell repertoires for TV9 and the characteristics of constituent clonotypes were assessed by ex vivo priming of circulating CD8+ T cells from healthy seronegative donors. TV9-specific CTLs capable of suppressing viral replication in vitro were readily generated, suggesting that the cognate T cell repertoire is not limiting. However, these cultures contained multiple discrete populations with a range of binding avidities for the TV9 tetramer and correspondingly distinct functional dependencies on the CD8 coreceptor. The lack of dominant clonotypes was not affected by the stage of maturation of the priming dendritic cells. Cultures primed by dendritic cells transduced to present endogenous TV9 were also incapable of clonal maturation. Thus, a diffuse TCR repertoire appeared to be an intrinsic characteristic of TV9-specific responses. These data indicate that subdominance is not a function of poor immunogenicity, cognate TCR repertoire availability, or the potential avidity properties thereof, but rather suggest that useful responses to this epitope are suppressed by competing CD8+ T cell populations during HIV-1 infection.
Virus-specific CD8+ T cells play an important role in the suppression of HIV-1 replication during active infection. Studies have clearly shown an association between CD8+ CTL depletion and viremia in macaques, and links between certain HLA class I alleles and disease progression have been identified consistently (reviewed in Refs. 1–3). It is also well established that the virus can rapidly evade host CTL responses through mutation of targeted epitopes, resulting in diminished viral recognition and reduced immune control (4–10). Because CTL escape mutations that do not significantly compromise viral fitness tend to be maintained when transmitted to a HLA-matched recipient (11–13), common HLA class I types are more likely to accumulate variants over time than rare alleles, which may account for HLA-related risk of progression to AIDS (14, 15).
HLA-A2, the most prevalent allele worldwide (16), must be considered in terms of coverage in the development of HIV-1 vaccines. Although reduced infection risk with possession of the HLA-A2 supertype was reported for two Nairobi cohorts (17), HLA-A2-restricted CTL responses to clade B virus infection do not appear to suppress virus effectively in infected individuals (18–20). This has led to the suggestion that useful HLA-A2-restricted epitopes in circulating clade B viruses may have been lost because the clade B epidemic is historically older than the clade C epidemic and HLA-A2 is more prevalent in the West than in Africa (19, 20). Loss of epitopes restricted by MHC alleles with high frequencies was also supported by studies in HIV-1 clade C-infected cohorts (21, 22) and in SIV-infected macaques (23).
HIV-1-specific CTLs primed directly ex vivo from healthy sero-negative donors can provide complementary insights to findings in infected people, particularly into the pre-existing repertoire of T cell clonotypes and the immunological characteristics of T cells immediately after priming, in the absence of immune responses engendered to competing epitopes (24). With this approach, we have shown that CTLs to the HLA-A2-restricted immunodominant SL9 epitope in Gag p1777−85 are aberrantly sensitive to cytokine-mediated activation-induced cell death in vitro. At the same time, these T cells are capable of producing sufficient autocrine factors to support prolonged periods of proliferation (24, 25). These characteristics provide a plausible explanation for the paucity of circulating SL9-specific CD8+ T cell reactivity during the proinflammatory acute phase of infection, as well as its paradoxical dominance during the CD4-diminished chronic phase of infection (18, 26, 27). The observation that SL9 and its mutations are highly conserved across clades (28), often emerging sequentially or even re-emerging within the same infection (18, 29), is consistent with the idea that this epitope may oscillate around an optimal viral “solution” to a host population rich in HLA-A2 alleles (22). Indeed, SL9 and its common variants may be “optimized” to elicit ineffectual CTL responses (30). Furthermore, immunodominant SL9-specific CTLs may be expected to mask responses directed to subdominant epitopes (31, 32). In doing so, dominant epitopes may have ironically preserved efficacious subdominant target sequences within the viral genome. Consistent with this notion, subdominant epitopes that can control virus in vivo were recently reported in infected patients (21).
Gag-specific CTLs may be especially important for HIV-1 control (33, 34). In particular, the extreme conservation and intolerance of escape variations in Gag p24 indicate that it may be a critical target for vaccine design (35, 36). There is only one well-defined HLA-A2-restricted epitope in p24, abbreviated as TV9 (TLNAWVKVV, Gag p2419−27). This epitope resides in the first of seven α-helices in the N terminus (37), a region under rigid functional constraints (38). It is not surprising that TV9 is highly conserved across clades, with one common variant (155 of 371 (28)) where valine in position 9 is “conservatively substituted” by isoleucine (39). Of interest, TV9 shares five residues in common with the HLA-B57-restricted ISPRTLNAW (IW9) peptide recognized by early CD8+ T cell responses in long-term nonprogressors (9, 40−42). However, there have only been sporadic reports of TV9-specific CD8+ T cell responses during infection. TV9 reactivity was detected, but was not the dominant HLA-A2-restricted response, in HLA-A2+-exposed seronegative sex workers (43, 44). Persistently high numbers of TV9-specific IFN-γ-producing cells were reported for one patient (45). Most intriguingly, a vigorous TV9-specific response in one HLA-A2+ individual was associated with absence of infection after accidental parenteral exposure to high HIV-1 load (46). To understand this dominance pattern in more detail, we used ex vivo priming to determine whether TV9-specificity is underrepresented in the T cell repertoire of healthy seronegative donors. Understanding how human T cells respond to such a subdominant HLA-A2-restricted epitope may provide insights that will help in the design of HIV-1 vaccines for HLA-A2+ individuals (47).
Materials and Methods
Healthy seronegative donors
Heparinized blood was collected from healthy seronegative HLA-A*0201 volunteers in the Detroit area. High-resolution HLA genotyping was performed by the Immunogenetics Laboratory at the National Cancer Institute. This study was approved by the Human Investigation Committee at Wayne State University School of Medicine, and all subjects provided written informed consent before enrollment.
Ex vivo priming of CD8+ T cells with monocyte-derived dendritic cells (DCs)3
PBMCs were isolated using lymphocyte separation medium (Mediatech). Immature DCs (iDCs) were derived from plastic-adherent monocytes after 7 days in RPMI 1640 medium with 10% autologous serum (“complete medium”) supplemented with GM-CSF (1000 U/ml; Amgen/Immunex) and IL-4 (500 U/ml; R&D Systems). Matured DCs (mDCs) were generated by an overnight exposure to 1 μg/ml LPS (Escherichia coli serotype 026: B6, Sigma-Aldrich). Isolated T cells were expanded with 30 ng/ml anti-CD3 mAb (Orthoclone OKT3, Ortho Biotech) and 50 U/ml IL-2 (Chiron) as described previously (48). Positively selected CD8+ T cells (Dyna-beads; Dynal Biotech) were primed with irradiated (4000 cGy) peptide-pulsed or transduced DCs at a T cell-DC ratio of 5:1 in 48-well or 96-well cluster plates. Cells were cultured in complete medium containing 10 ng/ml IL-7 (Genzyme) and restimulated every 7−10 days with autologous monocytes pulsed with peptides or transduced DCs (24, 25). IL-2 (20 U/ml) was added 1 and 4 days poststimulation.
Assays of cultured T cells
Cytotoxicity was determined by the chromium release assay (24). Target cells were T2 cells, Jurkat cells cotransfected with HLA-A*0201 and the HIV-1 proviral clone R7hyg (JA2/R7/Hyg) (49), and C1R cells expressing full-length wild-type HLA-A*0201 (C1R-A2wt) or the mutant D227K/T228A HLA-A*0201 (C1R-A2CD8null) that does not bind the CD8 core-ceptor (50).
Wild-type tetrameric recombinant HLA-A*0201-TV9 complexes labeled with allophycocyanin were provided by the National Institutes of Health/National Institute of Allergy and Infectious Diseases Tetramer Core Facility. Tetramer stains were performed on ice for 30 min at a concentration of ∼1 μg/ml with respect to the peptide MHC class I (pMHCI) component. Wild-type HLA-A*0201-TV9 and mutant D227K/T228A HLA-A*0201-TV9 tetramers conjugated to PE were synthesized as described previously (51). Each preparation was titered and used at the lowest effective concentration. T cells were stained with these tetramers at 37°C for 20 min, washed, and then stained for CD8 at 4°C for an additional 20 min (52).
IFN-γ secretion was determined by ELISA with an OptEIA Set (BD Pharmingen) after stimulating T cells with peptide-pulsed T2 or C1R cells for 48 h. EC50s (concentrations of peptide required for 50% of the maximal reactivity) were calculated with GraphPad Prism software.
Directly conjugated mAbs to CD8 (FITC- and PE-RPA-T8), CD54 (PE-HA58), CD80 (PE-L307.4), CD83 (PE-HB15a), CD86 (PE-IT2.2), CD107a (FITC-H4A3), CD107b (FITC-H4B4), HLA-DR (FITC-G46−6), HLA-class I (FITC-BB7.2), IFN-γ (PE-B27), IL-2 (PE-MQ1−17H12), and TNF-α (PE-MAb11) were purchased from BD Pharmingen. TCR Vβ family-specific mAbs were purchased from Beckman Coulter. Stained cells were analyzed with a FACScan or FACSCalibur flow cytometer (BD Biosciences) and WinMDI software. Intracellular cytokine production and degranulation were determined after stimulation for 4 h with peptide-pulsed (10 μg/ml) T2 or C1R cells at 37°C. Anti-CD107a/b mAbs and monensin (GolgiStop; BD Pharmingen) were added at the initiation of the incubation period as described previously (53). Gating was performed on CD8+ T cells, and >10,000 events were collected for each sample.
For IFN-γ ELISPOT analysis, cultured CD8+ T cells were plated at 100,000 cells/well in 96-well polyvinylidene plates (Millipore) coated with anti-IFN-γ mAb (Diaclone). Cells were incubated overnight in the presence of peptides (20 μg/ml) at 37°C in a CO2 incubator. Wells containing CD8+ T cells and medium alone were included as negative controls. ELISPOT plates were developed using Diaclone reagents according to the manufacturer's instructions. Spots were counted using an ImmunSpot Series 1 Analyzer (Cellular Technology), and the numbers of specific spot-forming cells (sfc) were calculated by subtracting those detected in the negative control wells.
Direct ex vivo IFN-γ ELISPOT assays
Patients responsive to the overlapping adapted 18-mer overlapping peptide (OLP) no. 21 were identified with peptide test sets consisting of a peptide matrix containing 410 OLPs spanning the entire HIV-1 clade B and clade C consensus sequences as described previously (21). PBMCs (100,000) were incubated for 16 h with nonameric TV9 peptide at serial 10-fold dilutions from 100 μg/ml to 10 pg/ml in ELISPOT plates. Plates were developed with Mabtech reagents (Mabtech). Half-maximal stimulatory Ag doses (SD50) were determined as the peptide concentration needed to achieve a half-maximal number of spots in the ELISPOT (54). Thresholds for positive responses were determined as at least five spots (50 sfc/106) per well and responses exceeding a mean of negative wells plus 3 SDs.
T2 stabilization assay to assess peptide binding to HLA-A*0201
Exponentially growing T2 cells were incubated with or without peptide at the saturating concentration of 200 μM overnight at 37°C. Cells were then stained with anti-HLA-A2 BB7.2 mAb, followed by a secondary FITC-labeled goat anti-mouse IgG Ab for analysis by flow cytometry (55, 56). The binding activity of each peptide was calculated as a fluorescence ratio (mean fluorescence intensity (MFI) of T2 incubated with peptide/MFI without peptide).
Peptides
The HIV-1 peptides TLNAWVKVV (TV9, Gag p2419−27), TLNAWVKVI (9I), TLNAWVKLV (HIV-2 Gag, 8L), SLYNTVATL (SL9, Gag p1777−85), SLFNTVATL (3F), SLYNTVAAL (SL9 agonist, p41), ILKEPVHGV (IV9, Pol476−484), the influenza matrix peptide GILGFVFTL (GL9, Flu MP58−66), and the tyrosinase368−376 peptide YMNGTMSQV (YV9) were synthesized by Genemed Synthesis. The purity and identity of each peptide were verified by electrospray mass spectrometry interfaced with liquid chromatography. Lyophilized peptides were dissolved in DMSO (10 mg/ml), aliquoted, and stored at −80°C.
HIV-1 vector and DC transduction
The HIV-1 proviral construct pNL4−3 vpr− was pseudotyped with vesicular stomatitis virus glycoprotein envelope and is referred to as pNL4−3 E− (57). Production in HEK293T cells was described previously (57). Virus titers were measured using the HIV-1 reporter cell line cMAGI (AIDS Research and Reference Reagent Program, National Institutes of Health). Four-day-old iDCs were infected with pNL43 E− at a multiplicity of infection of 2, and the percent-transduced DCs were determined 3 days later by flow cytometric determination of intracellular p24 Ag using an anti-p24 Ab (FITC-conjugated mAb KC57; Coulter).
Virus suppression assay
The capacity of TV9-specific CTLs to inhibit HIV-1 replication in acutely infected cells was determined as described previously (58, 59). CD8+ lymphocytes were depleted from PBMCs of HLA-A2+, HIV-1-negative donors with MACS CD8 microbeads (Miltenyi Biotec) and the AutoMACS system (Miltenyi Biotec) in accordance with the manufacturer's protocol. CD8-depleted cells were activated with PHA in complete medium containing IL-2 (25 U/ml) for 2 days and then infected with HIV-1 by overnight incubation with HIV-1JR-CSF (80 TCID50/ml) at 37°C as described previously (60). Infected PBMCs were washed, resuspended in complete medium containing IL-2 (25 U/ml), and plated in a 24-well plate (5 × 105 cells/well); the indicated CTL clones were added at a 1:1 ratio. Supernatant was removed every 2−4 days and analyzed for p24 Ag concentration by ELISA.
Results
TV9 reactivity in HIV-1-infected subjects
Reactivity to TV9 was sought with a set of 410 OLPs spanning the entire HIV-1 protein sequence in a cohort of 313 patients (21). HLA typing was possible for 280 of these samples, of which 121 were HLA-A2+. OLP21 (PRTLNAWVKVVEEKAP, p2417−32) was recognized by 24 subjects, with most of this reactivity (14 of 24) likely directed against the HLA-B*1503-restricted VKV VEEKAF epitope frequently seen in HLA-B*1503-expressing subjects (21). Reactivity to the nonameric TV9 peptide was confirmed in one of two HLA-A2+ HLA-B*1503− samples tested that reacted to OLP21 (L8 47, 500 sfc/106 PBMCs; Fig. 1A). Compared with other targets of the HLA-A2-restricted response, these data identify TV9 as an infrequently targeted epitope in natural HIV infection (61). Interestingly, the frequency and functional sensitivity of TV9-reactive cells were relatively high with a SD50 value of ∼0.0001 μg/ml (54) in L8 47, indicating that the in vivo CD8+ T cell response to this epitope can be highly avid despite its sub-dominance in natural infection. Notably, TV9 bound to HLA-A*0201 with a similar affinity to SL9 (fluorescence ratio of 1.2 ± 0.02 vs 1.3 ± 0.01) as determined by the T2 stabilization assay (Fig. 1B).
FIGURE 1.

A, CD8+ TV9-specific responses in four selected patients by IFN-γ ELISPOT assay. One hundred thousand live PBMCs were incubated overnight with the TV9 peptide at concentrations ranging from 0.01 to 1000 ng/ml. B, Relative binding of TV9, the TV9 variant (9I), the HIV-2 TV9 homolog (8L), SL9, and the flu MP (GL9) peptides to HLA-A*0201 determined by the T2 stabilization assay. GL9 served as the positive control. The fluorescence ratio was calculated as described in the Materials and Methods. Assays were performed in triplicate, and the values represent the mean ± SD. This experiment was repeated twice with essentially identical results.
Ex vivo primed TV9-specific CTLs
To address whether the rare responses to TV9 were due to the absence of an appropriate T cell repertoire, positively selected CD8+ T cells from seronegative donors were stimulated with TV9-pulsed iDCs and restimulated weekly thereafter with peptide-pulsed (10 μg/ml) autologous monocytes as described in Materials and Methods. Fig. 2A shows CD8+ tetramer-binding cells in TV9-immunized cultures from three representative donors (TV9−2, TV9−4.1, and TV9−5) at five time points over 50 days. Significant percentages of the cells were TV9 specific, as indicated by binding to tetramer as early as day 14 (5−12%). The proportion of tetramer+ cells generally increased progressively, representing between 31 and 93% of all cells by day 50. In this study, TV9-specific CTLs were generated from 7−10 donors, suggesting that most people are capable of mounting an immune response to this peptide. TV9-primed tetramer+ populations stained with a broad range of intensities as shown for TV9−2 and TV9−4.1 (Fig. 2A) and TV9−1 and TV9−3 (Fig. 2C). Tetramer staining was more homogeneous for TV9−5, in which a distinct cloud of cognate CD8+ T cells was observed, but this was the only exception in the cultures studied (Fig. 2A). For comparison, established cultures specific for SL9, p41, 3F, or YV9 stained as single homogeneous populations (Fig. 2B). Therefore, the CD8+ T cell repertoire for TV9 appears to be structurally diverse in most people, with a broad range of TCR avidities. Curiously, these diverse binding properties persisted despite prolonged propagation of these cells in vitro, thereby suggesting a lack of competitive advantage for more highly avid clonotypes within these particular populations.
FIGURE 2.
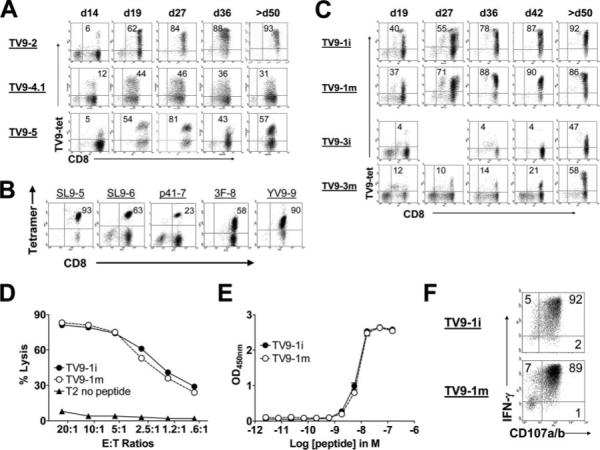
Characteristics of ex vivo-primed TV9-specific CD8+ T cell cultures. A, Tetramer-binding cells in TV9−2, TV9−4.1, and TV9−5 over time. T cells were stained 6 days after restimulation. Numbers in the upper right quadrants are the percentages of CD8+tetramer+ T cells. B, Tetramer-binding cells in SL9-, p41-, 3F-, and YV9-specific CD8+ T cell cultures. Cells were stained with cognate tetramers and anti-CD8 mAb. C, Parallel cultures primed with iDCs or mDCs from donor 1 (TV9−1i and TV9−1m, top two rows) and donor 3 (TV9−3i and TV9−3m, bottom two rows). Cultures were monitored for >50 days. D, Cytotoxicity against T2 cells in the presence of 1 μg/ml cognate peptide by TV9−1i and TV9−1m. This experiment was repeated four times with identical results. E, Functional avidity of TV9−1i and TV9−1m determined by IFN-γ secretion at the E:T ratio of 1:10. EC50s were 6.6 × 10−9 and 7.3 × 10−9 M, respectively. F, Scatter plots showing induction of CD107a/b expression and IFN-γ production by TV9−1i and TV9−1m after stimulation by C1R-A2wt cells pulsed with 10 μg/ml cognate peptide at the E:T ratio of 1:1. Numbers in the upper right quadrants represent the percentages of CD107a/b+ IFN-γ-secreting cells. Nonspecific activation with an irrelevant peptide (YV9) resulted in <1% CD107a/b+ IFN-γ-secreting T cells in both cultures (data not shown).
CTLs specific for the highly immunogenic SL9 epitope can be primed only by iDCs and not mDCs (24). To ascertain whether the maturity of DCs affects induction of TV9-specific CD8+ T cells or the profile of constituent clonotypes, parallel cultures of CD8+ T cells were established with peptide-pulsed iDCs or mDCs from two donors. Fig. 2C shows the percentage of TV9-tetramer-binding CD8+ T cells from donor 1 (TV9−1i and TV9−1m) and donor 3 (TV9−3i and TV9−3m) over time. TV9−1i and TV9−1m contained ∼40% tetramer+ cells soon after one restimulation (day 19), suggesting a high precursor frequency in this individual. Over time, the parallel cultures exhibited almost identical tetramer-binding profiles. The TV9−3i culture from donor 3 contained fewer tetramer-binding cells than TV9−3m at all time points tested (Fig. 2C). mDCs from this donor provoked a stronger and visibly more complex response than iDCs, although high and low tetramer-binding cells were present in both cultures. Nonetheless, the parallel cultures from donor 1 were equally cytotoxic to T2 cells pulsed with TV9 over a range of E:T ratios (Fig. 2D), showed equivalent Ag density requirement (functional avidity) for activation of IFN-γ secretion (Fig. 2E), and contained essentially identical proportions of CD107a/b+ IFN-γ-secreting cells after specific activation (Fig. 2F). Thus, both iDCs and mDCs primed low- and high-avidity TV9-specific CD8+ T cells, although their composition appeared to differ somewhat. These CTLs, however, were indistinguishable as measured by standard functional assays.
TV9 is naturally processed and presented in the context of HLA-A2
To demonstrate that TV9 is naturally processed and presented by APCs, this reactivity was sought in cultures derived from purified circulating CD8+ T cells primed with DCs transduced with a vesicular stomatitis virus glycoprotein-pseudotyped pNL4−3 virus (pNL4−3 E−) encoding Gag and Pol (57). Transduction was verified by staining for intracellular Gag p24. iDCs from the first donor did not mature posttransduction, expressing CD80, CD86 (Fig. 3A, top panels), HLA-A2, HLA-DR, and CD83 (data not shown) at levels equal to DCs that were not transduced in the same culture. Similarly, the uninfected parallel culture also displayed equivalent percentages of CD80- and CD86-expressing cells (Fig. 3A, bottom panels). Although only 20% of the DCs were infected, IFN-γ-secreting cells specific for TV9 (and to the HLA-A2-restricted IV9 epitope in Pol) were detected in TV9−7t as early as 8 days postpriming and 8 days after restimulation with transduced iDCs (day 16 postpriming; Fig. 3B). The frequency of TV9-specific cells increased 5-fold (510−2560 sfc/106 T cells) while that for IV9 increased 3-fold (690−2180 sfc/106 T cells) with restimulation. The number of sfc after stimulation with the non-HIV-1 HLA-A2-restricted peptide, YV9, was <50. Of interest, no reactivity to SL9 was detected, most likely due to the presence of exogenous IL-2 (24). This experiment clearly demonstrates that TV9 is naturally presented and capable of eliciting a specific CD8+ response in vitro. However, this TV9-specific culture failed to expand sufficiently to allow for more detailed characterization. In our experience, transduced DCs are poor APCs for restimulation of cultured peptide-specific CD8+ T cells (24).
FIGURE 3.

Priming of TV9-specific CTLs by DCs transduced with the pNL43 E− vector. A, CD86 and CD80 expression by iDCs from donor 7 4 days after transduction (top panels). Transduction was visualized by intracellular p24 expression. A parallel culture of nontransduced iDCs is shown in the bottom panels. B, Reactivity of TV9−7t CD8+ T cells to SL9, TV9, and IV9 as determined by IFN-γ ELISPOT assay after one (d8) and two (d16) stimulations with transduced DCs. C, Transduced iDCs from donor 2; details as for A above. D, Tetramer-binding TV9−2t CD8+ T cells on days 26 and 54. E, Cytotoxicity of TV9−2t against T2 cells in the presence of cognate peptide. T2 cells without peptide were included as a negative control.
The next T cell culture (TV9−2t) was restimulated with TV9-pulsed monocytes after priming by transduced DCs. DCs from this donor were transduced efficiently, with >90% of the cells staining positively for intracellular p24 (Fig. 3C, top panels). Moreover, they acquired a matured phenotype after transduction, as judged by increased expression of CD80, CD86 (Fig. 3C, top panels), CD54 and HLA-DR (data not shown) as compared with uninfected cells (Fig. 3C, bottom panels). The TV9−2t culture primed by these DCs and expanded by TV9-pulsed autologous monocytes contained TV9-specific cells as determined by tetramer staining (18 and 72% on days 26 and 54, respectively; Fig. 3D). As with TV9 cultures primed by TV9 peptide-pulsed DCs, TV9−2t contained TV9-specific cells with a range of avidities for the tetramer. Moreover, TV9-specific CD8+ T cells in TV9−2t were cytotoxic for TV9-pulsed T2 cells specifically (Fig. 3E). These results show that multiple populations of functionally competent TV9-specific CD8+ T cells were primed by DCs presenting “natural” levels of TV9: MHCI complexes. Therefore, the complex profile of tetramer binding cells is likely a reflection of a diverse human CD8+T cell repertoire for this epitope and not an artifact resulting from excessive stimulation by peptide-pulsed DCs expressing an unnatural density of TV9:MHCI complexes.
Role of the CD8 coreceptor in the structurally diverse TV9 response
CD8 contributes substantially to the mobilization of CD8+ T cell effector functions, i.e., cytolytic activity and cytokine expression, in response to cognate Ag engagement (62). The degree to which individual CD8+ T cells depend on the pMHCI/CD8 interaction for stable tetramer binding can be evaluated by using pMHCI tetramers carrying mutations in the α3 domain that abrogate CD8 binding without affecting TCR recognition (CD8null) (51, 63–66). Fig. 4A shows four TV9 cultures stained with the wild-type (top row) or the CD8null TV9 tetramers (bottom row). Equal numbers of TV9−1 cells (89%) bound to both tetramers, indicating that the majority had sufficient avidity for the pMHCI ligand to bind without an absolute requirement for CD8 (high “intrinsic” avidity). In contrast, of the TV9−2 cells stained by the wild-type tetramer (96% of total), only a fraction (6% of total) could bind to the point-mutated CD8null reagent. Thus, most of the cells in TV9−2 required CD8 compensation to stabilize the TCR/pMHCI interaction, consistent with a lower intrinsic avidity compared with TV9−1 cells. For TV9−4.1 and TV9−5, approximately half of the TV9-specific CD8+ T cells were capable of binding to CD8null tetramers. Two populations with distinct (high and low) fluorescence intensities for the A2wt tetramer were noted in TV9−5. Analysis of CD107a/b expression after stimulation with TV9-pulsed C1R-A2wt cells also revealed two functional subsets in this culture (data not shown). Because binding by tetramer activates T cells, tetramer was not used for this analysis. It is highly likely that the two populations of high and low tetramer-binding cells are also functionally distinct.
FIGURE 4.
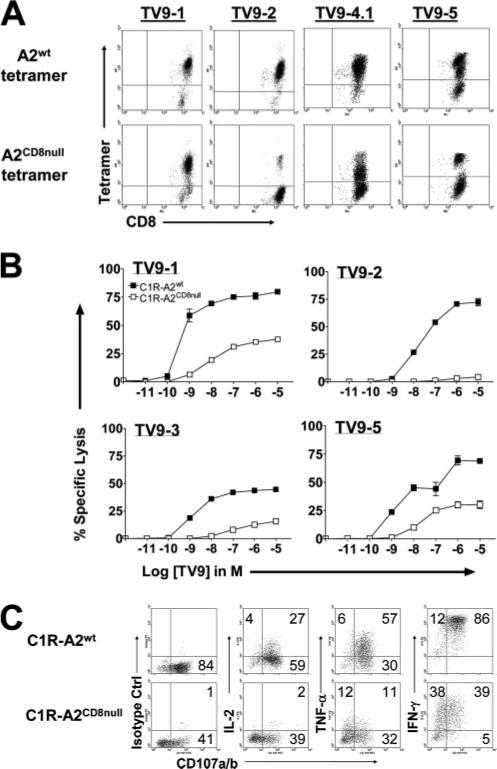
Participation of the CD8 coreceptor in tetramer binding and functional activation of TV9-specific T cells. A, TV9-specific cultures stained with wild-type or CD8null tetramers and anti-CD8 mAb. B, Lysis of C1R-A2wt or C1R-A2CD8null cells pulsed with a range of TV9 concentrations by TV9−1, TV9−2, TV9−3, and TV9−5 at the E:T ratio of 2.5:1. Experiments were repeated at least twice for TV9−1, TV9−2, and TV9−5 with similar results. C, Flow cytometric analysis of IL-2, TNF-α, and IFN-γ production and degranulation (CD107a/b) by TV9−3 after a 4-h stimulation with C1R-A2wt or C1R-A2CD8null cells loaded with a saturating concentration of TV9 (10 μg/ml). The number in each quadrant represents the percentage of cells within that quadrant. Plots are gated on live CD8+ T cells.
The relationship between TCR engagement of the pMHCI ligand and the triggering of downstream functional events was further analyzed by testing the ability of TV9-specific T cells to respond to C1R cells generated to express equivalent levels of wild-type or CD8null HLA-A*0201 molecules (51, 67). First, the role of CD8 in cytotoxicity under limiting antigenic stimulation was assessed using these target cells loaded over a range of peptide concentrations (10–12–10–5 M; Fig. 4B). Functional avidities (peptide concentrations at 50% maximal lysis) of the cultures were estimated for both sets of target cells. For C1R cells expressing wild-type HLA-A*0201, the range of functional avidities for TV9-specific cultures was broader than those published for SL9-specific CTLs (10−8−10−10 as compared with 10−8−10−9 M (24)), consistent with different representation of low-avidity cells among TV9 cultures. Substantial differences in functional sensitivity according to the nature of the target cells were also observed. C1R cells bearing mutant HLA-A*0201 were invariably poorer targets than C1R cells expressing wild-type HLA-A*0201 at equivalent peptide concentrations as shown for four TV9 cultures (Fig. 4B). Lysis of the CD8null C1R cells required two to three orders of magnitude greater Ag density. In fact, TV9−2 was completely noncytotoxic to CD8null cells, despite the presence of 10−5 M peptide. Among the TV9 cultures, dependence on CD8 for cytotoxicity was greatest for TV9−2 cells, which was also the least able to bind to the CD8null tetramer (Fig. 4A). For each E:T cell combination, CD8 was more important for T cell activation under limiting Ag density, consistent with previous reports (51, 65).
Cytokines secreted by activated CD8+ T cells are tightly regulated because they are cytopathic and invariably associated with some degree of immunopathology (68). Therefore, we examined whether CD8 plays a different role relating Ag avidity to sensitivity with respect to degranulation (cytotoxicity) and production of three cytokines. Five TV9 cultures were stimulated with C1R cells loaded with a saturating concentration of peptide (10−5 M) for 4 h. The scatter plots in Fig. 4C show the distribution of TV9-specific CD8+ T cells that degranulated (CD107a/b+) and secreted one of three cytokines (IL-2, TNF-α, and IFN-γ) after specific stimulation with wild-type (top row) or mutant A*0201 C1R cells (bottom row) in a representative TV9 culture (TV9−3). Table I summarizes the results for five TV9 cultures. Of the 60−84% of the cells that degranulated when stimulated by C1R cells expressing wild-type HLA-A*0201, approximately half (49−61%) were activated by the CD8null counterpart. With one exception (TV9−2), degranulation was consistent with cytotoxicity shown in Fig. 4B. Fifty-two percent of the TV9−2 cells expressed CD107a/b after interacting with TV9-pulsed C1RCD8null cells, which was more than expected as judged by their poor binding to the CD8null tetramer and minimal cytotoxicity directed at C1RCD8null cells. Thus, degranulation was transduced by a TCR with an intrinsic avidity that was too low to affect physical binding to tetramer without help from CD8. This is consistent with the observation that CTLs can exhibit cytotoxicity without stable synapse formation (69). Overall, a significant proportion of the TV9-specific CD8+ T cells in all five cultures were dependent on CD8 to activate production of IL-2, TNF-α, and IFN-γ. This is consistent with the previously reported hierarchical differences in Ag sensitivity required for activation of these respective effector functions (70, 71).
Table I.
Degranulation and cytokine secretion by three-color flow cytometry after stimulation of TV9-T cell cultures with C1R-A2wt or C1R-A2CD8null cells pulsed with TV9
| % T Cells Expressing |
||||||||
|---|---|---|---|---|---|---|---|---|
| CTL Culture | HLA-A2 CIR Stimulator Cells | CD107a/b | IL-2 | CD107 a/b and IL-2 | TNF-α | CD107 a/b and TNF-α | IFN-γ | CD107 a/b and IFN-γ |
| TV9−1 | Wild type | 80 (100%) | 19 (100%) | 15 (100%) | 18 (100%) | 15 (100%) | 92 (100%) | 78 (100%) |
| CD8null | 49 (61%) | 3 (16%) | 3 (20%) | 10 (55%) | 7 (46%) | 56 (61%) | 39 (50%) | |
| TV9−2 | Wild type | 79 (100%) | 15 (100%) | 14 (100%) | 44 (100%) | 41 (100%) | 91 (100%) | 76 (100%) |
| CD8null | 41 (52%) | 0 (0%) | 0 (0%) | 16 (36%) | 11 (27%) | 55 (60%) | 35 (46%) | |
| TV9−3 | Wild type | 84 (100%) | 29 (100%) | 25 (100%) | 61 (100%) | 55 (100%) | 96 (100%) | 84 (100%) |
| CD8null | 41 (49%) | 1 (3%) | 1 (4%) | 22 (36%) | 10 (18%) | 76 (79%) | 38 (45%) | |
| TV9−4.1 | Wild type | 81 (100%) | 1 (NR)a | 1 (NR) | 12 (100%) | 11 (100%) | 43 (100%) | 41 (100%) |
| CD8null | 45 (56%) | 1 (NR) | 1 (NR) | 4 (33%) | 3 (27%) | 20 (47%) | 17 (41%) | |
| TV9−5 | Wild type | 60 (100%) | 36 (100%) | 33 (100%) | 49 (100%) | 47 (100%) | 87 (100%) | 60 (100%) |
| CD8null | 31 (51%) | 31 (86%) | 19 (58%) | 25 (51%) | 20 (43%) | 53 (61%) | 31 (52%) | |
NR, not relevant.
TCR Vβ usage by TV9 cultures
To examine clonotypic diversity, TCR Vβ usage of TV9 tetramer+ cells was assessed with a panel of mAbs specific for individual Vβ sequences representing ∼70% of TCRBV genes. The concentration of mAbs used in these experiments was determined a priori not to interfere with tetramer binding. Fig. 5 summarizes the results for four cultures. TV9−2 was comprised of 97% tetramer+ cells (Fig. 5A); of these, 85% were Vβ3+ (Fig. 5B) and 5% were Vβ8+ (Fig. 5C). The remaining 10% of tetramer+ cells were not recognized by the mAbs used. Judging from MFI, the numerically dominant Vβ3+ cells bound the cognate tetramer with lower avidity than Vβ8+ cells. Six Vβ chains were associated with tetramer-binding cells in TV9−4 (19%; Fig. 5A), none of which was particularly dominant. Three represented only 1% of the cells in culture (Vβ1, Vβ7, and Vβ16); Vβ8, Vβ14, and Vβ22 stained ∼4 −5%. TV9−1 was essentially tetramer homogeneous (98%; Fig. 5A). The seven Vβ sequences identified accounted for most of these cells. Dominant clonotypes were Vβ2 (22%), Vβ8 (20%), and Vβ22 (33%). With the exception of Vβ13.1+ cells, all stained strongly for the tetramer. Two discrete tetramer+ populations were identified among Vβ22+ (MFI of 193 and 1288) and Vβ2+ cells (MFI of 117 and 387), suggesting distinct clonotypes with common TCRBV gene usage and different avidities for Ag. The TV9−2t culture, primed with transduced DCs and expanded with TV9-pulsed monocytes, contained 95% tetramer+ cells. Five of the six Vβ sequences identified by the panel of mAbs represented subdominant populations present at 1−4% of the cells. The dominant population was Vβ14+ at 58%. These results showed that TV9-specific clonotypes that stained with different intensities by the tetramer were structurally diverse. Moreover, there was no correlation between the frequency of a particular clonotype and its avidity for tetramer, again suggesting a lack of interclonal competition in these cultures. Clonal diversity was maintained for at least 45 days of continuous culture during which the T cells were estimated to have undergone >20 rounds of division, more than the number of divisions reported for the full expansion phase of a primary CTL response (72). A diffuse staining pattern for CD8+ responses was reported recently for an immunodominant HIV-1 Nef epitope in HLA-B*08+ patients (73), indicating that a lack of clonal focusing may also be characteristic of certain CD8+ T cell responses in vivo.
FIGURE 5.

TCR Vβ composition of tetramer-binding cells in four representative TV9-specific cultures. Each culture was stained with tetramer, anti-CD8 mAb, and a TCR Vβ-specific mAb at a concentration shown a priori not to interfere with tetramer binding. Panels on the left show CD8+ tetramer+ cells in TV9−2, TV9−4, TV9−1, and TV9−2t cultures. Panels on the right are gated on CD8+ cells, and the inset numbers represent the percentages of tetramer+ cells that stain with each depicted TCR Vβ-specific mAb.
Characterization of constituent clonotypes with different TCR Vβ usage
To dissect further the interclonal relationships in these TV9-specific CD8+ T cell populations, Vβ-specific T cells were sorted from TV9−2 for expansion by anti-CD3 mAb and IL-2. In this manner, two homogeneous Vβ-specific CD8+ T cell subcultures were derived: TV9−2(Vβ3) and TV9−2 (Vβ8). Fig. 6A shows the binding avidity of TV9−2(Vβ3) and (Vβ8) subsets to wild-type and CD8null tetramers. TV9−2(Vβ3) CTLs bound only to the wild-type and not at all to the CD8null tetramer. In contrast, TV9−2(Vβ8) cells stained with both tetramers equally, indicating a high intrinsic avidity for the cognate pMHCI molecules. Functionally, the more avid TV9−2(Vβ8) cells lysed TV9-pulsed C1R-A2wt targets (Fig. 6B, top panel) more efficiently than the parental (TV9−2) or TV9−2(Vβ3) culture over a range of peptide concentrations. Maximal lysis (50−60%) was achieved by the TV9−2 and its subcultures at 10−6 M. However, when the available peptide was limiting (10−9 M), low-avidity TV9−2(Vβ3) CTLs were noncytotoxic. These cultures were also tested for recognition of C1R-A2CD8null targets (Fig. 6B, bottom panel). In the absence of CD8 participation, cytotoxicity was reduced greatly for all cultures. TV9−2(Vβ8) cells were least dependent on CD8, lysing 50% of targets at 10−6 M. In contrast, TV9−2(Vβ3) cells exhibited minimal lysis (4%). Because the parental TV9−2 culture consisted of mostly low-avidity Vβ3+ cells, it was less cytotoxic than high-avidity Vβ8 cells with a maximum lysis of 30%. A parallel study of these cultures was performed to measure IFN-γ release following stimulation with peptide-pulsed C1R cells for 48 h (Fig. 6C). Although all TV9−2 lines produced essentially equal quantities of IFN-γ after stimulation by C1R-A2wt targets and high concentrations of the peptide, low-avidity TV9−2(Vβ3) CTLs produced the lowest amounts when peptide was limiting (Fig. 6C, top panel). Only TV9−2(Vβ8) CTLs were capable of responding to C1RA2CD8null targets pulsed by TV9 peptides. TV9−2(Vβ3) cells failed to secrete IFN-γ over the entire range of peptide concentrations, suggesting that cytokine secretion is particularly dependent on CD8 compensation for low-avidity T cells. Failure to secrete IFN-γ by the parental culture is likely explained by the predominance of low-avidity Vβ3+ T cells, although interclonal interference cannot be excluded. Lastly, Fig. 6D compares the suppression of viral replication by the parental TV9−2 and its constituent sub-clones in three allogeneic, HLA-A2-matched CD8-depleted PBMC cultures that were infected acutely with JR-CSF HIV-1. TV9−2 and TV9−2(Vβ8) CTLs were for the most part equally inhibitory against each of the three cultures (between 30 and 60%). In contrast, TV9−2(Vβ3) cells were minimally suppressive, if at all, in all three cultures. In summary, there was direct correlation between structural avidity for tetramer and the downstream functions measured. Moreover, participation by the CD8 coreceptor in the activation of low-avidity T cells was seemingly more crucial for the facilitation of less sensitive effector functions.
FIGURE 6.

Characterization of TV9-specific CD8+ T cells sorted according to different TCR Vβ usage from the TV9−2 culture. A, TV9−2(Vβ3+) and TV9−2(Vβ8+) T cells stained with the wild-type (top panels)orCD8null (bottom panels) tetramers and anti-CD8 mAbs. B, Lysis of C1R-A2wt (top panel) and C1R-A2CD8null cells (bottom panel) over a range of TV9 peptide concentrations by the parental TV9−2 culture and sorted TV9−2(Vβ3+) or TV9−2(Vβ8+) cells. The E:T ratio was 2.5:1. Lysis of C1R cells alone or pulsed with the irrelevant peptide YV9 (negative controls) was <7%. Specific lysis was calculated by subtracting lysis in the negative control from the overall lysis for each E:T ratio. Data are representative of three independent experiments and presented as mean ± SEM of triplicate assays. C, Secretion of IFN-γ by TV9-specific CTLs after stimulating for 48 h with C1R-A2wt (top panel) and C1R-A2CD8null (bottom panel) cells over a range of TV9 peptide concentrations. The E:T ratio used was 1:10. D, Suppression of HIV-1 JR-CSF replication in three acutely infected, allogeneic, HLA-A2-matched, CD8-depleted PBMC cultures by TV9−2, TV9−2(Vβ3+), and TV9−2(Vβ8+) CTLs measured 9 days postinfection. The concentrations of p24 in infected PBMC-1, PBMC-2, and PBMC-3 in the absence of CTLs were 311, 210, and 184 ng/ml, respectively. The difference in the median inhibition against the three cultures between the parental TV9−2 cells and the low-avidity Vβ3+ T cells was determined to be statistically significant by the Student t test with Holm's adjustment for pairwise comparison (p = 0.006). Similarly, the difference between the high-and low-avidity T cell subclones was also significant (p = 0.01).
Cross-reactivity of TV9-specific CTLs for naturally occurring Ag variants
Fig. 7A shows that TV9-specific CTLs were equally cytotoxic to T2 cells pulsed with TV9, its variant 9I, or the HIV-2 homolog 8L for a representative culture. All five cultures tested recognized these peptides equally well. However, because patients do not recognize TV9, there are no reports of CTL escape variants.
FIGURE 7.

A, Cross-recognition of variants of TV9 by a representative TV9-specific culture (TV9−1) determined by cytotoxicity at various E:T ratios. T2 cells pulsed with 1 μg/ml TV9, 9I, and 8L peptides were used as targets. B, Lysis of JA2/R7/Hyg cells by three TV9-specific cultures (TV9−1, TV9−2, and TV9−5) and a clone (clone 3.2) isolated from TV9−2.
Recognition of infected target cells by TV9-specific CTLs
To ascertain whether TV9 is presented at sufficient densities by infected cells to activate the CTLs, the ability of TV9−1, TV9−2, and TV9−5 cultures, as well as a TV9-specific clone derived from TV9−2 (clone 3.2) to lyse productively infected JA2/R7/Hyg cells, was determined (Fig. 7B). Maximum lysis was ∼25% for cultures and 40% for the clone 3.2. Cytotoxicity was HLA class I-mediated because it was blocked by ∼60% at all E:T ratios by 100 μg/ml anti-HLA class I mAb W6/32 (data not shown). Lastly, TV9-specific cultures were also capable of specific lysis of allogeneic HLA-A0201+ DCs transduced with pNL4−3 E− (data not shown).
Discussion
Immunodominant CTL responses specific for HIV-1 and SIV can exert clinically significant selection forces on the virus during primary infection, in the absence or presence of prior immunization (5, 6, 74, 75). Although subdominant epitopes elicit fewer responding T cells, they are ostensibly protective in particular cohorts of patients with active infection (5, 6, 9, 21, 76). The most direct evidence that sub-dominant responses are important for HIV-1 control comes from a recent report in macaques that spontaneously controlled pathogenic SIV. Virus recrudescence after CD8 depletion was suppressed with the return of CD8+ cells targeting subdominant epitopes (77). Thus, understanding the characteristics of subdominant responses and the conditions under which they play a complementary role in protection have fundamental implications in vaccine development because immunodominant hierarchies are operative in the setting of vaccination as well (78). In this study, the subdominant status of the TV9 epitope, at least in chronic infection, was confirmed in a large cohort of HLAA2+ individuals. Despite the paucity of reports, there are indications that reactivity to this determinant can control virus; in particular, an elevated and sustained TV9-specific response was detected in an individual who remained uninfected despite exposure to highly replicating HIV-1 (46). In this study, we show that the CD8+ TV9-specific response can be highly avid in an infected individual.
TV9-specific CTLs were first described in a HIV-1 exposed but persistently seronegative HLA-A*0202+ Nairobi sex worker (43). This A2 subtype is found at much higher frequency among East African populations as compared with Caucasians (79) and differs from the predominant Caucasian subtype, HLA-A*0201, by three amino acid residues (80). Of interest, expression of HLA-A*0202 is associated clearly with a decreased risk of infection in sexually exposed adults and perinatally exposed infants (17). Although TV9 might elicit protective responses in the context of HLA-A*0202, it is paradoxically silent in patients from industrialized countries. One explanation is that the TV9:HLA-A*0202 complex may elicit a more effective T cell repertoire than the corresponding TV9: HLA-A*0201 complex. Indeed, there is precedence that closely related HLA class I alleles (HLA-B*5701 and HLA-B*5703) can recruit dissimilar and differentially effective CD8+ T cell repertoires during infection (81).
Characterization of subdominant epitopes such as TV9 is particularly difficult due to the small number of reactive T cells and/or responders. For this reason, we generated T cells specific for this determinant from healthy donors using a culture system capable of immunizing HIV-1-specific T cells de novo (24). Study of these early responses should capture the TCR repertoires at their most diverse. Our results show that the subdominant status of TV9 is not due to lack of recognition because robust and homogeneous tetramer-binding CD8+ T cell cultures were expanded readily from most donors (7 of 10 studied). Moreover, in contrast to those directed against other HLA-A2-restricted specificities examined under identical conditions, all but one of the TV9-specific cultures remained oligoclonal after >20 rounds of division (24, 53) beyond that reported for a primary CTL response (72). Thus, TV9 provokes a large though structurally diverse precursor pool in HLA-A2 carriers. Although immunodominance has been reported to be a direct function of precursor T cell numbers in murine studies (82), our results suggest that an intrinsic bias of its precursor pool may be a determining factor for the subdominance of TV9.
Understanding the limitations and predisposition of the CTL repertoire to HIV-1 determinants helps to evaluate their relevance as vaccine targets. Initial appraisal of TCR usage by direct flow cytometric analysis of uncloned short-term TV9-specific CD8+ T cell cultures showed a diverse set of available Vβ sequences. There was large variability in the numbers of reactive clonotypes among cultures from different donors. Emergence of common Vβ usage dominating the repertoire was not observed. Almost invariably, the clonotypes were heterogeneous in terms of avidity of binding to the TV9 tetramer within each culture. These features are not unique to TV9-reactive T cells and are shared by the well-characterized human CD8+ T cell repertoire specific for the HLAA2-restricted Melan-A/MART-1 peptide (83). The factors that determine T cell clonal selection within an epitope-specific response are understood incompletely, but activation of cross-reactive TCR by a large family of peptide mimics naturally occurring in self-and/or microbial-derived peptides may play an important role (83). Regardless, absence of preferential TCRBV usage by TV9-specific cells is consistent with “private” (unique to an individual) responses in our donors. Private repertoires may be preferentially elicited by structurally intricate pMHCI complexes (84, 85). From this perspective, TV9 would be structurally more complex than the immunodominant SL9 epitope, which primed at most a few highly avid clones from each donor under identical conditions.
It has been proposed that cross-reactivity may explain why T cell responses to some epitopes in human viral infections have a narrow oligoclonal TCR repertoire while others are diverse (86). It is intriguing to speculate that TV9 may be a cross-reactive determinant that HIV-1 shares with a heterologous organism(s) (87). Thus, variability in cross-reactive T cell expansion unique to an individual may influence the character of the TV9-specific response on encountering HIV-1. Modulation of epitope hierarchy by heterologous immunity (88) may help to focus the avidly diffuse TV9-specific primary T cell response, thus accounting for the highly avid response in some individuals.
The progression of an ongoing T cell response is linked generally to an increase in the overall avidity of Ag-specific T cells for pMHC complex. Because T cells do not somatically mutate their receptors, avidity maturation relies on the competition and eventual emergence of high-avidity clonotypes at the expense of their low-avidity counterparts (89, 90). The basic mechanism for this affinity maturation remains incompletely understood. In contrast to immunodominant responses, which are frequently composed of one or two prevalent clonotypes with high avidity for cognate Ags (29, 51, 91, 92), a narrowing of the TCR repertoire in TV9-specific cultures did not occur with repeated antigenic restimulation. Failure of high-avidity clonotypes to predominate may explain why TV9 responses were not detected readily during infection. We used point-mutated pMHCI Ags in both soluble and cell-associated forms to assess the contribution of CD8 to activation of both lowand high-avidity TV9-specific CD8+ T cells. A differential compensatory role of CD8 was observed for less sensitive effector functions in low-avidity clonotypes, confirming previous results in CD8+ T cell populations specific for persistent DNA viruses (51). All of the TV9-specific clonotypes, including those with the lowest avidities, expressed similar levels of CD8 as determined by immunostaining and produced Tc1-type cytokines. Thus, TV9 does not appear to elicit CD8low regulatory T cells generated by suboptimal restimulations (93). Although CD8 participation enabled low-avidity T cells to proliferate as rapidly as their high-avidity counterpart and persist in these cultures, ligation of CD8 was insufficient to overcome low avidity for activation of some downstream effector functions, such as the suppression of virus replication.
Because Gag-specific immunity may be particularly effective for the control of HIV-1 infection (8, 22, 36), the highly conserved TV9, which overlaps with the HLA-B57-restricted p24 peptide recognized by long-term nonprogressors (9, 40–42), may be a potentially useful vaccine target for HLA-A2 carriers. The fact that it is subdominant in patients may be particularly advantageous in a therapeutic vaccine setting because the responding repertoire would not have been exhausted by the infection. Our results suggest that there is no intrinsic limitation to the population size of the T cell repertoire for TV9. However, for TV9-specific response to be effective, it may be necessary to devise immunization strategies to elicit secondary clonotypes with high avidity. One approach may involve mimic agonists to selectively stimulate high-avidity TV9-cross-reactive T cells (85). Understanding the human T cell repertoire to subdominant CTL determinants of HIV-1 may provide important insights into vaccine design.
Acknowledgments
We are grateful to Dr. Tinghua Cao, David Craig, and Melissa Bajcz for their excellent help in generating some of the data. We also thank Dr. Malcolm S. Mitchell for his helpful discussion of the manuscript and Dr. Ori Rosen for his statistical analyses.
Footnotes
This work was supported by Michigan Life Sciences Corridor Program 1659 (to J.K.-M.) and National Institutes of Health Grant R01-AI064069 (to J.K.-M.) and made possible by Grant 5G12RR008124 from the National Center for Research Resources, a component of the National Institutes of Health. D.A.P. is a Medical Research Council (U.K.) Senior Clinical Fellow.
Abbreviations used in this paper: DC, dendritic cell; iDC, immature DC; mDC, matured DC; pMHCI, peptide MHC class I; sfc, spot-forming cell; tetramer, tetrameric HLA-A*0201-peptide complex; TV9, HIV-1 p24 Gag19−27 epitope (TLNAWVKVV); OLP, overlapping peptide.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Letvin NL. Progress toward an HIV vaccine. Annu. Rev. Med. 2005;56:213–223. doi: 10.1146/annurev.med.54.101601.152349. [DOI] [PubMed] [Google Scholar]
- 2.Brander C, Frahm N, Walker BD. The challenges of host and viral diversity in HIV vaccine design. Curr. Opin. Immunol. 2006;18:430–437. doi: 10.1016/j.coi.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 3.McMichael AJ. HIV vaccines. Annu. Rev. Immunol. 2006;24:227–255. doi: 10.1146/annurev.immunol.24.021605.090605. [DOI] [PubMed] [Google Scholar]
- 4.Phillips RE, Rowland-Jones S, Nixon DF, Gotch FM, Edwards JP, Ogunlesi AO, Elvin JG, Rothbard JA, Bangham CR, Rizza CR, et al. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991;354:453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- 5.Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MB, Shaw GM. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 6.Price DA, Goulder PJ, Klenerman P, Sewell AK, Easterbrook PJ, Troop M, Bangham CR, Phillips RE. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrich TC, Dodds EJ, Yant LJ, Vojnov L, Rudersdorf R, Cullen C, Evans DT, Desrosiers RC, Mothe BR, Sidney J, et al. Reversion of CTL escape-variant immunodeficiency viruses in vivo. Nat. Med. 2004;10:275–281. doi: 10.1038/nm998. [DOI] [PubMed] [Google Scholar]
- 8.Goulder PJ, Brander C, Tang Y, Tremblay C, Colbert RA, Addo MM, Rosenberg ES, Nguyen T, Allen R, Trocha A, et al. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature. 2001;412:334–338. doi: 10.1038/35085576. [DOI] [PubMed] [Google Scholar]
- 9.Leslie AJ, Pfafferott KJ, Chetty P, Draenert R, Addo MM, Feeney M, Tang Y, Holmes EC, Allen T, Prado JG, et al. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 2004;10:282–289. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- 10.Oxenius A, Price DA, Trkola A, Edwards C, Gostick E, Zhang HT, Easterbrook PJ, Tun T, Johnson A, Waters A, et al. Loss of viral control in early HIV-1 infection is temporally associated with sequential escape from CD8+ T cell responses and decrease in HIV-1-specific CD4+ and CD8+ T cell frequencies. J. Infect. Dis. 2004;190:713–721. doi: 10.1086/422760. [DOI] [PubMed] [Google Scholar]
- 11.Klenerman P, Wu Y, Phillips R. HIV: current opinion in escapology. Curr. Opin. Microbiol. 2002;5:408–413. doi: 10.1016/s1369-5274(02)00339-9. [DOI] [PubMed] [Google Scholar]
- 12.Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296:1439–1443. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- 13.Allen TM, Altfeld M, Geer SC, Kalife ET, Moore C, O'Sullivan M, Desouza KI, Feeney ME, Eldridge RL, Maier EL, et al. Selective escape from CD8+ T cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J. Virol. 2005;79:13239–13249. doi: 10.1128/JVI.79.21.13239-13249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trachtenberg E, Korber B, Sollars C, Kepler TB, Hraber PT, Hayes E, Funkhouser R, Fugate M, Theiler J, Hsu YS, et al. Advantage of rare HLA supertype in HIV disease progression. Nat. Med. 2003;9:928–935. doi: 10.1038/nm893. [DOI] [PubMed] [Google Scholar]
- 15.Scherer A, Frater J, Oxenius A, Agudelo J, Price DA, Gunthard HF, Barnardo M, Perrin L, Hirschel B, Phillips RE, McLean AR. Quantifiable cytotoxic T lymphocyte responses and HLA-related risk of progression to AIDS. Proc. Natl. Acad. Sci. USA. 2004;101:12266–12270. doi: 10.1073/pnas.0404091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Browning M, Krausa P. Genetic diversity of HLA-A2: evolutionary and functional significance. Immunol. Today. 1996;17:165–170. doi: 10.1016/0167-5699(96)80614-1. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald KS, Fowke KR, Kimani J, Dunand VA, Nagelkerke NJ, Ball TB, Oyugi J, Njagi E, Gaur LK, Brunham RC, et al. Influence of HLA supertypes on susceptibility and resistance to human immunodeficiency virus type 1 infection. J. Infect. Dis. 2000;181:1581–1589. doi: 10.1086/315472. [DOI] [PubMed] [Google Scholar]
- 18.Goulder PJ, Altfeld MA, Rosenberg ES, Nguyen T, Tang Y, Eldridge RL, Addo MM, He S, Mukherjee JS, Phillips MN, et al. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J. Exp. Med. 2001;193:181–194. doi: 10.1084/jem.193.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brander C, Walker BD. Gradual adaptation of HIV to human host populations: good or bad news? Nat. Med. 2003;9:1359–1362. doi: 10.1038/nm941. [DOI] [PubMed] [Google Scholar]
- 20.Altfeld M, Allen TM, Kalife ET, Frahm N, Addo MM, Mothe BR, Rathod A, Reyor LL, Harlow J, Yu XG, et al. The majority of currently circulating human immunodeficiency virus type 1 clade B viruses fail to prime cytotoxic T lymphocyte responses against an otherwise immunodominant HLA-A2-restricted epitope: implications for vaccine design. J. Virol. 2005;79:5000–5005. doi: 10.1128/JVI.79.8.5000-5005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frahm N, Kiepiela P, Adams S, Linde CH, Hewitt HS, Sango K, Feeney ME, Addo MM, Lichterfeld M, Lahaie MP, et al. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nat. Immunol. 2006;7:173–178. doi: 10.1038/ni1281. [DOI] [PubMed] [Google Scholar]
- 22.Leslie A, Kavanagh D, Honeyborne I, Pfafferott K, Edwards C, Pillay T, Hilton L, Thobakgale C, Ramduth D, Draenert R, et al. Transmission and accumulation of CTL escape variants drive negative associations between HIV polymorphisms and HLA. J. Exp. Med. 2005;201:891–902. doi: 10.1084/jem.20041455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barouch DH, Powers J, Truitt DM, Kishko MG, Arthur JC, Peyerl FW, Kuroda MJ, Gorgone DA, Lifton MA, Lord CI, et al. Dynamic immune responses maintain cytotoxic T lymphocyte epitope mutations in transmitted simian immunodeficiency virus variants. Nat. Immunol. 2005;6:247–252. doi: 10.1038/ni1167. [DOI] [PubMed] [Google Scholar]
- 24.Kan-Mitchell J, Bisikirska B, Wong-Staal F, Schaubert KL, Bajcz M, Bereta M. The HIV-1 HLA-A2-SLYNTVATL is a help-independent CTL epitope. J. Immunol. 2004;172:5249–5261. doi: 10.4049/jimmunol.172.9.5249. [DOI] [PubMed] [Google Scholar]
- 25.Kan-Mitchell J, Bajcz M, Schaubert KL, Price DA, Brenchley JM, Asher TE, Douek DC, Ng HL, Yang OO, Rinaldo CR, Jr., et al. Degeneracy and repertoire of the human HIV-1 Gag p1777−85 CTL response. J. Immunol. 2006;176:6690–6701. doi: 10.4049/jimmunol.176.11.6690. [DOI] [PubMed] [Google Scholar]
- 26.Dalod M, Dupuis M, Deschemin JC, Goujard C, Deveau C, Meyer L, Ngo N, Rouzioux C, Guillet JG, Delfraissy JF, et al. Weak anti-HIV CD8+ T cell effector activity in HIV primary infection. J. Clin. Invest. 1999;104:1431–1439. doi: 10.1172/JCI7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altfeld MA, Livingston B, Reshamwala N, Nguyen PT, Addo MM, Shea A, Newman M, Fikes J, Sidney J, Wentworth P, et al. Identification of novel HLA-A2-restricted human immunodeficiency virus type 1-specific cytotoxic T lymphocyte epitopes predicted by the HLA-A2 supertype peptide-binding motif. J. Virol. 2001;75:1301–1311. doi: 10.1128/JVI.75.3.1301-1311.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Los Alamos National Laboratory . HIV Immunology and HIV/SIV Vaccine Databases 2003. Los Alamos National Laboratory, Theoretical Biology and Biophysics; Los Alamos, NM: 2003. [Google Scholar]
- 29.Douek DC, Betts MR, Brenchley JM, Hill BJ, Ambrozak DR, Ngai KL, Karandikar NJ, Casazza JP, Koup RA. A novel approach to the analysis of specificity, clonality, and frequency of HIV-specific T cell responses reveals a potential mechanism for control of viral escape. J. Immunol. 2002;168:3099–3104. doi: 10.4049/jimmunol.168.6.3099. [DOI] [PubMed] [Google Scholar]
- 30.McMichael A, Klenerman P. HIV/AIDS: HLA leaves its footprints on HIV. Science. 2002;296:1410–1411. doi: 10.1126/science.1072492. [DOI] [PubMed] [Google Scholar]
- 31.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez F, Harkins S, Slifka MK, Whitton JL. Immunodominance in virus-induced CD8+ T cell responses is dramatically modified by DNA immunization and is regulated by γ interferon. J. Virol. 2002;76:4251–4259. doi: 10.1128/JVI.76.9.4251-4259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of functional CD8+ T cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 2002;76:2298–2305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramduth D, Chetty P, Mngquandaniso NC, Nene N, Harlow JD, Honeyborne I, Ntumba N, Gappoo S, Henry C, Jeena P, et al. Differential immunogenicity of HIV-1 clade C proteins in eliciting CD8+ and CD4+ cell responses. J. Infect. Dis. 2005;192:1588–1596. doi: 10.1086/496894. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Picado J, Prado JG, Fry EE, Pfafferott K, Leslie A, Chetty S, Thobakgale C, Honeyborne I, Crawford H, Matthews P, et al. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J. Virol. 2006;80:3617–3623. doi: 10.1128/JVI.80.7.3617-3623.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuniga R, Lucchetti A, Galvan P, Sanchez S, Sanchez C, Hernandez A, Sanchez H, Frahm N, Linde CH, Hewitt HS, et al. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J. Virol. 2006;80:3122–3125. doi: 10.1128/JVI.80.6.3122-3125.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Z, Jin L, Peterson DL, Lawson CL. Model for lentivirus capsid core assembly based on crystal dimers of EIAV p26. J. Mol. Biol. 1999;286:83–93. doi: 10.1006/jmbi.1998.2443. [DOI] [PubMed] [Google Scholar]
- 38.Furuta RA, Shimano R, Ogasawara T, Inubushi R, Amano K, Akari H, Hatanaka M, Kawamura M, Adachi A. HIV-1 capsid mutants inhibit the replication of wild-type virus at both early and late infection phases. FEBS Lett. 1997;415:231–234. doi: 10.1016/s0014-5793(97)01132-0. [DOI] [PubMed] [Google Scholar]
- 39.Tangri S, Ishioka GY, Huang X, Sidney J, Southwood S, Fikes J, Sette A. Structural features of peptide analogs of human histocompatibility leukocyte antigen class I epitopes that are more potent and immunogenic than wild-type peptide. J. Exp. Med. 2001;194:833–846. doi: 10.1084/jem.194.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein MR, van der Burg SH, Hovenkamp E, Holwerda AM, Drijfhout JW, Melief CJ, Miedema F. Characterization of HLAB57-restricted human immunodeficiency virus type 1 Gag- and RT-specific cytotoxic T lymphocyte responses. J. Gen. Virol. 1998;79:2191–2201. doi: 10.1099/0022-1317-79-9-2191. [DOI] [PubMed] [Google Scholar]
- 41.Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, Hallahan CW, Selig SM, Schwartz D, Sullivan J, Connors M. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA. 2000;97:2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Draenert R, Le Gall S, Pfafferott KJ, Leslie AJ, Chetty P, Brander C, Holmes EC, Chang SC, Feeney ME, Addo MM, et al. Immune selection for altered antigen processing leads to cytotoxic T lymphocyte escape in chronic HIV-1 infection. J. Exp. Med. 2004;199:905–915. doi: 10.1084/jem.20031982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowland-Jones SL, Dong T, Fowke KR, Kimani J, Krausa P, Newell H, Blanchard T, Ariyoshi K, Oyugi J, Ngugi E, et al. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J. Clin. Invest. 1998;102:1758–1765. doi: 10.1172/JCI4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaul R, Dong T, Plummer FA, Kimani J, Rostron T, Kiama P, Njagi E, Irungu E, Farah B, Oyugi J, et al. CD8+ lymphocytes respond to different HIV epitopes in seronegative and infected subjects. J. Clin. Invest. 2001;107:1303–1310. doi: 10.1172/JCI12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rinaldo CR,, Jr., Huang XL, Fan Z, Margolick JB, Borowski L, Hoji A, Kalinyak C, McMahon DK, Riddler SA, Hildebrand WH, et al. Anti-human immunodeficiency virus type 1 (HIV-1) CD8+ T lymphocyte reactivity during combination antiretroviral therapy in HIV-1-infected patients with advanced immunodeficiency. J. Virol. 2000;74:4127–4138. doi: 10.1128/jvi.74.9.4127-4138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Missale G, Papagno L, Penna A, Pilli M, Zerbini A, Vitali P, Pieroni G, Urbani S, Uggeri J, Pinheiro S, et al. Parenteral exposure to high HIV viremia leads to virus-specific T cell priming without evidence of infection. Eur. J. Immunol. 2004;34:3208–3215. doi: 10.1002/eji.200424889. [DOI] [PubMed] [Google Scholar]
- 47.Yewdell JW. Confronting complexity: real-world immunodominance in antiviral CD8+ T cell responses. Immunity. 2006;25:533–543. doi: 10.1016/j.immuni.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Kan-Mitchell J, Huang XQ, Steinman L, Oksenberg JR, Harel W, Parker JW, Goedegebuure PS, Darrow TL, Mitchell MS. Clonal analysis of in vivo activated CD8+ cytotoxic T lymphocytes from a melanoma patient responsive to active specific immunotherapy. Cancer Immunol. Immunother. 1993;37:15–25. doi: 10.1007/BF01516937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsomides TJ, Aldovini A, Johnson RP, Walker BD, Young RA, Eisen HN. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J. Exp. Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purbhoo MA, Boulter JM, Price DA, Vuidepot AL, Hourigan CS, Dunbar PR, Olson K, Dawson SJ, Phillips RE, Jakobsen BK, et al. The human CD8 coreceptor effects cytotoxic T cell activation and antigen sensitivity primarily by mediating complete phosphorylation of the T cell receptor ζ chain. J. Biol. Chem. 2001;276:32786–32792. doi: 10.1074/jbc.M102498200. [DOI] [PubMed] [Google Scholar]
- 51.Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J. Exp. Med. 2005;202:1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whelan JA, Dunbar PR, Price DA, Purbhoo MA, Lechner F, Ogg GS, Griffiths G, Phillips RE, Cerundolo V, Sewell AK. Specificity of CTL interactions with peptide-MHC class I tetrameric complexes is temperature dependent. J. Immunol. 1999;163:4342–4348. [PubMed] [Google Scholar]
- 53.Mitchell MS, Lund TA, Sewell AK, Marincola FM, Paul E, Schroder K, Wilson DB, Kan-Mitchell J. The cytotoxic T cell response to peptide analogs of the HLA-A*0201-restricted MUC1 signal sequence epitope, M1.2. Cancer Immunol. Immunother. 2006;56:287–301. doi: 10.1007/s00262-006-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bihl F, Frahm N, Di Giammarino L, Sidney J, John M, Yusim K, Woodberry T, Sango K, Hewitt HS, Henry L, et al. Impact of HLA-B alleles, epitope binding affinity, functional avidity, and viral coinfection on the immunodominance of virus-specific CTL responses. J. Immunol. 2006;176:4094–4101. doi: 10.4049/jimmunol.176.7.4094. [DOI] [PubMed] [Google Scholar]
- 55.Brander C, Pichler WJ, Corradin G. Identification of HIV protein-derived cytotoxic T lymphocyte (CTL) epitopes for their possible use as synthetic vaccine. Clin. Exp. Immunol. 1995;101:107–113. doi: 10.1111/j.1365-2249.1995.tb02285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarobe P, Pendleton CD, Akatsuka T, Lau D, Engelhard VH, Feinstone SM, Berzofsky JA. Enhanced in vitro potency and in vivo immunogenicity of a CTL epitope from hepatitis C virus core protein following amino acid replacement at secondary HLA-A2.1 binding positions. J. Clin. Invest. 1998;102:1239–1248. doi: 10.1172/JCI3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Majumder B, Janket ML, Schafer EA, Schaubert K, Huang XL, Kan-Mitchell J, Rinaldo CR, Jr., Ayyavoo V. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T cell activation: implications for viral immune escape. J. Virol. 2005;79:7990–8003. doi: 10.1128/JVI.79.13.7990-8003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang OO, Kalams SA, Rosenzweig M, Trocha A, Jones N, Koziel M, Walker BD, Johnson RP. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J. Virol. 1996;70:5799–5806. doi: 10.1128/jvi.70.9.5799-5806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang OO, Kalams SA, Trocha A, Cao H, Luster A, Johnson RP, Walker BD. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Browning Paul J, Wang EJ, Pettoello-Mantovani M, Raker C, Yurasov S, Goldstein MM, Horner JW, Chan J, Goldstein H. Mice transgenic for monocyte-tropic HIV type 1 produce infectious virus and display plasma viremia: a new in vivo system for studying the postintegration phase of HIV replication. AIDS Res. Hum. Retroviruses. 2000;16:481–492. doi: 10.1089/088922200309142. [DOI] [PubMed] [Google Scholar]
- 61.Frahm N, Korber BT, Adams CM, Szinger JJ, Draenert R, Addo MM, Feeney ME, Yusim K, Sango K, Brown NV, et al. Consistent cytotoxic T lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J. Virol. 2004;78:2187–2200. doi: 10.1128/JVI.78.5.2187-2200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kienzle N, Baz A, Kelso A. Profiling the CD8low phenotype, an alternative career choice for CD8 T cells during primary differentiation. Immunol. Cell Biol. 2004;82:75–83. doi: 10.1111/j.1440-1711.2004.01210.x. [DOI] [PubMed] [Google Scholar]
- 63.Pittet MJ, Rubio-Godoy V, Bioley G, Guillaume P, Batard P, Speiser D, Luescher I, Cerottini JC, Romero P, Zippelius A. α3 domain mutants of peptide/MHC class I multimers allow the selective isolation of high avidity tumor-reactive CD8 T cells. J. Immunol. 2003;171:1844–1849. doi: 10.4049/jimmunol.171.4.1844. [DOI] [PubMed] [Google Scholar]
- 64.Choi EM, Chen JL, Wooldridge L, Salio M, Lissina A, Lissin N, Hermans IF, Silk JD, Mirza F, Palmowski MJ, et al. High avidity antigen-specific CTL identified by CD8-independent tetramer staining. J. Immunol. 2003;171:5116–5123. doi: 10.4049/jimmunol.171.10.5116. [DOI] [PubMed] [Google Scholar]
- 65.Wooldridge L, Hutchinson SL, Choi EM, Lissina A, Jones E, Mirza F, Dunbar PR, Price DA, Cerundolo V, Sewell AK. Anti-CD8 antibodies can inhibit or enhance peptide-MHC class I (pMHCI) multimer binding: this is paralleled by their effects on CTL activation and occurs in the absence of an interaction between pMHCI and CD8 on the cell surface. J. Immunol. 2003;171:6650–6660. doi: 10.4049/jimmunol.171.12.6650. [DOI] [PubMed] [Google Scholar]
- 66.Wooldridge L, van den Berg HA, Glick M, Gostick E, Laugel B, Hutchinson SL, Milicic A, Brenchley JM, Douek DC, Price DA, Sewell AK. Interaction between the CD8 coreceptor and MHC class I stabilizes TCR-antigen complexes at the cell surface. J. Biol. Chem. 2005;280:27491–27501. doi: 10.1074/jbc.M500555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Purbhoo MA, Sewell AK, Klenerman P, Goulder PJ, Hilyard KL, Bell JI, Jakobsen BK, Phillips RE. Copresentation of natural HIV-1 agonist and antagonist ligands fails to induce the T cell receptor signaling cascade. Proc. Natl. Acad. Sci. USA. 1998;95:4527–4532. doi: 10.1073/pnas.95.8.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slifka MK, Whitton JL. Antigen-specific regulation of T cell-mediated cytokine production. Immunity. 2000;12:451–457. doi: 10.1016/s1074-7613(00)80197-1. [DOI] [PubMed] [Google Scholar]
- 69.Krogsgaard M, Huppa JB, Purbhoo MA, Davis MM. Linking molecular and cellular events in T cell activation and synapse formation. Semin. Immunol. 2003;15:307–315. doi: 10.1016/j.smim.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Valitutti S, Muller S, Dessing M, Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J. Exp. Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Price DA, Sewell AK, Dong T, Tan R, Goulder PJ, Rowland-Jones SL, Phillips RE. Antigen-specific release of β-chemokines by anti-HIV-1 cytotoxic T lymphocytes. Curr. Biol. 1998;8:355–358. doi: 10.1016/s0960-9822(98)70138-1. [DOI] [PubMed] [Google Scholar]
- 72.Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meyer-Olson D, Brady KW, Bartman MT, O'Sullivan KM, Simons BC, Conrad JA, Duncan CB, Lorey S, Siddique A, Draenert R, et al. Fluctuations of functionally distinct CD8+ T cell clonotypes demonstrate flexibility of the HIV-specific TCR repertoire. Blood. 2006;107:2373–2383. doi: 10.1182/blood-2005-04-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allen TM, O'Connor DH, Jing P, Dzuris JL, Mothe BR, Vogel TU, Dunphy E, Liebl ME, Emerson C, Wilson N, et al. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;407:386–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- 75.Barouch DH, McKay PF, Sumida SM, Santra S, Jackson SS, Gorgone DA, Lifton MA, Chakrabarti BK, Xu L, Nabel GJ, Letvin NL. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J. Virol. 2003;77:8729–8735. doi: 10.1128/JVI.77.16.8729-8735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones NA, Wei X, Flower DR, Wong M, Michor F, Saag MS, Hahn BH, Nowak MA, Shaw GM, Borrow P. Determinants of human immunodeficiency virus type 1 escape from the primary CD8+ cytotoxic T lymphocyte response. J. Exp. Med. 2004;200:1243–1256. doi: 10.1084/jem.20040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watkins DI. Cellular immune responses that successfully control AIDS virus replication.. 5th International AIDS Vaccine Conference, August 29–September 1, 2006.; International AIDS Vaccine Initiative, Amsterdam, The Netherlands. 2006. [Google Scholar]
- 78.Yewdell JW, Del Val M. Immunodominance in TCD8+ responses to viruses: cell biology, cellular immunology, and mathematical models. Immunity. 2004;21:149–153. doi: 10.1016/j.immuni.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 79.MacDonald KS, Matukas L, Embree JE, Fowke K, Kimani J, Nagelkerke NJ, Oyugi J, Kiama P, Kaul R, Luscher MA, et al. Human leucocyte antigen supertypes and immune susceptibility to HIV-1, implications for vaccine design. Immunol. Lett. 2001;79:151–157. doi: 10.1016/s0165-2478(01)00277-2. [DOI] [PubMed] [Google Scholar]
- 80.Robinson J, Waller MJ, Parham P, de Groot N, Bontrop R, Kennedy LJ, Stoehr P, Marsh SG. IMGT/HLA and IMGT/MHC: sequence databases for the study of the major histocompatibility complex. Nucleic Acids Res. 2003;31:311–314. doi: 10.1093/nar/gkg070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu XG, Lichterfeld M, Chetty S, Williams KL, Mui SK, Miura T, Frahm N, Feeney ME, Tang Y, Pereyra F, et al. Mutually exclusive T cell receptor induction and differential susceptibility to human immunodeficiency virus type 1 mutational escape associated with a two-amino-acid difference between HLA class I subtypes. J. Virol. 2007;81:1619–1631. doi: 10.1128/JVI.01580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.La Gruta NL, Kedzierska K, Pang K, Webby R, Davenport M, Chen W, Turner SJ, Doherty PC. A virus-specific CD8+ T cell immunodominance hierarchy determined by antigen dose and precursor frequencies. Proc. Natl. Acad. Sci. USA. 2006;103:994–999. doi: 10.1073/pnas.0510429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pittet MJ, Zippelius A, Valmori D, Speiser DE, Cerottini JC, Romero P. Melan-A/MART-1-specific CD8 T cells: from thymus to tumor. Trends Immunol. 2002;23:325–328. doi: 10.1016/s1471-4906(02)02244-5. [DOI] [PubMed] [Google Scholar]
- 84.Turner SJ, Kedzierska K, Komodromou H, La Gruta NL, Dunstone MA, Webb AI, Webby R, Walden H, Xie W, McCluskey J, et al. Lack of prominent peptide-major histocompatibility complex features limits repertoire diversity in virus-specific CD8+ T cell populations. Nat. Immunol. 2005;6:382–389. doi: 10.1038/ni1175. [DOI] [PubMed] [Google Scholar]
- 85.Welsh RM. Private specificities of heterologous immunity. Curr. Opin. Immunol. 2006;18:331–337. doi: 10.1016/j.coi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 86.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim SK, Clute SC, Welsh RM. Memory of mice and men: CD8+ T cell cross-reactivity and heterologous immunity. Immunol. Rev. 2006;211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Welsh RM, Selin LK. No one is naive: the significance of heterologous T cell immunity. Nat. Rev. Immunol. 2002;2:417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 88.Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat. Immunol. 2002;3:627–634. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- 89.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 90.Kedl RM, Kappler JW, Marrack P. Epitope dominance, competition and T cell affinity maturation. Curr. Opin. Immunol. 2003;15:120–127. doi: 10.1016/s0952-7915(02)00009-2. [DOI] [PubMed] [Google Scholar]
- 91.Trautmann L, Rimbert M, Echasserieau K, Saulquin X, Neveu B, Dechanet J, Cerundolo V, Bonneville M. Selection of T cell clones expressing high-affinity public TCRs within human cytomegalovirus-specific CD8 T cell responses. J. Immunol. 2005;175:6123–6132. doi: 10.4049/jimmunol.175.9.6123. [DOI] [PubMed] [Google Scholar]
- 92.Wallace ME, Bryden M, Cose SC, Coles RM, Schumacher TN, Brooks A, Carbone FR. Junctional biases in the naive TCR repertoire control the CTL response to an immunodominant determinant of HSV-1. Immunity. 2000;12:547–556. doi: 10.1016/s1074-7613(00)80206-x. [DOI] [PubMed] [Google Scholar]
- 93.Maile R, Pop SM, Tisch R, Collins EJ, Cairns BA, Frelinger JA. Low-avidity CD8lo T cells induced by incomplete antigen stimulation in vivo regulate naive higher avidity CD8hi T cell responses to the same antigen. Eur. J. Immunol. 2006;36:397–410. doi: 10.1002/eji.200535064. [DOI] [PubMed] [Google Scholar]


