Abstract
Objective
To evaluate the cost-effectiveness of various melanoma screening strategies proposed in the United States.
Design
We developed a computer simulation Markov model to evaluate alternative melanoma screening strategies.
Participants
Hypothetical cohort of the general population and siblings of patients with melanoma.
Intervention
We considered the following 4 strategies: background screening only, and screening 1 time, every 2 years, and annually, all beginning at age 50 years. Prevalence, incidence, and mortality data were taken from the Surveillance, Epidemiology, and End Results Program. Sibling risk, recurrence rates, and treatment costs were taken from the literature.
Main Outcome Measures
Outcomes included life expectancy, quality-adjusted life expectancy, and lifetime costs. Cost-effectiveness ratios were in dollars per quality-adjusted life year ($/QALY) gained.
Results
In the general population, screening 1 time, every 2 years, and annually saved 1.6, 4.4, and 5.2 QALYs per 1000 persons screened, with incremental cost-effectiveness ratios of $10 100/QALY, $80 700/QALY, and $586 800/QALY, respectively. In siblings of patients with melanoma (relative risk, 2.24 compared with the general population), 1-time, every-2-years, and annual screenings saved 3.6, 9.8, and 11.4 QALYs per 1000 persons screened, with incremental cost-effectiveness ratios of $4000/QALY, $35 500/QALY, and $257 800/QALY, respectively. In higher risk siblings of patients with melanoma (relative risk, 5.56), screening was more cost-effective. Results were most sensitive to screening cost, melanoma progression rate, and specificity of visual screening.
Conclusions
One-time melanoma screening of the general population older than 50 years is very cost-effective compared with other cancer screening programs in the United States. Screening every 2 years in siblings of patients with melanoma is also cost-effective.
Melanoma is the only cancer for which incidence and mortality rates are rising unabated, while screening, the potential means for reducing the burden of disease, continues to be under-used.1 In contrast to other early detectable cancers, including breast, prostate, co-lorectal, and cervical cancers, with recently decreasing mortality rates, the mortality rate for melanoma in the United States increased by 29% from 1975 to 2000.1,2
More than 62 000 new invasive melanoma cases are predicted for 2006, and the cost of treating melanoma exceeds $740 million annually in the United States.3,4 The prevalence and incidence of melanoma increase with age, particularly after age 50 years.1 Siblings of patients with melanoma are at increased risk of developing melanoma; the risk is at least double for first-degree relatives of patients with melanoma and is 5-fold higher if 2 first-degree relatives or more are affected.5
Identifiable risk factors, increasing incidence, and the availability of curative treatment for early melanoma have stimulated interest in melanoma screening programs by dermatologists during a brief focused visit.6 In a 3-year follow-up of almost 250 000 persons in the United States attending free skin cancer screenings by dermatologists, melanoma was diagnosed in 363 (1.5 cases per 1000 individuals screened).7
The lack of evidence of screening efficacy from a randomized trial has been cited as an obstacle to population-based melanoma screening, yet the cost of such a trial seems prohibitive.8 Additional concerns about the expansion of screening include questions of test accuracy, the cost of screening, and lead-time bias.8
To understand these obstacles to melanoma screening, we developed a simulation Markov model to examine the potential impact of melanoma screening by a dermatologist. Our objective was to evaluate the impact and cost-effectiveness of alternative melanoma screening programs in both the general population and a high-risk population including siblings of patients with melanoma.
METHODS
MODEL OVERVIEW
We developed a computer simulation, state-transition Markov model to evaluate alternative strategies for melanoma screening compared with background screening alone.9 Disease progression, rates of recurrence, mortality, and costs of melanoma treatment depended on disease stage and lesion thickness (Table 1). The model differentiated between local disease (stages I and II), metastatic disease with local lymph node involvement (stage III), and distant metastases (stage IV). Local disease was further divided into 4 groups according to lesion size, as follows: less than 0.76 mm (stage IA), 0.76 to 1.50 mm (stage IB), 1.51 to 3.99 mm (stage IIA), and 4.00 mm or larger (stage IIB).16 Most data available on melanoma stage-specific incidence, prevalence, and survival were based on the 1992 American Joint Committee on Cancer staging system.17 To capitalize on the best data to support the model structure, we designed the model using this staging system.
Table 1.
Base Case Parameter Estimates for Melanoma Screening*
| Parameter | Base Case | Source |
|---|---|---|
| 5-y Survival† | ||
| Stage 1 | 0.92 (0.81–0.99) | 10, 11 |
| Stage 2 | 0.72 (0.65–0.79) | 11 |
| Stage 3 | 0.50 (0.45–0.55) | 11 |
| Stage 4 | 0.13 (0.11–0.15) | 11 |
| Increased risk | ||
| General population | 1.00 | |
| Siblings‡ | 2.24 (1.00–10.00) | 5 |
| Siblings at high risk§ | 5.56 (1.00–10.00) | 5 |
| Screening | ||
| Sensitivity, % | 85 (70–100); beta (19, 3) | 12 |
| Specificity, % | 99 (70–100); beta (30, 1) | 12 |
| Cost, $ | 41 (21–123); normal (40, 15) | 13, 14 |
| Progression rate, % | 10 (0–50); beta (0.3, 2.7) | || |
| Treatment costs, $ | ||
| Stage 1 | 1732 (1299–2165) | 3 |
| Stage 2 | 4361 (3271–5451) | 3 |
| Stage 3 | 55 080 (41 310–68 850) | 3 |
| Stage 4 | 56 059 (42 044–70 074) | 3 |
| Health state utilities | ||
| Stages 1 and 2 | 0.937 | 15 |
| Stages 3 and 4 | 0.52 | 15 |
Values in parentheses are given as range used in sensitivity analysis; distribution used for probabilistic sensitivity analysis. All costs are reported in 2004 US dollars.
Based on the 1992 American Joint Committee on Cancer staging system.17
Those with 1 first-degree relative having been diagnosed with melanoma.
Those with 2 or more first-degree relatives having been diagnosed with melanoma.
Assumption based on expert opinion, varied in sensitivity analysis.
The model consisted of the following 5 mutually exclusive health states: melanoma free, undiagnosed melanoma, newly diagnosed melanoma, history of melanoma, and death (Figure 1). Transitions from the melanoma-free to the undiagnosed melanoma state were governed by age-dependent prevalence and incidence. Incidence rates ranged from 28.7 per 100 000 persons at age 50 years to 55.9 per 100 000 persons at age 80 years; prevalence rates ranged from 0.196% at age 50 years to 0.292% at age 80 years.1,11 Patients who developed melanoma remained in the undiagnosed state until a dermatologist confirmed the diagnosis and initiated treatment. False-negative screening results may also be obtained in patients with melanoma; these patients remained in the undiagnosed state. While in the undiagnosed state, the model allowed disease progression. Transition from the undiagnosed to the newly diagnosed state was defined by either background screening or a prespecified screening frequency. Patients having been diagnosed with melanoma were treated and transitioned to the history of melanoma state, in which risk of melanoma recurrence was increased.18 Patients with melanoma, regardless of diagnostic status, had higher mortality according to disease stage than did patients who were melanoma free. Mortality in the melanoma-free population was governed by age- and gender-specific US life tables.19
Figure 1.
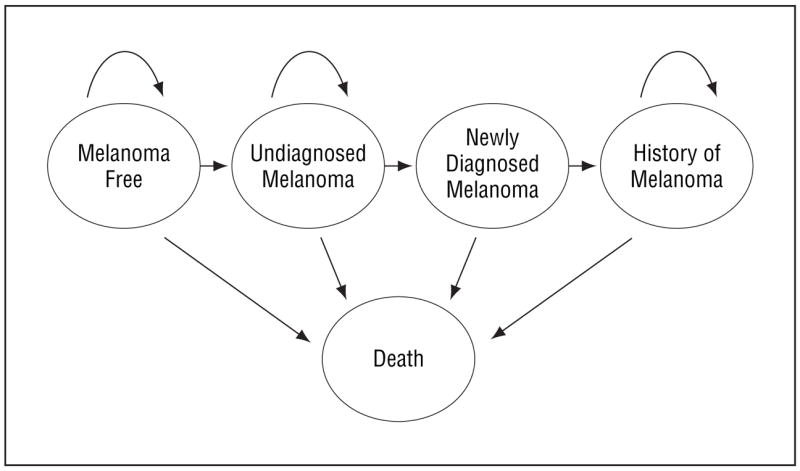
Model structure. See “Methods” section for details.
Outcomes from the model included projected life expectancy, quality-adjusted life expectancy, lifetime costs, and incremental cost-effectiveness ratios. The analyses were performed from a health care system perspective (third-party payer), excluding patient and time costs, with 3% discounting of costs and life expectancy.20
CLINICAL DATA
We used data from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute to derive age-specific incidence and prevalence of melanoma.1 We incorporated an increase in relative risk of melanoma in siblings of patients with melanoma of 2.24, and in those with multiple affected first-degree relatives of 5.56.5 Five-year survival ranged from 92% for stage I disease to 13% for stage IV disease and was derived from the Surveillance Epidemiology and End Results Program.1,11 Survival probabilities at 5 and 10 years were derived from a subset of data from this program (1988–1998) by one of us (F.C.B.). Accuracy of visual screening in the absence of a biopsy specimen was obtained from the Skin and Cancer Unit of New York University Medical Center, New York City, and included 85% sensitivity and 99% specificity.21 When visual inspection and biopsy results differed, the biopsy results were used in decision making. In patients in the undiagnosed melanoma state, we assumed a progression rate from one stage or thickness to the next of 10% per year. After discussion with experts, we used this estimate in the base case to ensure a conservative analysis. We applied age-adjusted quality-of-life weighting for the general population from the population-based Beaver Dam Health Outcomes Study, and for patients with melanoma from data presented nationally.15,22 Values for stage-based survival, incidence, prevalence, progression rates, increased risk in siblings of patients with melanoma, sensitivity and specificity of screening, and cost of screening and treatment were all varied in sensitivity analyses to determine which had the greatest impact on the results.
COSTS
The model included direct medical costs related to melanoma treatment. Treatment costs, which ranged from $1732 for stage I disease to $56 059 for stage IV disease, were obtained from published literature.3 We assumed costs as follows: screening, $41; biopsy, $245; follow-up visit, $109; and pathologic analysis, $68.13,14 All costs were updated to 2004 US dollars using the medical care component of the Consumer Price Index.
SCREENING STRATEGIES
We considered the following 4 screening strategies: background screening only; that is, skin examination at a routine nondermatologist physician visit, followed by referral to a dermatologist, on average, once every 5 years; and 1-time, every-2-years, and annual screening by a dermatologist, all beginning at age 50 years. We compared outcomes from the more frequent to less frequent screening strategies using incremental cost-effectiveness ratios. The incremental cost-effectiveness ratio for a strategy (the added cost divided by the added life expectancy) was calculated in comparison with the next most effective strategy after eliminating dominated strategies (more costly and less effective) and strategies with extended dominance (higher incremental cost-effectiveness ratios compared with more effective strategies).20–23 Outcomes for each strategy are expressed in years of life saved and in quality-adjusted life years (QALY). These outcomes enabled comparison of the cost-effectiveness of melanoma screening with other health care interventions.20
POPULATIONS CONSIDERED
We considered 3 populations: a general population, siblings of patients with melanoma (1 first-degree relative having been diagnosed as having melanoma, hereafter called siblings), and higher risk siblings of patients with melanoma (at least 2 first-degree relatives having been diagnosed as having melanoma, hereafter called siblings at high risk). The 2 populations of siblings of patients with melanoma differ from each other and from the general population with respect to an increased risk of melanoma applied to both prevalence and incidence (Table 1).3,5,10–15
RESULTS
BASE-CASE ANALYSIS: GENERAL POPULATION
In the general population, no screening was associated with a projected discounted quality-adjusted life expectancy, from age 50 years, of 13.537 QALYs, with lifetime skin cancer and screening-related costs of $236 per person. These costs are from occasional referrals to a dermatologist and comprise all direct costs associated with these referrals. One-time, every-2-years, and annual screening increased projected life expectancy to 13.539, 13.542, and 13.543 QALYs, respectively, saving 1.6, 4.4, and 5.2 QALYs, respectively, per 1000 persons screened. The incremental cost-effectiveness ratios were $10 100/QALY gained ($7400 per unadjusted year of life saved [YLS]) for 1-time screening at age 50 years compared with no screening, $80 700/QALY gained ($54 700/YLS) for every-2-years screening compared with 1-time screening, and $586 800/QALY gained ($398 600/YLS) for annual screening compared with every-2-years screening (Table 2).
Table 2.
Cost-effectiveness of Screening for Melanoma in the General Population and in Siblings of Patients With Melanoma
| Screening Frequency | Total Cost, $* | Life Expectancy, y | Quality-Adjusted Life Expectancy, y | Cost-effectiveness Ratio ($/QALY) |
|---|---|---|---|---|
| General population | ||||
| No screening program | 236 | 18.932 | 13.537 | … |
| 1 Time, at age 50 y | 252 | 18.935 | 13.539 | 10 100 |
| Every 2 years beginning at age 50 y | 481 | 18.939 | 13.542 | 80 700 |
| Every year beginning at age 50 y | 905 | 18.940 | 13.543 | 586 800 |
| Siblings of patients with melanoma | ||||
| No screening program | 316 | 18.921 | 13.529 | … |
| 1 Time, at age 50 y | 331 | 18.926 | 13.533 | 4000 |
| Every 2 years beginning at age 50 y | 550 | 18.935 | 13.539 | 35 500 |
| Every year beginning at age 50 y | 970 | 18.938 | 13.541 | 257 800 |
| Higher risk siblings of patients with melanoma | ||||
| No screening program | 537 | 18.890 | 13.507 | … |
| 1 Time, at age 50 y | 545 | 18.903 | 13.516 | 900 |
| Every 2 years beginning at age 50 y | 753 | 18.924 | 13.530 | 14 700 |
| Every year beginning at age 50 y | 1162 | 18.930 | 13.534 | 99 800 |
Abbreviations: QALY, quality-adjusted life year; ellipses, an incremental cost-effectiveness ratio cannot be calculated for the baseline strategy.
All costs are reported in 2004 US dollars.
SIBLINGS OF PATIENTS WITH MELANOMA
In siblings, lifetime costs varied from $316 per person for the no-screening strategy to $970 per person with annual screening. One-time, every-2-years, and annual screening saved 3.6, 9.8, and 11.4 QALYs per 1000 persons screened, respectively. Incremental cost-effectiveness ratios were $4000/QALY gained ($2900/YLS) for 1-time screening compared with no screening and $35 500/QALY gained ($24 000/YLS) for screening every 2 years compared with screening 1 time. Annual screening had an incremental cost-effectiveness ratio of $257 800/QALY gained ($174 900/YLS) compared with screening every 2 years (Table 2). In siblings at high risk, the corresponding incremental cost-effectiveness ratios were, respectively, $900/QALY gained ($700/YLS), $14 700/QALY gained ($9800/YLS), and $99 800/QALY gained ($67 600/YLS) (Table 2).
SENSITIVITY ANALYSES
In sensitivity analyses, we varied model parameters across a wide range (Table 1). We found that screening cost, melanoma progression rate, and specificity for melanoma detection by visual screening were the 3 most important parameters in the analysis (Figure 2). When we varied the cost of the visual screening from $21 (half of the $41 base case) to $123 (3 times the base case), the cost-effectiveness ratios for 1-time screening in both the general population and siblings of patients with melanoma remained less than $50 000/QALY gained. The cost-effectiveness ratio for screening siblings every 2 years remained less than $100 000/QALY gained the screening cost was no more than 2½ times the base case value (Figure 2A).
Figure 2.
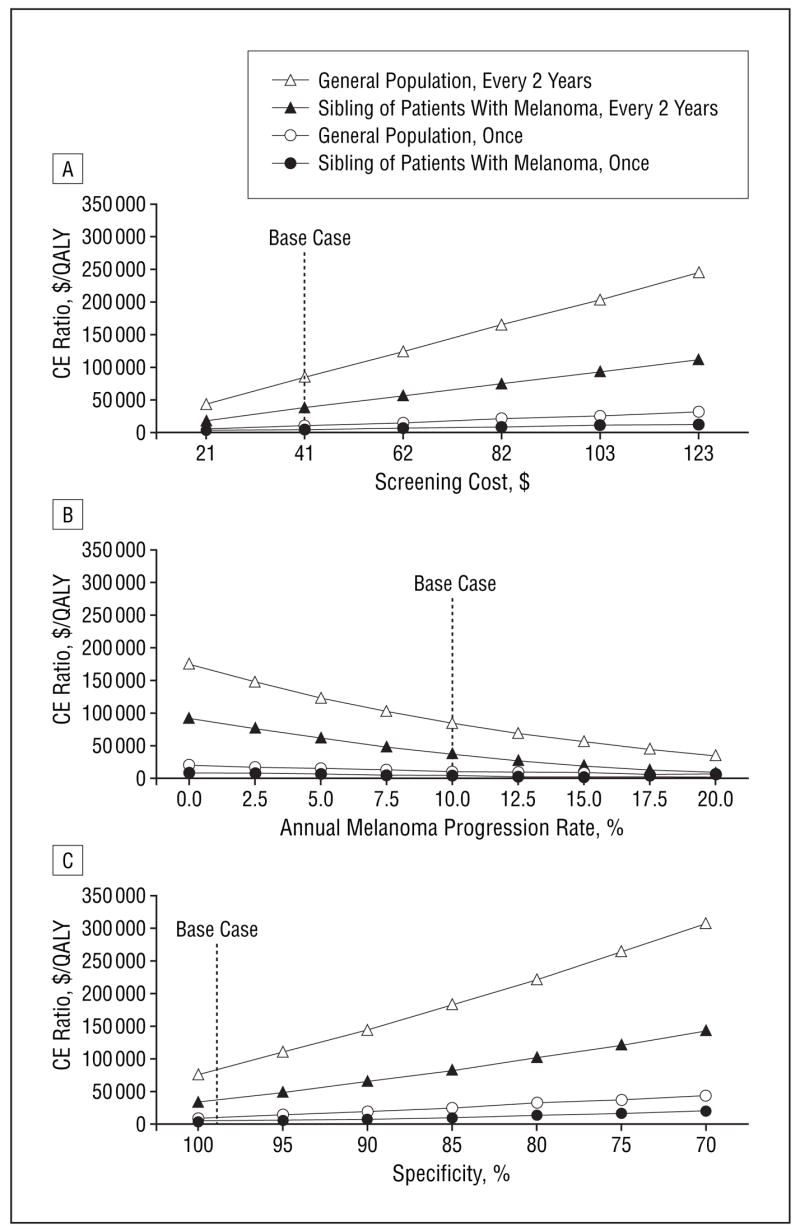
Sensitivity analyses on the impact of the screening cost (A), the annual melanoma progression rate (B), and the specificity for melanoma detection by visual screening (C) on the cost-effectiveness of melanoma screening. CE indicates cost-effectiveness; QALY, quality-adjusted life year.
As the annual progression rate of melanoma decreased, screening every 2 years became less cost-effective (Figure 2B). At a progression rate of 5% per year, screening every 2 years of the general population cost $120 600/QALY gained compared with screening 1 time. When the progression rate dropped to less than 7% per year, the incremental cost-effectiveness ratio for screening every 2 years for siblings increased to more than $50 000/QALY gained compared with screening 1 time. For siblings at high risk, when the progression rate dropped to less than 2% per year, the incremental cost-effectiveness ratio increased to more than $50 000/QALY gained compared with 1-time screening. We also examined higher progression rates (data not shown in figure). For the general population, when progression rates exceeded 36% per year, the less frequent screening strategies (no screening and 1-time screening) were dominated by screening every 2 years and annually. For siblings, the incremental cost-effectiveness ratio comparing screening annually with every 2 years was $224 600/QALY gained at a 36% progression rate. To further examine the sensitivity of the results to assumptions about the progression rate, we assumed no progression of undiagnosed melanoma in lesions less than 0.76 mm thick. This changed the results of the analysis minimally, increasing the cost-effectiveness ratio of 1-time screening in the general population by 6% and of screening every 2 years in siblings of patients with melanoma by 10%. When we doubled the progression rate of undiagnosed melanoma in lesions less than 0.76 mm thick to 20%, the cost-effectiveness ratios of 1-time screening in the general population and screening every 2 years in siblings of patients with melanoma decreased by 4% and 7%, respectively.
We also varied the specificity for melanoma detection by visual screening from 100% to 70% (Figure 2C). As specificity decreased, the cost-effectiveness ratio for 1-time screening compared with no screening in the general population remained less than $50 000/QALY gained, increasing from $9200/QALY gained at 100% specificity to $44 500/QALY gained at 70% specificity. For siblings, the incremental cost-effectiveness of screening every 2 years compared with 1-time screening remained less than $100 000/QALY gained at specificity values of 80% and greater, with the cost-effectiveness ratio ranging from $32 500/QALY gained at 100% specificity to $137 000/QALY gained at 70% specificity. The sensitivity of melanoma detection by visual screening had little effect on the results. The cost-effectiveness ratio for 1-time screening in the general population ranged from $9300/QALY gained to $11 100/QALY gained for sensitivity values of 100% and 70%, respectively. The incremental cost-effectiveness for screening every 2 years compared with 1-time screening in siblings was less than $50 000/QALY gained when sensitivity was greater than 72%. At a sensitivity of 70%, the incremental cost-effectiveness ratio for screening every 2 years compared with 1-time screening in siblings was $53 400/QALY gained. Results were not sensitive to changes in survival by stage, incidence and prevalence of disease, discount rate, costs of care, and quality-of-life estimates.
SENSITIVITY OF RESULTS TO POTENTIAL LEAD-TIME BIAS AND LENGTH BIAS
To examine the effect of potential lead-time bias on the results (the screened group of siblings had the potential to be diagnosed earlier), we assumed that less frequent screening would be used in patients with slower progressing disease (progression rate of 7%, a 30% reduction from the base case). The corresponding incremental cost-effectiveness ratios were, respectively, $24 500/QALY gained, $49 200/QALY gained, and $260 900/QALY gained for screening 1 time, every 2 years, and annually. From a policy perspective, these results are similar to the base case results.
To assess the effect of length bias on the results (faster progressing disease will be identified less often by screening), we considered a hypothetical population of siblings with fast progressing disease (progression rate of 15%, a 50% increase from the base case). In this population, the quality-adjusted life expectancies were, respectively, 13.526, 13.530, 13.539, and 13.540 QALYs per person for no screening and for screening 1 time, every 2 years, and annually. The cost-effectiveness ratios were $2400/QALY gained, $18 600/QALY gained, and $253 300/QALY gained for screening 1 time, every 2 years, and annually.
PROBABILISTIC SENSITIVITY ANALYSIS
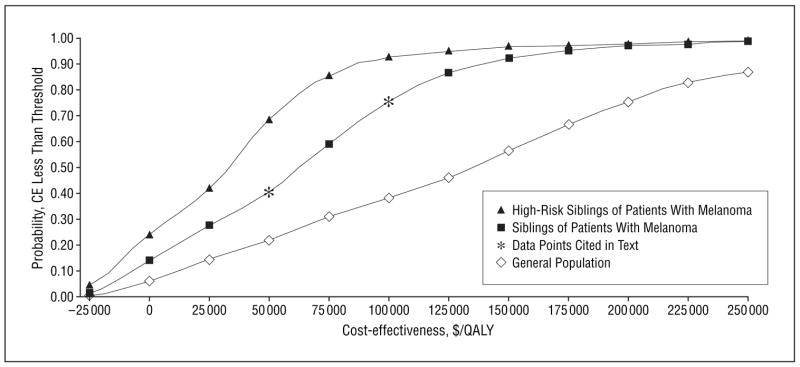
To further examine the sensitivity of our results to variation in imperfect model parameters, we also performed a probabilistic sensitivity analysis, drawing values from distributions for screening cost, sensitivity, specificity, and melanoma progression rate (Table 1). Comparing screening every 2 years with screening 1 time in siblings, the probability that the incremental cost-effectiveness ratio was less than a threshold of $100 000/QALY gained was 0.77 and the probability that it was less than $50 000/QALY gained was 0.42 (Figure 3). These results suggest that if decision makers are willing to pay $100 000 or $50 000 for each additional QALY gained, screening siblings every 2 years will be cost-effective compared with 1-time screening 77% or 42% of the time, respectively.
Figure 3.
Cost-effectiveness acceptability curve for screening every 2 years compared with 1-time screening in 3 populations. CE indicates cost-effectiveness; QALY, quality-adjusted life year.
COMMENT
We developed a state-transition model to assess melanoma disease progression with time and used it to estimate the impact and value of various melanoma screening programs. We found that 1-time melanoma screening by dermatologists in the US general population at age 50 years and screening of siblings of patients with melanoma every 2 years have cost-effectiveness ratios of $10 100/QALY gained and $35 500/QALY gained, respectively. These ratios are comparable to those for other types of cancer screening, including breast, cervical, and colorectal cancers, all of which are recommended by the US Preventive Services Task Force (Table 3).29 If screening costs could be reduced below the base case estimate of $41 per screening, screening would be even more cost-effective.
Table 3.
Cost-effectiveness of Cancer Screening Programs and US Preventive Services Task Force Ratings
| Screening Program | Description | Cost-effectiveness Ratio, $* | Source | USPSTF Rating† |
|---|---|---|---|---|
| Breast cancer | Mammogram every 2 years, ages 50–69 y | 30 500/QALY | 25 | B |
| Cervical cancer | Papanicolaou test every year, lifetime | 24 100/QALY | 26 | A |
| Colorectal cancer | Fecal occult blood test plus sigmoidoscopy every 5 years after age 50 y | 47 400/YLS | 27 | A |
| Melanoma | ||||
| Siblings of patients with melanoma | Visual screening every 2 years after age 50 y | 35 500/QALY | Current study | I |
| General population | Visual screening 1 time, at age 50 y | 10 100/QALY | Current study | I |
| Prostate cancer | Combined digital rectal examination and prostate-specific antigen determination 1 time, age 50–59 y | 20 400/YLS | 28 | I |
Abbreviations: QALY, quality-adjusted life year; USPSTF, US Preventive Services Task Force; YLS, year of life saved.
All costs are reported in 2004 US dollars.
USPSTF ratings28: A indicates the USPSTF strongly recommends that clinicians provide [the service] to eligible patients. The USPSTF found good evidence that [the service] improves important health outcomes and concludes that benefits substantially outweigh harm. B indicates the USPSTF recommends that clinicians provide [the service] to eligible patients. The USPSTF found at least fair evidence that [the service] improves important health outcomes and concludes that benefits outweigh harm. I indicates the USPSTF concludes that the evidence is insufficient to recommend for or against routinely providing [the service]. Evidence that [the service] is effective is lacking, of poor quality, or conflicting and the balance of benefits and harm cannot be determined.
Cost-effectiveness analysis is particularly useful when randomized controlled trials cannot be done because of ethical or logistic considerations. In the case of melanoma, the low overall disease prevalence and incidence would require more than 360 000 study participants followed up for 10 years to identify statistically significant differences in the outcome of screening. Cost-effectiveness analysis is most often used when decisions are being made in the absence of randomized trials with mortality end points and when the best available data can be combined from numerous sources to inform policy. Such analyses have been used to guide clinical decision making in colon cancer screening, breast cancer screening, and human immunodeficiency virus disease management, among others.28,30,31 Using this method, interventions in the United States are generally considered cost-effective at less than $50 000/QALY gained or less than $100 000/QALY gained.32,33
Several previous studies have estimated the cost-effectiveness of melanoma screening. Freedberg et al13 estimated a cost-effectiveness ratio of $39 600/YLS for 1-time screening in a population at high risk. That study was limited to 1-time screening, was applied in a younger population, and did not account for increased progression and recurrence of melanoma. Beddingfield34 estimated a cost-effectiveness ratio of $220 700/YLS for 1-time screening of a white population of all ages at average risk. However, the cost-effectiveness for older patients was much lower, at $28 700/YLS. While this estimate differs from that in the current analysis, owing to differences in defining the higher risk population and screening cost, the policy recommendations are similar. Other cost-effectiveness analyses of melanoma prevention strategies have been done in Australia and Italy. The Australian study, by Girgis et al,35 found the cost-effectiveness of screening every 2 years for melanoma by family practice physicians (60% sensitivity) to be $15 000/YLS for men aged 50 years and $25 800/YLS for women aged 50 years. While these results are similar to those in the current study, this is likely owing to both the increased prevalence and incidence of melanoma in Australia compared with the United States and the lower sensitivity of screening by family practice physicians in their study.35 Cristofolini et al,36 in Italy, evaluated the cost-effectiveness of an educational campaign for early diagnosis of melanoma and did not address screening strategies.
Several US national committees have debated melanoma screening but have not included it in recommended guidelines. The Third United States Preventive Services Task Force in 2001 concluded that “evidence is lacking that skin examination by clinicians is effective in reducing mortality or morbidity from skin cancer,”37 (p44) but called for studies to help identify patients, especially the elderly, at high risk for melanoma.37 The Institute of Medicine reached similar conclusions in 2000 about general screening recommendations but conceded that “clinicians and patients should continue to be alert to the common signs of skin cancer with a particular emphasis on older white males and on melanoma.”38(p62)
To our knowledge, there are no trials of melanoma screening in the United States, and melanoma screening was not included as part of the National Cancer Institute Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial.39 Without conclusive evidence, committees have had to rely on observational evidence that suggests but does not prove the effectiveness of melanoma screening in the detection of thinner melanomas, the main determinant of prognosis.40
This study has several limitations. Although they represent the best available published data, these data were derived from multiple sources. Lead-time and length bias may have a role in survival benefit from earlier diagnosis of melanoma, although sensitivity analyses suggest that these have modest effects on the general policy conclusions of this analysis. The actual rate of melanoma progression is unknown; while this had little effect on 1-time screening, the results of screening every 2 years were sensitive to the progression rate. Although in the base-case analysis our model assumed a constant progression rate of 10% from one stage to another, results of the sensitivity analysis confirmed that varying the progression rate between stages did not affect the conclusions. Because the progression rate for any malignancy is unknown, researchers must make specific assumptions about progression rates to examine the cost-effectiveness of screening strategies. Assumptions similar to ours were used in analyses of the cost-effectiveness of screening for breast and colon cancers.24,41 The comprehensive set of sensitivity analyses varying progression rate in this article provides important insight into the role of the progression rate in assessment of the cost-effectiveness of screening for malignant melanoma (Figure 2B).
Recent evidence also suggests that patients with a family history of melanoma are at increased risk for second primary melanomas.42 While not explicitly modeled in this analysis, to the extent that second primary cases represent a major problem and that the incidence of a second primary melanoma in a patient with melanoma is higher than the incidence of a first primary melanoma in otherwise similar patients, screening programs will be even more cost-effective.42,43 While costs of false-positive results are included in this analysis, quality-of-life decrements are not. Given that false-positive results are observed in only about 1% of persons screened and that a false-positive result provides only a small decrement in overall quality of life, the effect of false-positive results on the quality-adjusted life expectancy of the cohort is minimal. Finally, the model did not account for the detection of nonmelanoma skin cancer, which is approximately 20 times more common than melanoma and shares many of the same risk factors.44 Nonmelanoma skin cancer may be diagnosed in a melanoma screening program, adding both costs and benefits. Because the benefits from detection of these lesions are uncertain and there are insufficient data to quantify them, they were not included. Furthermore, the study by Beddingfield,34 which included costs of non-melanoma skin cancer, reported that, while the cost-effectiveness ratio increased by about 25% ($16 900/YLS), the policy recommendations did not change.
The improvements in life expectancy suggested by the results of this study were moderate because only those individuals who develop melanoma gain a survival benefit from screening. Since the prevalence of melanoma in the United States is low, mean life expectancy improvements across the population were limited. In general, increases in life expectancy as a result of screening are smaller than increases as a result of treatment because all patients receiving treatment have the disease.45
What is the future of melanoma screening? The Institute of Medicine did not endorse screening because of lack of efficacy data, yet there is no ongoing effort to obtain such data in the United States. This study suggests that 1-time screening of the general US population at age 50 years for malignant melanoma is very cost-effective and that screening every 2 years of siblings of patients with melanoma may also be cost-effective, depending on disease progression rates. Either screening programs should be expanded or efforts to perform a definitive efficacy trial should be initiated.
Acknowledgments
Funding/Support: This study was supported by grant R01CA76333 from the National Cancer Institute and grants K25AI50436, K23AI01794, and K24AI062476 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Author Contributions: Study concept and design: Losina, Walensky, Gilchrest, and Freedberg. Acquisition of data: Losina, Geller, and Beddingfield. Analysis and interpretation of data: Losina, Walensky, Beddingfield, Wolf, Gilchrest, and Freedberg. Drafting of the manuscript: Losina, Geller, and Wolf. Critical revision of the manuscript for important intellectual content: Losina, Walensky, Beddingfield, Gilchrest, and Freedberg. Statistical analysis: Losina. Obtained funding: Losina, Geller, and Freedberg. Administrative, technical, and material support: Losina, Beddingfield, Wolf, Gilchrest, and Freedberg. Study supervision: Losina and Freedberg. Framing issues: Gilchrest.
Financial Disclosure: None reported.
References
- 1.Ries LA, Eisner MP, Kosary CL, et al. SEER Cancer Statistics Review, 1975–2000. Bethesda, Md: National Cancer Institute; 2003. [Google Scholar]
- 2.Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control [published correction appears in J Natl Cancer Inst. 2003;95:1641] J Natl Cancer Inst. 2003;95:1276–1299. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 3.Tsao H, Rogers GS, Sober AJ. An estimate of the annual direct cost of treating cutaneous melanoma. J Am Acad Dermatol. 1998;38:669–680. doi: 10.1016/s0190-9622(98)70195-1. [DOI] [PubMed] [Google Scholar]
- 4.Atlanta, Ga: American Cancer Society; 2006. [Accessed March 13, 2006]. Cancer Facts and Figures. http://www.cancer.org/downloads/STT/CAFF2006PWSecured.pdf. [Google Scholar]
- 5.Ford D, Bliss JM, Swerdlow AJ, et al. The International Melanoma Analysis Group (IMAGE) Risk of cutaneous melanoma associated with a family history of the disease. Int J Cancer. 1995;62:377–381. doi: 10.1002/ijc.2910620403. [DOI] [PubMed] [Google Scholar]
- 6.Koh HK. Cutaneous melanoma. N Engl J Med. 1991;325:171–182. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- 7.Geller AC, Sober AJ, Zhang Z, et al. Strategies for improving melanoma education and screening for men age ≥50 years: findings from the American Academy of Dermatological National Skin Cancer Screening Program. Cancer. 2002;95:1554–1561. doi: 10.1002/cncr.10855. [DOI] [PubMed] [Google Scholar]
- 8.Geller AC. Screening for melanoma. Dermatol Clin. 2002;20:629–640. doi: 10.1016/s0733-8635(02)00034-7. [DOI] [PubMed] [Google Scholar]
- 9.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute, Dept of Cancer Control and Population Science, Surveillance Research Program, Cancer Statistics Branch. Surveillance, Epidemiology, and End Results (SEER) Program public-use data (1973–1999) [Accessed October 24, 2006]; Released April 2001, based on the November 2000 submission. http://seer.cancer.gov/csr/1973_1999/melanoma.pdf.
- 11.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 12.Koh HK, Geller AC. Melanoma and skin cancer control: an international perspective. Cancer Control. 1995;2:385–391. doi: 10.1177/107327489500200501. [DOI] [PubMed] [Google Scholar]
- 13.Freedberg KA, Geller AC, Miller DR, Lew RA, Koh HK. Screening for malignant melanoma: a cost-effectiveness analysis. J Am Acad Dermatol. 1999;41:738–745. doi: 10.1016/s0190-9622(99)70010-1. [DOI] [PubMed] [Google Scholar]
- 14.Dobson A, DaVanzo J, Kerns J. Cost Estimates for Expanded Medicare Benefits: Skin Cancer Screening, Medically Necessary Dental Benefits, and Immunosuppressive Therapy for Transplant Recipients. Washington, DC: Institute of Medicine, National Academy Press; 1999. [Google Scholar]
- 15.Chen SC, Bendeck SE, Hadley JC, et al. Can melanoma patients predict the quality of life impact of an alternate melanoma stage?. Presented at the 26th Annual Meeting of the Society for Medical Decision Making; October 18, 2004; Atlanta, Ga. [Google Scholar]
- 16.Balch CM, Houghton AN, Milton GW, Sober AJ, Soong SJ, editors. Cutaneous Melanoma. 2. Baltimore, Md: Lippincott Williams & Wilkins; 1992. [Google Scholar]
- 17.American Joint Committee on Cancer. Manual for Staging of Cancer. 4. Philadelphia, Pa: JB Lippincott; 1992. [Google Scholar]
- 18.Shumate CR, Urist MM, Maddox WA. Melanoma recurrence surveillance: patient or physician based? Ann Surg. 1995;221:566–571. doi: 10.1097/00000658-199505000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arias E. United States Life Tables, 2000. Vol. 51. Hyattsville, Md: National Center for Health Statistics; 2002. [Accessed October 25, 2006]. Life table for the total population: United States, 2000 [Table 1] pp. 7–8. National Vital Statistics Reports. No. 3. http://www.cdc.gov/nchs/data/nvsr/nvsr51/nvsr51_03.pdf. [Google Scholar]
- 20.Gold MR, Siegel JE, Russel LB, Weinstein M. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 21.Grin CM, Kopf AW, Welkovich B, Bart RS, Levenstein MJ. Accuracy in the clinical diagnosis of malignant melanoma. Arch Dermatol. 1990;126:763–766. [PubMed] [Google Scholar]
- 22.Fryback DG, Dasbach EJ, Klein R, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13:89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 23.Cantor SB. Cost-effectiveness analysis, extended dominance, and ethics: a quantitative assessment. Med Decis Making. 1994;14:259–265. doi: 10.1177/0272989X9401400308. [DOI] [PubMed] [Google Scholar]
- 24.Salzmann P, Kerlikowske K, Phillips K. Cost-effectiveness of extending screening mammography guidelines to include women 40 to 49 years of age. Ann Intern Med. 1997;127:955–965. doi: 10.7326/0003-4819-127-11-199712010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol. 2004;103:619–631. doi: 10.1097/01.AOG.0000120143.50098.c7. [DOI] [PubMed] [Google Scholar]
- 26.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284:1954–1961. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 27.Coley CM, Barry MJ, Fleming C, Fahs MC, Mulley AG. Early detection of prostate cancer. Part II: Estimating the risks, benefits, and costs. American College of Physicians. Ann Intern Med. 1997;126:468–479. doi: 10.7326/0003-4819-126-6-199703150-00010. [DOI] [PubMed] [Google Scholar]
- 28.US Preventive Services Task Force. Guide to clinical preventive services. [Accessed March 14, 2006]; http://www.ahrq.gov/clinic/cps3dix.htm#cancer.
- 29.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2002;137:96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- 30.Mandelblatt J, Saha S, Teutsch S, et al. The cost-effectiveness of screening mammography beyond age 65 years: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2003;139:835–842. doi: 10.7326/0003-4819-139-10-200311180-00011. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein MC, Goldie SJ, Losina E, et al. Use of genotypic resistance testing to guide HIV therapy: clinical impact and cost-effectiveness. Ann Intern Med. 2001;134:440–450. doi: 10.7326/0003-4819-134-6-200103200-00008. [DOI] [PubMed] [Google Scholar]
- 32.Goldman L. Cost-effectiveness in a flat world: can ICDs help the United States get rhythm? N Engl J Med. 2005;353:1513–1515. doi: 10.1056/NEJM2e058214. [DOI] [PubMed] [Google Scholar]
- 33.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163:1637–1641. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 34.Beddingfield FC., III Melanoma: a decision analysis to estimate the effectiveness and cost-effectiveness of screening and an analysis of the relevant epidemiology of the disease. [Accessed March 14, 2006]; http://www.rand.org/publications/RGSD/RGSD167/
- 35.Girgis A, Clarke P, Burton RC, Sanson-Fisher RW. Screening for melanoma by primary health care physicians: a cost-effectiveness analysis. J Med Screen. 1996;3:47–53. doi: 10.1177/096914139600300112. [DOI] [PubMed] [Google Scholar]
- 36.Cristofolini M, Bianchi R, Boi S, et al. Analysis of the cost-effectiveness ratio of the health campaign for the early diagnosis of cutaneous melanoma in Trentino, Italy. Cancer. 1993;71:370–374. doi: 10.1002/1097-0142(19930115)71:2<370::aid-cncr2820710217>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 37.US Preventive Services Task Force. Screening for skin cancer: recommendations and rationale. Am J Prev Med. 2001;20:44–46. [Google Scholar]
- 38.Field MJ, Lawrence RL, Zwanziger L, editors. Extending Medicare Coverage for Prevention and Other Services. Washington, DC: National Academy Press; 2000. Committee on Medicare Coverage Extension, Institute of Medicine. [PubMed] [Google Scholar]
- 39.Gohagan JK, Prorok PC, Hayes RB, Kramer BS. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials. 2000;21:251S–272S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 40.Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902–908. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ness RM, Holmes AM, Klein R, Dittus R. Cost-utility of one-time colonoscopic screening for colorectal cancer at various ages. Am J Gastroenterol. 2000;95:1800–1811. doi: 10.1111/j.1572-0241.2000.02172.x. [DOI] [PubMed] [Google Scholar]
- 42.Ferrone CR, Ben Porat L, Panageas KS, et al. Clinicopathological features of and risk factors for multiple primary melanomas. JAMA. 2005;294:1647–1654. doi: 10.1001/jama.294.13.1647. [DOI] [PubMed] [Google Scholar]
- 43.Slingluff CL, Jr, Vollmer RT, Seigler HF. Multiple primary melanoma: incidence and risk factors in 283 patients. Surgery. 1993;113:330–339. [PubMed] [Google Scholar]
- 44.Hill L, Ferrini RL. Skin cancer prevention and screening: summary of the American College of Preventive Medicine’s practice policy statements. CA Cancer J Clin. 1998;48:232–235. doi: 10.3322/canjclin.48.4.232. [DOI] [PubMed] [Google Scholar]
- 45.Wright JC, Weinstein MC. Gains in life expectancy from medical interventions: standardizing data on outcomes. N Engl J Med. 1998;339:380–386. doi: 10.1056/NEJM199808063390606. [DOI] [PubMed] [Google Scholar]