Abstract
Fourteen patients were treated over 2 years with cervical vagus nerve stimulation (VNS) for adjunctive therapy of severe, treatment-resistant depression. Here, we report the serendipitous observation that this treatment was associated with highly significant, gradual weight loss despite the patients’ report of not dieting or exercising. The weight loss was proportional to the initial BMI, that is, the more severe the obesity, the greater the weight loss. Weight loss did not correlate with changes in mood symptoms. The vagus nerve carries visceral information to and from the brain; modulation of its activity may alter eating behavior. Chronic cervical VNS may merit controlled study for the treatment of severe obesity.
Keywords: vagal, obesity treatment, treatment-resistant depression, neurostimulation
Fourteen patients (eight women and six men; average age 43 years, s.d. ± 10) underwent vagus nerve stimulation (VNS) over 1 year according to standard protocols as approved by the FDA for the adjunctive treatment of severe, treatment-resistant depression. All patients provided signed informed consent as dictated by the Institutional Review Board. Demographic and other clinical details appear in Table 1. Psychiatric diagnoses were determined using a structured clinical interview (SCID).1 One patient had bipolar depression; all others had recurrent, unipolar, major depression. These severely depressed patients (mean Hamilton2 depression score 29, range 24-37) had failed standard treatments: average duration of illness, 24 years; average duration of current episode, 2.5 years; average number of failed, but adequate, medications trials, 4. The medications on intake and psychiatric co-morbidities are also listed in Table 1.
Table 1.
Demographics and clinical characteristics of the patients
| Agea | Gender | Ethnicity | Diagnoses | Medications | Ht (cm) | I-Wt (kg) | ΔWt | I-BMI | ΔBMI | I-HmD | ΔHmD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 54 | M | Euroam | MDD recurrent PTSD |
No medicines | 178 | 83 | 3 | 26.1 | 0.9 | 24 | 1 |
| 33 | F | Euroam | MDD recurrent h/o OCD (remitted) |
Sertraline Quetiapine | 160 | 46 | -1 | 18.1 | -0.4 | 28 | 13 |
| 32 | F | Euroam | MDD recurrent ED (per history BN) (per SCID AN) |
Lamotrigine Paroxetine Mirtazipine Diazepam, Gabapantin |
165 | 55 | -6 | 20.3 | -2.2 | 37 | -1 |
| 42 | F | Euroam | MDD recurrent Sleep apnea |
Clonazepam | 183 | 131 | -13 | 38.1 | -3.8 | 37 | 12 |
| 58 | M | Middle eastern | MDD recurrent Diabetes mellitus |
Citalopram Quetiapine | 183 | 117 | -9 | 35 | -2.6 | 36 | -13 |
| 46 | F | Euroam | MDD recurrent Bradycardia |
Citalopram Topiramate Bupropion | 164 | 94 | 6 | 35.5 | 2.1 | 31 | -4 |
| 33 | M | Euroam | Bipolar disorder NOS h/o alcohol and cocaine dependence Dysthymic disorder Sexual paraphilia |
Fluoxetine Carbemazepine Quetiapine Clonazepam |
183 | 101 | -10 | 30 | -2.9 | 30 | 14 |
| 46 | F | Euroam | MDD recurrent | Bupropion Venlafaxine | 165 | 67 | 3 | 24.5 | 1.2 | 25 | 11 |
| 55 | M | Euroam | MDD recurrent h/o alcohol abuse/dependence polysubstance abuse |
Quetiapine Sertraline Bupropion Terazosin Provocholinel |
170 | 79 | -2 | 27.1 | -0.8 | 34 | 12 |
| 35 | F | Euroam | MDD recurrent h/o ED (remitted since 1990) h/o alcohol dependence |
Modafinil Fluoxetine Disulfiram prn |
165 | 81 | -6 | 29.6 | -2.3 | 24 | -10 |
| 52 | F | Euroam | MDD recurrent | Mirtazipine Fluvoxamine | 169 | 137 | -24 | 48.7 | -8.4 | 32 | -4 |
| 47 | F | Euroam | MDD recurrent h/o panic disorder |
Citalopram Trazodone Clonazepam |
170 | 121 | -19 | 43.1 | -6.8 | 24 | -11 |
| 54 | F | Hispanic | MDD recurrent h/o alcohol dependence h/o PTSD |
Methylphenidae Coumadin | 138 | 93 (end of 12 weeks) | -17 | 37.7 | -6.8 | 25 | 7 |
| 27 | M | Euroam | MDD recurrent OCD Panic disorder |
Sertraline | 170 | 80 | 2 | 27.5 | 0.5 | 24 | 22 |
Abbreviations: AN, anorexia nervosa; ΔBMI, change in body mass index (final-initial; kg/m2); BN, bulimia nervosa; ED, eating disorder; Euroam, Euroamerican; F, female; h/o, history of; Ht, height; ΔHmD, change in Hamilton depression score (final minus initial); I-BMI, initial body mass index; I-HmD, initial Hamilton depression score; I-Wt, initial weight (kg); M, male; MDD, major depressive disorder; NOS, not otherwise specified, OCD, obsessive compulsive disorder; PTSD, post-traumatic stress disorder; ΔWt, change in weight (final-initial; kg).
In years.
The VNS device (NCP Model 101, Cyberonics Inc., Houston, TX, USA) was approved in 2005 for the adjunctive therapy of severe depression refractory to conventional therapies. It consists of a pacemaker-like device implanted subcutaneously just below the left clavicle. An electrode from the device courses subcutaneously and attaches to the trunk of the left vagus nerve about half way between the clavicle and the mastoid. The right vagus is not used typically because of greater vagal side effects upon the heart. The device is programmed to deliver preset stimuli using an external, magnetic, programming wand. The protocol and device parameters have been previously described.3 The output current frequently varied either up or down depending upon the patients’ side effects and depression symptoms, since the precise relationship between dose and efficacy remains unsettled. At 1 year, the current outputs varied between 0.25 and 1.5 mA. The stimulation frequency was 30 Hz with a pulse width of 500 μs, except for two patients who had 250 μs. The device cycled continuously with stimulation on for 30 s and off for 5 min.
After continuous VNS therapy for 6-12 months, several obese patients showed marked weight loss. The mean weight on intake was 91 kg (s.d. ± 27, range 46-137 kg) with a body mass index (BMI) of 43 kg/m2 (s.d. ± 5, range 18-49 kg/m2). The average weight loss at 1 year was 7 kg (s.d.± 3, range -6 to +24) with mean drop in BMI at 1 year of 2 kg/m2 (s.d.± 3, range -2 to +8). The heaviest patient weighed initially 137 kg (BMI 49 kg/m2) and after 1 year of VNS, 114 kg (BMI 40 kg/m2), a decrease of 23 kg. Figure 1 shows that the decrease in BMI at 1 year of individual patients wasconsistently proportional to their initial BMI at enrollment (Pearson r = 0.73; d.f. 12, P<0.003), or equivalently, the loss of weight was proportional to the initial weight. This linear relationship accounted for the majority of the variance (53%). Of note, patients with normal weight or overweight (<30 mg/m2) were affected little by VNS.
Figure 1.
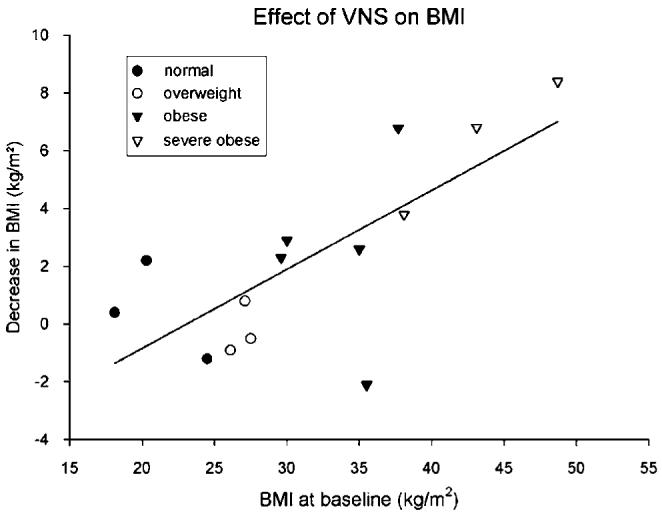
Relationship between BMI (or weight) loss after 1 year of VNS and BMI (or weight) at study entry. Normal weight, 19-24 kg (●); overweight, 25-29 (○); obese, 30-38 (▲); severely obese, 40(△). BMI, body index; VNS, vagus nerve stimulation.
These patients were followed for an additional year. To better address the significance of the weight changes over time, a repeated-measures, mixed model regression analysis of weight change following VNS implantation was performed on all BMI measurements available (that is, not limited to initial and at one year; 2-6 observations per patient for a total of 57 observations over 2 years). BMI at baseline, controlling for gender, and age at baseline, was 32 kg/m2 (s.d. = 8). BMI at baseline was a significant predictor of weight loss over time (baseline BMI × time interaction, P = 0.0006). Patients with the average BMI at baseline (BMI = 2 kg/m2) lost an estimated 3.7 kg/year. An additional 1 kg/year (s.e. = 0.3) was lost for each unit increase of BMI at baseline. Neither age at baseline nor gender was significant predictor of weight loss over time (both P>0.20). The stimulation parameters did not predict weight loss. For example, the two patients losing the most weight in Table 1 had at 1 year both the highest (1.5 mA, 500 μs) and the lowest (0.25 mA, 250 μs) settings used in this study.
As with any serendipitous, uncontrolled observation, many factors could confound these observations. The data were collected without the benefit of an a priori hypothesis, control group or blinding. These patients had psychiatric comorbidities; severely obese patients free of psychiatric diagnosis may not show the effect. An improvement in mood may be associated with less excessive eating. However, the change in weight during VNS was not significantly related to changes in the level of depression as measured by the Hamilton2 depression rating (d.f. 12; at 1 year: r = -0.44, P = 0.11; at 2 years: r = -0.13, P = 0.66).. Anecdotally, patients felt they satiated with less food. However, neither activity nor diet was controlled. Medications are another potential confound. If anything, most of the medications the patients were taking would be expected to induce weight gain rather than weight loss. The observed weight loss might reflect regression to the mean. However, the weight of obese patients typically increases, rather than decreases, over time given the induction of the metabolic syndrome. The small number of cases may limit generalization to other samples. Clearly, there remains a need for a well-designed study of the effects of VNS on weight in the severely obese. Such a study would optimally use two matched groups in a double-blind, crossover design (±stimulation) over at least 6 months per period with careful measurement of additional dependent measures such as body composition, activity level, hormone levels, metabolic rate and so on.
The extant literature provides some support that these observations are meaningful. Normal meal termination involves activation of the vagus nerve.4 Inflation of the stomach with a balloon, which increases vagal activity, causes an immediate decrease of hunger in fasting, healthy humans.5 In animal studies, intra-abdominal vagal stimulation by electrical pacing over 1 month reduces food intake and body mass at the expense of body fat without a change in metabolic rate.6,7 Subdiaphragmatic vagal deafferentation reduces food intake (g/kg) and weight gain without affecting insulin sensitivity of obese, but not lean, Zucker (fa/fa) rats.8 Alternatively, changes in vagal efferent activity by VNS may lead to alterations in the metabolism of intra-abdominal fat.9-11 There is even one clinical note suggesting that VNS for the treatment of epilepsy, its other approved indication, can cause weight loss as a side effect.12 So, modulation of vagus nerve activity, whether afferent or efferent, has complex effects on eating behaviors, some of which may prove effective for the treatment of obesity.
There are extensive safety data on VNS from its application to over 25 000 patients. Yet, severe obesity carries high morbidity, mortality and other complications. Furthermore, the prevalence of obesity continues to increase iatrogenically through the use of atypical neuroleptics, which are prescribed widely. Given the invasiveness and side effects of intra-abdominal procedures such as bariatric surgery13 or gastric pacing,14 chronic cervical VNS, if proved effective, could be a reasonable, safer alternative.
Acknowledgements
This work was supported in part by NARSAD, the Department of Veterans Affairs. Mark A Nugent Medical Foundation (St Paul, MN), NIH (Minnesota Obesity Center P30 DK 50456-02) and a research grant from Cyberonics Inc. (Houston, TX, USA) to the Minnesota Veterans Research Institute.
References
- 1.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV. American Psychiatric Association; Washington, DC: 1997. [Google Scholar]
- 2.Hamilton M. A rating scale for depression. J Neuro Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rush AJ, Marangell LB, Sackheim HA, George MS, Brannan SK, Davis SM, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized controlled acute phase trial. Biol Psychiatry. 2005;58:347–354. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Peters JH, McKay BM, Simasko SM, Ritter RC. Leptin-induced satiation mediated by abdominal vagal afferents. Am J Physiol Regul Integr Comp Physiol. 2005;288:R879–R884. doi: 10.1152/ajpregu.00716.2004. [DOI] [PubMed] [Google Scholar]
- 5.Stephan E, Pardo JV, Faris PL, Hartman BK, Kim SW, Ivanov EH, et al. Functional neuroimaging of gastric distention. J Gastrointest Surg. 2003;7:740–749. doi: 10.1016/s1091-255x(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 6.Sobocki J, Thor PJ, Uson J, Díaz-Guemes I, Lipinski M. Calles C et al. Microchip vagal pacing reduces food intake and body mass. Hepatogastroenterology. 2001;48:1783–1787. [PubMed] [Google Scholar]
- 7.Sobocki J, Fourtanier G, Estany J, Otal P. Does vagal stimulation affect body composition and metabolism? Experimental study of a new potential technique in bariatric surgery. Surgery. 2006;139:209–216. doi: 10.1016/j.surg.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari B, Arnold M, Carr RD, Langhans W, Pacini G, Bódvarsdottir TB, et al. Subdiaphragmatic vagal deafferentation affects body weight gain and glucose metabolism in obese male Zucker (fa/fa) rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1027–R1034. doi: 10.1152/ajpregu.00736.2004. [DOI] [PubMed] [Google Scholar]
- 9.Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat-functional implications. J Clin Invest. 2002;110:1243–1250. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallen EL. Vagal afferent stimulation as a cardioprotective strategy? Introducing the concept. Ann Noninvasive Electrocardiol. 2005;10:441–446. doi: 10.1111/j.1542-474X.2005.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uno K, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Imai J, et al. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science. 2006;312:1656–1659. doi: 10.1126/science.1126010. [DOI] [PubMed] [Google Scholar]
- 12.Burneo JG, Faught E, Knowlton R, Morawetz R, Kuznieky R. Weight loss associated with vagus nerve stimulation. Neurology. 2002;59:463–464. doi: 10.1212/wnl.59.3.463. [DOI] [PubMed] [Google Scholar]
- 13.Schauer PR, Ikramuddin S. Laparoscopic surgery for morbid obesity. Surg Clin N Am. 2001;81:1145–1179. doi: 10.1016/s0039-6109(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 14.Saber AA. Gastric pacing: a new modality for the treatment of morbid obesity. J Invest Surg. 2004;17:57–59. doi: 10.1080/08941930490422032. [DOI] [PubMed] [Google Scholar]


