Abstract
Recent clinical and pathological studies have suggested that frontotemporal lobar degeneration (FTLD) and corticobasal syndrome (CBS) show clinical and pathological overlap. We present four years of longitudinal clinical, cognitive and anatomical data in the case of a 56-year-old woman, AS, whose clinical picture evolved from FTLD to CBS. For the first three years, AS showed a progressive speech and language disorder compatible with a diagnosis of the nonfluent aphasia variant of FTLD. At year four, 10 years after her first symptom, AS developed the classical clinical signs of CBS, including alien limb phenomenon and dystonia. Voxel-based morphometry (VBM) applied to AS’s four annual scans showed progression of atrophy from the inferior posterior frontal gyrus, to the left insula and finally to the medial frontal lobe. This case demonstrates the clinical overlap between FTLD and CBS and shows that the two can appear in the same patient at different stages of the disease in relation to the progression of anatomical damage.
Introduction
Pick was the first to describe cases of the aphasic variants (Pick, 1892) of what is now called frontotemporal lobar degeneration (FTLD; Neary et al., 1998). Neary and colleagues (1998) established the clinical criteria for FTLD and outlined three distinctive clinical presentations: 1) frontotemporal dementia (FTD), characterized by a progressive behavioral syndrome; 2) semantic dementia (SD), characterized by loss of word, object and/or face meaning with sparing of fluency and syntactic skills; and 3) progressive nonfluent aphasia (NFPA), characterized by labored speech, anomia and/or agrammatism with late behavioral changes similar to FTD. The presence of motor symptoms is a supportive feature and not exclusion criteria for an FTLD diagnosis. Typically, patients with SD and NFPA present with isolated speech and language symptoms for at least two years from onset and therefore also meet criteria for primary progressive aphasia (PPA; Mesulam, 1991).
On neuroimaging, the three variants of FTLD show different degrees and distributions of frontotemporal atrophy: right frontal and orbitofrontal in FTD (Ishii et al., 1998; Rosen et al., 2002) bilateral anterior and orbitofrontal in SD (Mummery et al., 2000; Chan et al., 2001; Gatton et al.,) 2001; Rosen et al., 2002); and left posterior frontal lobe and insula in NFPA (Gorno-Tempini et al., 2004; Nestor, et al., 2003). Pathologically, the FTLD spectrum of diseases has been associated with classic Pick’s disease with tau inclusions (Pick’s bodies; Graff-Radford et al., 1990; Kertesz et al., 1994), FTLD with ubiquitin-only-immunoreactive neuronal changes (Jackson et al., 1996; Josephs et al., 2004), dementia lacking distinctive histology (DLDH; Turner et al., 1996; Rossor et al., 2000; Snowden et al., 1992) and neurofilament inclusion body disease (Jaros et al., 2000; Cairns et al., 2003). Subcortical involvement is also common and in nearly 80 percent of all cases of DLDH there is involvement of the midbrain (Knopman et al., 1990).
In contrast, clinical definitions of corticobasal degeneration (CBD), also referred to as corticobasal syndrome (CBS; Boeve et al., 2003), emphasized motor manifestations (Rebeiz et al., 1968; Gibb et al., 1989; Riley et al., 1990; Rinne et al., 1994). Typical motor features include asymmetrical, non-L-dopa responsive extrapyramidal syndrome, myoclonus and dystonia. Cortical features that suggest a focal cortical neurodegenerative disorder, include limb apraxia, cortical sensory loss and alien limb phenomenon (Rebeiz et al., 1968; Gibb et al., 1989). More recently, speech disorders and aphasia, most often of the non-fluent type (Lippa et al., 1991; Bergeron et al., 1996; Sakurai et al., 1996; Black, 2000; Kertesz et al., 2000; Ozsdncak, et al., 2000; Mimura et al., 2001; Graham et al., 2003), and behavioral abnormalities (Kertesz et al., 1999, 2000; Mathuranath et al., 2000) also have been reported, and dementia has been described as the most common CBD presentation (Grimes et al., 1999). Motor, cognitive and behavioral manifestations often occur simultaneously. However, patients presenting with a motor syndrome have been shown to develop aphasia or behavioral symptoms later in the disease course (Kertesz et al., 2000) and cases initially showing relatively isolated speech and language, visuo-spatial and behavioral disorders have been described (Lang, 1992; Bergeron et al., 1996; Mathuranath et al., 2000; Mimura et al., 2001; Kertesz and Munoz, 2003; Tang-Wai et al., 2003). The variability in cortical and basal ganglia involvement in CBD remains a source of diagnostic confusion (Litvan et al., 1997; Boeve et al., 1999).
Neuroimaging findings in CBS show asymmetrical frontoparietal and basal ganglia atrophy or hypometabolism contralateral to the side first affected (Eidelberg et al., 1991; Sawle et al., 1991). Recent reports stress the presence of significant frontal involvement in CBS, including posterior frontal regions (inferior frontal gyrus and motor and premotor cortices) and extending to the medial wall and the supplementary motor regions (Garraux et al., 2000; Kitagaki et al., 2000; Peigneux et al., 2001). Pathologically, CBS is characterized by neuronal loss and gliosis in the cortex, basal ganglia and substantia nigra and by the presence of ballooned neurons, and argyrophilic and tau-immunoreactive astrocytic plaques (Dickson et al., 2002). The clinical, anatomical and neuropathological similarities between FTLD, PPA, CBD and Pick’s disease have led to the introduction of the concept of “Pick complex” disorder to subsume these overlapping syndromes (Kertesz et al., 1994).
Here we present longitudinal data from a single patient who provides a striking example of the clinical and anatomical overlap between FTLD and CBS, documenting the cognitive and anatomical evolution from one to the other. AS is a 56-year-old, right-handed woman who first presented with a slowly progressive nonfluent aphasia. Four years into her illness she developed an asymmetrical extrapyramidal syndrome, alien limb phenomenon, apraxia and dystonia compatible with a CBS diagnosis. VBM demonstrated that the anatomical evolution strictly corresponded to the progression of the clinical signs.
Case Report
AS first presented to the UCSF Memory and Aging Center in January of 1999 with speech output difficulties. In 1994, at the age of 51, AS observed difficulty in “expressing her thoughts.” At first, her difficulties were not evident to others but after about one year, her slowness in communicating became obvious to her children who told their mother to “just spit it out.” At this time, she had no difficulty with language comprehension or naming and she was able to function normally at her job as an executive. Her husband noted that she was “not as conversant.” By the age of 52, AS had trouble speaking in public. She compensated by rehearsing what she wanted to say and by slowing down her speech. She delegated more responsibilities to vice principals, but was considered “fine” by her coworkers. When AS first sought medical help at age 53, she was diagnosed as suffering from a functional disorder due to depression. Frustrated by this diagnosis, AS came to UCSF in January of 1999. Past medical history and familiar history were unremarkable. AS had a master’s degree in business, never smoked nor drank and was married with three children. On examination, AS was a pleasant, cooperative and socially adept woman. Her speech was slow and deliberate with subtle loss of prosody. There were no clear signs of dysarthria or apraxia of speech and comprehension and the basic neurological examination were normal. Over the next year, AS observed that her speech became increasingly slow and stilted, especially during public speaking.
By early 2000 at the age of 53, AS was no longer able to talk to her clients and she sought work reassignment. She began working in a simpler job where less contact with the public was required. Despite increasing difficulties at work, AS was still able to manage the house well, cook and clean. By mid-2000, mild compulsive behaviors were first noted, such as the need to place dishes in the dishwasher in a particular arrangement and to move groceries to particular areas of her car. Over the next six months she gained 15 pounds, her mood improved and her sex drive decreased. Neurological examination in late 2000 showed slower and more effortful speech compared to the previous year but still no signs of speech apraxia or dysarthria. Bedside naming, repetition and comprehension remained intact. AS showed mild slowing of fine finger movements on the right hand. No Parkinsonian signs, such as cogwheeling, hypophonia, hypomimia or seborrhea were found. Furthermore, no limb or bucco-facial apraxias were noted.
By early 2001 behavioral symptoms became more pronounced. Judgment worsened and she began driving aggressively. Her compulsions now included inappropriately sweeping up dishes at restaurants. Her speech and writing slowed and she encountered greater word-finding difficulties. Over the next year AS’s speech and language impairments declined. She retired in late 2001 at the age of 55. Neurological examination in November 2001 showed that her language output was clearly nonfluent, with mild speech apraxia. AS spoke only if interrogated. Naming, repetition and comprehension abilities were still normal. AS showed slightly greater difficulty with fine finger movements of the right hand, mildly decreased arm swing on the right, a subtle, general slowing of movement but no rigidity. On the UCSF bedside examination (which evaluates transitive and intransitive movements, such as waving goodbye, saluting, brushing teeth, combing hair and cutting bread) she showed mild limb apraxia, with obligatory use of hand as body part. She was gradually brought up to 0.25 mg t.i.d. of generic Mirapex, a dopamine agonist.
By early 2002, problems with using her right hand increased and she started using her left upper limb during cooking and eating. Motor deficits also led to increasing difficulty dressing and tying her shoes. AS began exhibiting greater signs of a frontal executive disorder and became more compulsive. Her behavior worsened and she stared inappropriately at others and left tissues in her nose to stop a nasal drip. Neurological examination showed slow, effortful, nonfluent speech with significant speech apraxia. Ideomotor apraxia on the right side was noted, as was mild buccofacial apraxia limited to movements that involved the posterior part of the mouth and throat, such as coughing. She showed increased latency in initiating lateral gaze, particularly toward the right. Extra-pyramidal symptoms in her right arm were slightly worse, resulting in mild slowness and cogwheeling. The dosage of Mirapex was increased to 0.625 mg t.i.d. with decreased rigidity and cogwheeling and faster finger movements in the right limb. Speech output was not improved by the medication.
In late November 2002, AS changed dramatically. Her gait became unstable with frequent falls, while motor symptoms in the right limbs became prominent. Speech was effortful, consisting mainly of single words and two or three-word sentences with severe speech apraxia and mild hyperkinetic dysarthria. She showed right upper alien limb phenomenon, with involuntary groping and incontrollable grasping, accompanied by a feeling of estrangement from the right hand. Her right hand continuously groped at visible objects, and when it grabbed items, the hand had to be physically pried off because AS was unable to spontaneously release them. Her right arm became rigid and began to exhibit dystonic posturing. AS’s frontal executive syndrome increased and she developed ritualistic behaviors around feeding and making her bed. Her appetite also increased. By end of January 2003, AS’s balance and rigidity had further deteriorated and she was wheelchair bound. Her husband reported no further benefit from the 1 mg Mirapex t.i.d. Her alien right upper limb remained and was associated with painful dystonia and unintentional groping. Her right leg also showed increased tone. Her speech continued to decline and she was now functionally mute. When she did speak she was echolalic, producing only single words or repeated stereotypical expressions such as “you have to.” AS could perform only simple movements to command. Her right arm wandered over the arm of the wheelchair but could not be moved on command. When asked to move the right hand or to produce a sound, AS showed an effortful face expression and movement of her whole trunk and mouth but could not comply. She gave the clinical impression of understanding what she was asked to do but could not perform the correct movements. Her husband confirmed this impression by saying that she laughed and cried appropriately in social situations. AS could still understand one-and two-step commands. At this time, she could not initiate any horizontal eye movements. Botulinum toxin injection helped to decrease painful contraction of the dystonic hand.
Methods
General Neuropsychological and Functional Evaluation
AS underwent four annual neuropsychological and functional evaluations from 1999 to 2002. Comprehensive evaluations were performed in 1999, 2000 and 2001. In 2002, AS’s extreme slowness and significant motor impairments prevented the administration of many cognitive tests. Though many of the tests have published normative data available to allow standardization of the patient’s scores, the same battery also was administered to 15 age- and education-matched healthy normal control subjects in order to provide comparable normative data for the tests without published norms.
General intellectual function was assessed using the Mini-Mental State Examination (Folstein et al., 1975). Assessment of visuospatial abilities included copying a modified version of the Rey-Osterreith figure, as well as performing the WAIS-III Block Design Test, in which the subject is asked to replicate a two-dimensional geometric figure using colored cubes (Wecshler, 1997a). The patient also was tested using the Number Location test from the Visual Object Space Perception Battery, for which she was asked to precisely locate a stimulus on a two-dimensional plane (Warrington et al., 1991), and she also performed the first 6 (non-rotated) items from the Benton Faces test (Benton et al., 1983). Nonverbal episodic memory was measured by asking the patient to make a free recall drawing of the modified Rey-Osterreith figure after a 10-minute delay. The Visual Reproduction and Faces tests from the Wechsler Memory Scale—Third Edition (Wecshler, 1997b) also were administered at some sessions. Verbal memory was measured using the California Verbal Learning Test—Mental Status (CVLT-MS) Version (Delis et al., 2000a, b). A variety of measures were used to assess executive functioning. Auditory and visual working memory were evaluated using the Digit Span and Spatial Span tests from the WMS-III. The first Design Fluency subtest (5 filled dots) from the Delis-Kaplan Executive Function Scales was used (Delis et al., 2001), as well as a version of the Trailmaking test modified for a geriatric population to include numbers and days of the week. The Color-Word trial of the Stroop test also was administered. Abstract reasoning was assessed by asking the patient to evaluate two similarities, one metaphor, and one proverb for a total of four points. Ability to perform five arithmetic calculations was also assessed. Praxis was evaluated by asking patients to perform seven buccofacial, transitive limb, and intransitive limb praxis tasks, each of which was rated on a two-point scale. At the time of clinical assessment, the patient underwent a functional assessment that included a structured caregiver interview based on the Washington University, St. Louis (WUSTL) worksheets for calculation of the Clinical Dementia Rating Scale (CDR; Morris, 1993).
Speech and Language Assessment
A complete speech and language assessment was performed only in year 2001. In 1999 and 2000 limited tests were performed and in 2002 AS’s conditions allowed the collection of only few tests. Articulation abilities were tested using the Motor Speech Evaluation (MSE; Wertz et al., 1984), which elicits speech samples with such tasks as vowel prolongation, repetition of syllables, words, and phrases; oral reading; and picture description. The examiner determines the presence or absence of dysarthria and apraxia of speech as well as a severity rating (1–7) for each. The Verbal Agility component of the Boston Diagnostic Aphasia Examination (BDAE; Goodglass et al., 1983) also was performed in 1999. Spontaneous speech and syntactic production were evaluated using the spontaneous speech section (including answering questions and describing the picnic scene) from the Western Aphasia Battery (WAB; Kertesz, 1980). Written language production was tested using the written version of the WAB picture description test. The repetition subtests of the BDAE and WAB were used to assess word and sentence repetition skills. Confrontation naming was evaluated using the 60- or 15-item versions of the Boston Naming Test (BNT). To test visual semantic abilities, the three-picture version of the Pyramid and Palm Trees test was administered (Howard and Patterson, 1992). Comprehension of spoken single words was tested with the Auditory Word Recognition subtest of the WAB. Sentence and syntactic comprehension abilities were tested using the Sequential Command subtest of the WAB and, more extensively, by selected subtests of the Curtiss-Yamada Comprehensive Language Evaluation-Receptive (CYCLE-R; Curtiss and Yamada, 1988). Eleven subtests of the CYCLE-R were administered containing five sentences each, for a total of 55 sentences. All subtests require that the patient listen to a sentence presented verbally and select the line drawing that matches the meaning of the sentence from an array of three- or four-line drawings. The subtests span a range of sentence types comprising different levels of morphosyntactic complexity, from simple constructions, such as simple declaratives and possession (CYCLE level 2 and 3), more elaborated structures (active voice, agentless and agentive passive voice, double embedding: CYCLE level 4, 5, 6) and the most complex structures (object clefting, subject relative clauses, negative passives, object relative clauses and object relative clauses with relativized object: CYCLE level 7, 8, 9). The numbers denominating the levels correspond to the age at which children normally learn to comprehend the considered type of sentence. Single-word reading was tested by the Regularity and Reading, Lexical Morphology and Grammatical Class subtests of the Psycholinguistic Assessments of Language Processing in Aphasia (PALPA; Kay et al., 1992). Reading of a passage also was administered as part of the MSE. Phonological and phonological memory skills were assessed using the Homophone Decision subtest of the PALPA and the Gathercole and Baddeley’s Non-Word Repetition task (Gathercole et al., 1994). The 40 nonwords included in the test, which vary in length between two and five syllables with 10 nonwords at each syllable length, were presented to AS in random order.
All cognitive and neuroimaging evaluations reported as belonging to same year were acquired within a three-month period.
MRI Scanning and Voxel-based Morphometry
MRI scans were obtained on a 1.5-T Magnetom VISION system (Siemens Inc., Iselin, NJ, USA) equipped with a standard quadrature head coil. Structural MRI sequences included a volumetric magnetization prepared rapid gradient echo MRI (MPRAGE, TR/TE/TI = 10/4/300 ms) to obtain T1-weighted images of the entire brain, 15° flip angle, coronal orientation perpendicular to the double spin echo sequence, 1.0 × 1.0 mm2 in-plane resolution and 1.5 mm slab thickness. Longitudinal imaging data was obtained and images were acquired for four consecutive years in 2000, 2001, 2002 and 2003. Each image was obtained within three months from the clinical and cognitive evaluations.
Voxel-based morphometry (VBM) is a technique for the detection of regional brain atrophy by voxel-wise comparison of grey matter volumes between groups of subjects (Ashbumer and Friston, 2000; Good et al., 2002). The technique comprises an image preprocessing step (spatial normalization, segmentation, modulation and smoothing) followed by statistical analysis. Both stages were implemented in the SPM99 software package (www.fil.ion.ucl.ac.uk/spm). To optimize the spatial normalization of the subjects’ images into a common anatomical space, we created an ad hoc a priori template image, which was used as the template for the subsequent normalization of images obtained from the patient and 15 age-matched healthy women. AS’s images obtained in 2000, 2001 and 2002, within a two-month period from the language and neuropsychological evaluations, were first coregistered to the image obtained in 1999. Affine and nonlinear transformations were applied to the image obtained in 1999 in order to spatially normalize it to the template image. The same normalization parameters were then applied to the images obtained in 2000, 2001 and 2002. Affine and nonlinear transformations were also applied to spatially normalize the control images. Each normalized image was then segmented into gray, white and CSF compartments. Since the nonlinear spatial transformation step can alter the volume of certain brain regions, a further “modulation” step was performed. This involved multiplying gray matter voxel values by the Jacobian determinants derived from the spatial normalization step. Spatially-normalized, segmented and modulated gray matter images were then spatially smoothed with a 12 mm FWHM isotropic Gaussian kernel. This step allowed intersubject anatomical comparison and application of the theory of Gaussian fields. Each scan obtained from AS was compared to 16 age-matched female controls (mean age 55). Age and total intracranial volume were entered into the design matrix as nuisance variables. Regionally specific differences in gray matter volumes were assessed using the general linear model and the significance of each effect was determined by using the theory of Gaussian fields. VBM has been proven to be sensitive to differences in gray matter volumes between groups of subjects. Recently, it also has been validated for single subjects analysis, as long as a sufficient smoothing kernel has been applied (Salmond et al., 2002). We accepted a level of significance of p < 0.05 corrected for multiple comparisons but, because of the risk of false negatives in single subject analysis, we also report effects at p < 0.001 uncorrected. AS’s scans from each year were compared to controls.
Results
Cognitive Assessment
When AS first presented to UCSF in 1999, her speech production complaints were mainly subjective. AS performed within normal limits in all our general neuropsychological and functional tests, though her performance on complex executive tasks such as the Trailmaking and Stroop tasks was slow (see Table 1). A limited formal speech and language evaluation was performed in 1999 and AS was impaired only in the Verbal Agility subtest of the BDAE, being able to repeat only eight times the words “thanks” and “huckleberry” in five seconds. She showed borderline performance on a phonemic word generation task, though her semantic word production was average. Confrontation naming and semantic functions were spared. AS also showed perfect scores in the single-word and sentence repetition subtests of the BDAE.
Table 1.
AS demographic, functional, general cognitive and language data compared to published normative data, or to fifteen age- and education-matched normal control subjects. Impaired scores are indicated in bold
| Demographics and functional data (max): | ||||
|---|---|---|---|---|
| Year 1 (1999)
Score (%) |
Year 2 (2000)
Score (%) |
Year 3 (2001)
Score (%) |
Year 4 (2002)
Score (%) |
|
| Age | 53 | 54 | 55 | 56 |
| Geriatric Depression Scale* | 7 (16) | — | 8 (8) | NA |
| MMSE (30) | 29 (50) | 29 (50) | 27 (9) | 16 ( < 1) |
| CDR | 0 | 0.5 | 0.5 | — |
| Visuospatial functions (max): | ||||
| Modified Rey-O Copy (16)* | 16 ( ≥ 69) | 15 (37) | 13 (2) | NA |
| Block Design (Scaled Score) | 13 (84) | — | 9 (37) | NA |
| Benton Faces (6)* | — | — | 6 ( ≥ 50) | 4 (2) |
| VOSP Number Location (10) | — | — | 10 ( ≥ 71) | 8 (10) |
| Visual memory: | ||||
| Modified Rey-O Delay (17)* | 17 ( ≥ 93) | 17 ( ≥ 93) | 15 (74) | NA |
| WMS-III Visual Reproductions II (scaled score) | 14 (91) | — | — | NA |
| WMS-III Faces II (scaled score) | — | — | 18 ( > 99) | NA |
| Verbal memory: | ||||
| CVLT-MS: | ||||
| 4-trial total correct (36) | 31 (78) | 31 (78) | 25 (8) | 13 ( < 1) |
| 30″ free recall (9) | 9 ( ≥ 98) | 9 ( ≥ 98) | 7 (63) | 2 ( < 1) |
| 10′ free recall (9) | 9 ( ≥ 84) | 9 ( ≥ 84) | 8 (63) | 0 ( < 1) |
| 10′ recognition (9) | 9 ( ≥ 63) | 9 ( ≥ 63) | 9 ( ≥ 63) | 9 ( ≥ 63) |
| Working memory: | ||||
| Spatial span backward (# digits) | 7 (98) | — | 6 (75) | NA |
| Digit span backward (# digits) | 5 (56) | 5 (56) | 3 (16) | D/C |
| Executive functions: | ||||
| Design fluency (designs/minute scaled score) | 10 (63) | 8 (37) | 7 (25) | NA |
| Modified trails speed (lines/minute)* | 26 (10) | 24 (6) | 18 (1) | NA |
| Abstraction total (4)* | 4 ( ≥ 81) | 3 (32) | 3 (32) | NA |
| Stroop Color-Word (# words/min)* | 37 (6) | 24 (1) | 17 ( < 1) | NA |
| Calculation (5)* | 5 ( ≥ 73) | 5 ( ≥ 73) | 5 ( ≥ 73) | NA |
| Praxis(14)* | 14 ( ≥ 64) | 14 ( ≥ 64) | 11 ( < 1) | NA |
| Speech and language production | ||||
| MSE Speech Apraxia Rating (7) | — | — | 2 | 6 |
| MSE Dysarthria Rating (7) | — | — | 0 | 2 |
| BDAE verbal agility | 12 ( < 1) | — | 10 ( < 1) | — |
| WAB speech fluency (10) | — | — | 10 | 5 |
| WAB speech information content (10) | — | — | 9 | 2 |
| Phonemic word production: “D” words* | 10 (7) | 9 (4) | 4 ( < 1) | NA |
| Semantic word production: animals | 18 (34) | 13 (7) | 11 (3) | NA |
| Phonological memory skills | ||||
| Gathercole & Baddeley Nonword repetition (40) | 39 (NA) | 38 (NA) | ||
| PALPA Homophone decision (60) | — | — | 47 (NA) | |
| Lexical retrieval, verbal comprehension and semantics | ||||
| Boston Naming Test | 60/60 ( > 90) | 15/15 ( > 90) | 60/60 ( > 90) | 8/15 (< 1) |
| Pyramid and Palm Trees Pictures (52) | 52 ( > 75) | 52 ( > 75)† | 51 (9)† | — |
| WAB Yes/No Comprehension (60) | 57 (< 1) | 57 (< 1) | ||
| WAB Auditory Word Recognition (60) | 60 9 (NA) | 60 (NA) | ||
| WAB Repetition (100) | 88 (< 1) | 72 (< 1) | ||
| Sentence comprehension | ||||
| WAB Sequential Commands (80) | 67 (< 1) | 62 (< 1) | ||
| Comprehension of sentences (7)* | 7 (61) | 7 (61) | 5 (< 1) | — |
| CYCLE (syntactic task)* Total (55) | 45 (< 1) | 33 (< 1) | ||
| Cycle 2, 3 (10) | 10 (NA) | 10 (NA) | ||
| Cycle 4 (15) | 14 (NA) | 13 (NA) | ||
| Cycle 5, 7 (10) | 8 (NA) | 4 (NA) | ||
| Cycle 8 (10) | 8 (< 1) | 5 (< 1) | ||
| Cycle 9 (10) | 6 (< 1) | 2 (< 1) | ||
| Reading | ||||
| PALPA Spelling to sound: regular words (max = 30) | 29 (< 1) | NA | ||
| exception words (max = 30) | 29 (< 1) | NA | ||
| PALPA lexical morphology (max = 60) | 54 (NA) | NA | ||
| PALPA grammatical class (max = 80) | 78 (NA) | NA | ||
Percentiles calculated using normal control data; otherwise, published norms were used.
In 2000 AS’s general neuropsychological testing was largely normal. Mini Mental State Examination (MMSE) was still 29 but CDR went from 0 to 0.5. Phonemic word production and the Stroop were accurate but had become impaired due to her slow speech. Her speech was more deliberate but no formal speech assessment was conducted. AS still obtained perfect scores on confrontation naming and semantic tests. She made one error in the Gathercole and Baddeley nonword repetition task and her span of digits backward remained normal, indicating good short-term phonological memory skills. At this time, she sought reassignment to a less demanding position at work.
In September 2001 AS’s MMSE was 27 and her CDR still 0.5. Scores on the Stroop task and phonemic word production continued to decline. At this point, semantic word production also fell below normal limits, and her trailmaking speed had slowed to the impaired range. Scores on other tasks requiring motor skills declined as well, including visuoconstruction, block design, and right-handed praxis. Her score on visuoper-ceptual tasks with no motor component remained perfect. There was a notable discrepancy between visual and auditory working memory scores, with spatial span backward remaining in the high average range, while digit span backward decreased to low average. Verbal memory scores were still average, and she performed in the high average to superior range on tests of visuospatial memory. Calculation remained intact. Formal speech and language assessment was conducted. AS’s speech was slow, effortful and showed mild signs of speech apraxia. Phrase length was reduced but she did not show clear signs of agrammatism in spontaneous speech production nor writing. However, AS showed hesitancies, article omissions and surface dysgraphic errors in the written picture description (see Figure 1).
Fig. 1.

AS’s oral and written description of the WAB picnic picture in 2001.
AS made few agrammatic errors when reading a passage and repeating sentences. While her lexical retrieval, semantic abilities on pictures and single-word comprehension were still normal, she clearly showed deficits in comprehending sentences (WAB and CYCLE tests). She had particular difficulty comprehending sentences requiring comprehension of prepositions in the WAB (i.e., “point to the comb with the pen”) and sentences with complex morphosyntactic structures in the CYCLE (i.e., CYCLE 9: negative passives as “The girl is not being led by the boy”; see Table 1). Short-term phonological memory abilities were still spared as she was able to correctly repeat 38 nonwords. However, she had difficulty with the PALPA homophone decision test, especially with the nonword pairs (13/20) and regular word pairs (15/20), while she performed perfectly on the irregular words pairs (20/20). AS made grammatical errors while reading a passage and also had difficulty in single-word reading tasks, especially in PALPA lexical morphology lists.
In December 2002, she declined in motor and praxis skills. Speech output was limited to few stereotypes, such as “I have to” or “you have to” and single words. She had severe speech apraxia and mild dysarthria. Her MMSE score was 16/30. Visuoconstruction tasks could not be performed, but she seemed to retain some function on visuoperceptual tests. She could not draw the modified Rey-Osterreith figure either as a copy or after a delay, but recognized the original stimulus after 10 minutes when given a four-item multiple choice trial. Similarly, she had difficulty speaking the words during the learning and free recall trials of the verbal memory test, but recognized 9/9 words on the yes–no recognition trial, suggesting relative preservation of verbal learning and memory. Due to the heavy motor and verbal demands of executive tasks, testing in this domain was not attempted. AS’s score in the BNT dropped to 8/15, but she was still able to name an additional four items with phonemic cueing, and the last three items when shown written multiple choice options to which she could point. Her score on a single-word comprehension test that did not require verbalization (WAB word recognition) was still perfect. AS’s syntactic comprehension skills declined significantly. Reading and writing tasks were not attempted. By January 2003 she was mute and wheelchair bound.
Voxel-based Morphometry
When AS’s image from late 1999 (year 1; see Table 2 and Figure 2) was compared to controls, no area showed decreased volume at a corrected level of significance. However, the left inferior frontal gyrus, insula, frontal and temporal poles and medial frontal lobe were significant at p < 0.001 uncorrected.
Table 2.
Voxel-based morphometry results for AS’s scan for each year compared to 15 age-matched controls.
| Brain area (BA) | YEAR 1
|
YEAR 2
|
YEAR 3
|
YEAR 4
|
||||
|---|---|---|---|---|---|---|---|---|
| coordinates | Z score | coordinates | Z score | coordinates | Z score | coordinates | Z score | |
| Left inf frontal gyrus | −53, 12, 18 | 4.4 | −53, 12, 18 | 4.9* | −54, 12, 16 | 5.2* | −54, 12, 14 | 5.7* |
| pars opercularis (44) | −57, 6, 9 | 3.7 | −57, 6, 9 | 4.2 | −57, 6, 9 | 4.5 | −44, 7, 4 | 5.5* |
| — | — | — | −47, 16, 5 | 5.5* | ||||
| pars triangularis (45) | — | — | — | −49, 27, 6 | 5.0* | |||
| pars orbitalis (47) | — | −47, 38, 3 | 3.1 | −47, 41, 4 | 3.4 | −45, 38, 3 | 4.5 | |
| Left insula | −44, 7, 4 | 3.3 | −44, 7, 4 | 4.0 | −44, 7, 4 | 4.9* | −44, 7, 4 | 5.5* |
| −34, 22, −7 | 2.7 | −34, 22, −7 | 3.1 | −34, 22, −7 | 3.8 | −35, 21, −8 | 5.0* | |
| Left inf central sulcus/subcentral gyrus (43) | — | −61, −19, 17 | 4.1 | −61, −19, −17 | 4.6 | −61, −19, 18 | 4.7 | |
| Left frontal pole (10) | −40, 56, −1 | 3.5 | −39, 56, 0 | 3.9 | −39, 56, 0 | 4.4 | −39, 56, 0 | 4.3 |
| — | — | — | −48, 38, 3 | 4.6 | ||||
| Medial frontal (6/8/9/10) | −6, 40, 20 | 3.5 | −6, 40, 20 | 3.7 | −6, 42, 20 | 3.7 | −6, 45, 24 | 3.6 |
| −5, −2, 60 | 3.2 | |||||||
| −6, 15, 48 | 3.7 | −6, 14, 49 | 3.4 | −5, 15, 51 | 3.7 | 6, 20, 55 | 5.1* | |
| 7, −2, 62 | 4.6 | |||||||
| — | — | 7, 65, −7 | 3.2 | 7, 65, −7 | 3.2 | 7, 63, −8 | 4.6 | |
| Left caudate | — | — | — | −14, 15, 5 | 3.7 | |||
| Right caudate | — | — | — | 15, 18, 2 | 3.1 | |||
| Left anterior temporal (38/21) | −39, 20, −36 | 4.1 | −38, 20, −36 | 4.3 | −39, 20, −36 | 4.5 | −38, 20, −37 | 4.8 |
| −53, 12, −28 | 4.0 | −53, 12, −28 | 4.0 | −53, 12, −28 | 4.1 | −53, 12, −27 | 4.6 | |
| — | −57, 7, 13 | 3.8 | −58, 6, −14 | 4.3 | −57, 7, −13 | 4.6 | ||
| Left thalamus | — | −6, −14, −2 | 3.7 | −5, −19, 5 | 3.9 | −5, −20, 7 | 4.1 | |
| −5, −20, 6 | 3.5 | −4, −22, 2 | 3.8 | −4, −24, 1 | 4.0 | |||
| Left angular gyrus (39) | — | — | −53, −68, 26 | 3.7 | −53, −68, 30 | 4.0 | ||
| Left inf temporal gyrus (37) | — | −65, −45, −13 | 3.7 | −65, −45, −11 | 3.9 | −66, −39,−16 | 4.6 | |
| Left sup parietal lobule (7) | — | −21, −55, 67 | 4.4 | −21, −55, 67 | 4.7 | −19, −51,69 | 4.3 | |
p < 0.05 corrected for multiple comparisons.
Fig. 2.
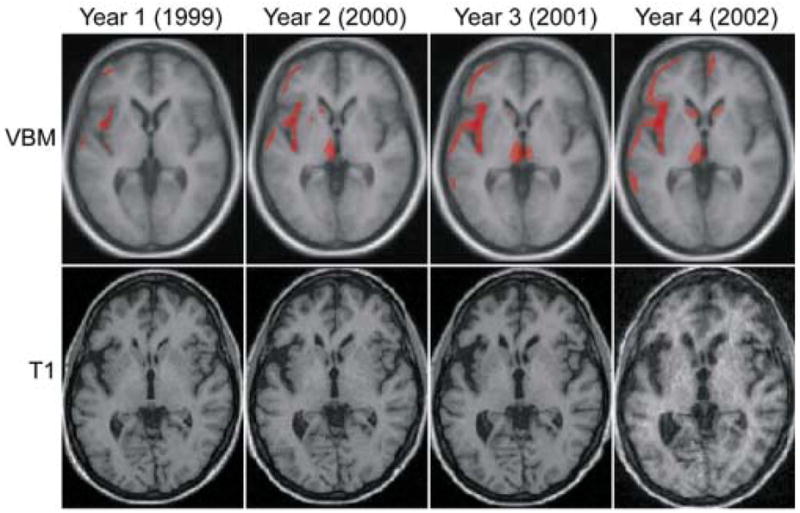
VBM results for the four annual scans of AS compared to controls. Scans were acquired in November or December of each year. The first line illustrates the VBM results thresholded at p < 0.005 uncorrected for multiple comparisons. The low level of significance was chosen to highlight the focality of the disease, even when a looser statistical threshold is applied. Areas of atrophied indicated in red are superimposed on axial sections (z = 0) of the mean image of all subjects used to create the template used for normalization. The second line shows AS’s original T1-weighted scans coregistered to show the same section as above.
In late 2000, the same cortical regions involved in 1999 became more significant (see Table 2), but only the pars opercularis of the inferior frontal gyrus reached a corrected level of significance. Furthermore, the left inferior premotor area, a more superior area in the medial frontal region, the thalamus, the posterior inferior temporal gyrus and the superior parietal lobule also showed decreased volume (p < 0.001 uncorrected).
In late 2001 all the same regions that were involved in 2000 showed either the same (medial frontal), or increased level of significance and extent. In particular, the left anterior insula now reached a corrected level of significance (see Table 2).
In the scan obtained in December 2002, atrophy corrected for multiple comparisons extended to left prefrontal regions, to more anterior regions in the left insula and the medial frontal lobe comprising supplementary motor area (SMA). It should be noted that the focus of most severe atrophy in the left medial frontal region is at a positive × coordinate while all other in the same region are at a negative coordinate. Because of the 12 mm smoothing kernel, we report all these regions as “medial frontal” and cannot comment on the lateralization of atrophy. The caudate nuclei were also atrophied but not at a corrected level of significance (p < 0.001 uncorrected; Z = 3.7 and 3.1).
Discussion
We report clinical, cognitive and anatomical longitudinal findings from a single patient who slowly evolved from NFPA to CBS. This case demonstrates that the FTLD and CBS clinical syndromes can present as different stages of the same disease in relation to the progression of anatomical damage. For the first three years of our evaluations, AS demonstrated an isolated, progressive speech and language disorder with mild behavioral and motor symptoms, compatible with a diagnosis of the non-NFPA variant of FTLD. Four years after presentation to our clinic and 10 years from the first reported symptom, AS developed a severe, right-sided extrapyramidal syndrome, limb apraxia, dystonia and alien limb phenomenon, compatible with a CBS clinical diagnosis. Remarkably this shift from NFPA emerged over less than one month. With this clinical evolution, VBM showed the progression of atrophy from the left posterior frontal gyrus, to the left insula and medial frontal lobe.
AS first complained of subjective speech output troubles when she was in her late forties, but her initial subjective speech output difficulties were attributed to depression. Six years after her first symptoms she still showed only slow speech output and borderline scores in verbally mediated executive tasks. VBM analysis of MRI did not reach a corrected level of significance but involved crucial regions for speech and language production, such as the left inferior frontal gyrus and the anterior insula. Damage to these regions has been shown to be involved in causing speech apraxia in acute and chronic stroke patients (Dronkers; 1996; Hillis et al., 2004). Furthermore, the left inferior frontal gyrus has a suspected role in syntactic processing (Tettamanti et al., 2002). In AS’s case mild damage to these regions was associated with slowness of speech.
The following year, speech output had further decreased but she still did not show clear signs of speech apraxia. There was mild difficulty with fine finger movements of her right hand and worsening behavior. Tests that involved left frontal lobe functions, such as phonemic word generation and the Stroop, now fell below normal range. Correspondently, atrophy in the left inferior frontal gyrus reached a corrected level of significance and atrophy spread posteriorly to primary motor regions.
By 2001, AS’s speech and language deficits became more evident and by this point she met criteria for the NFPA variant of FTLD, having clear signs of speech apraxia, mild agrammatism in reading, repetition and written production and difficulty understanding complex morphosyntactic structures. Confrontation naming was still intact. Since language deficits were mild, AS also could have been classified as having an isolated progressive speech disorder (Tyrell et al., 1991; Cohen et al., 1993; Chapman et al., 1997; Silveri et al., 2003). With the appearance of clear speech apraxia, atrophy in the left insula reached corrected level of significance, confirming the role of this region in programming motor movements for speech (Dronkers, 1996). At this point, AS showed mild right-sided extrapyramidal symptoms and mild limb and bucco-facial apraxia but they were not significant enough to justify a diagnosis of CBS.
However, by the end of 2002, AS’s clinical pictures changed dramatically: The extrapyramidal syndrome became severe with significant loss of balance and rigidity that constricted her to a wheelchair within a one-month period. She developed alien limb phenomenon in the right limb and, shortly after, painful dystonia. VBM showed the spread of significant atrophy to the medial frontal lobe and involvement of the basal ganglia bilaterally. This finding is consistent with the appearance of alien limb phenomenon. AS showed the anterior-grasping type of alien limb phenomenon that has been associated with damage to the medial motor areas including the supplementary motor region. It is remarkable that this region showed a significant level of volume loss in AS only when she developed alien limb phenomenon. At this time, AS met criteria for CBS. We can only speculate that the rapid development of CBS-type symptoms occurred because a critical number of neurons in SMA and basal ganglia became dysfunctional.
AS demonstrates that FTLD and CBS can represent two stages of the same disease that spreads anatomically from brain regions involved in high cognitive and behavioral functions to areas involved in motor control. Her case strongly supports the idea, first highlighted by Kertesz (1997), that FTLD and CBS should be considered as part of the same spectrum of disease (Kertesz and Munoz, 2003; Kertesz et al., 1994). This view is supported by the fact that patients diagnosed with CBS during life can show Pick’s disease, DLDH, CBD or neurofilament inclusion body disease (Boeve et al., 1999; Grimes et al., 1999; Kertesz et al., 1999; Jaros et al., 2000; Cairns et al., 2003). It has been further validated by the discovery of familial cases of FTLD linked to chromosome 17q21–22 (Lendon et al., 1998; Withelmsen et al., 1999; Spillantini et al., 2000). These cases present clinically with significant Parkin-sonism and a behavioral syndrome, demonstrating that the same disease can cause both. Pathologically, atrophy was found in the frontotemporal cortex, basal ganglia and sub-stantia nigra regions, and tau depositions were evident in both neurons and glia (Sima et al., 1996; Reed et al., 1998). Perhaps the most striking example of the existence of a common underlying disorder between FTLD and CBD comes from a family case in which a tau mutation presented as CBS in the father and as FTD in the son (Bugiani et al., 1999).
In conclusion, we presented a case of FTLD-NFPA that evolved clinically and anatomically into CBS. While only pathological confirmation can determine the nature of the disease, this case represents a compelling example of the overlap between these two clinical entities.
Acknowledgments
We thank patients and their families for the time and effort they dedicated to our research. We also thank Dr. Nina Dronkers and Jennifer Ogar for their advise on the speech and language evaluations. The study was supported by grants from the John Douglas French Alzheimer’s Foundation, the McBean Foundation, the Larry Hillblom Foundation, the Koret Foundation, the National Institute on Aging and the State of California.
References
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;1(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Annals of Neurology. 2003;54 Suppl 5:15–9. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- Black SE. Aphasia in coticobasal degeneration. In: Litvan I, Goetz CG, Lang AE, editors. Advances in neurology. Philadelphia: Lippincott; 2000. [Google Scholar]
- Bergeron C, Pollanen MS, Weyer L, Black SE, Lang AE. Unusual clinical presentations of cortical-basal ganglionic degeneration. Annals of Neurology. 1996;40(6):893–900. doi: 10.1002/ana.410400611. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Maraganore DM, Parisi JE, Ahlskog JE, Graff-Radford N, Caselli RJ. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology. 1999;53(4):795–800. doi: 10.1212/wnl.53.4.795. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher KS, Varney NR, Spreen O. Facial recognition: Stimulus and multiple choice pictures. In: Benton AL, Hamsher KS, Varney NR, Spreen O, editors. Contributions to neuropsychological assessment. New York: Oxford University Press; 1983. [Google Scholar]
- Bugiani O, Murrell J, Giaccone G, Hasegawa M, Ghigo G, Tabaton M. Fronto-temporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. Journal of Neuropathology Experimental Neurology. 1999;58(6):667–77. doi: 10.1097/00005072-199906000-00011. [DOI] [PubMed] [Google Scholar]
- Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Annals of Neurology. 2001;49(4):433–42. [PubMed] [Google Scholar]
- Cairns NJ, Perry RH, Jaros E, Burn D, McKeith IG, Lowe JS, et al. Patients with a novel neurofilamentopathy: dementia with neurofilament inclusions. Neuroscience Letters. 2003;341(3):177–80. doi: 10.1016/s0304-3940(03)00100-9. [DOI] [PubMed] [Google Scholar]
- Curtiss S, Yamada J. Curtiss-Yamada Comprehensive Language Evaluation. 1988. Unpublished test. [Google Scholar]
- Chapman SB, Rosenberg RN, Weiner MF, Shobe A. Autosomal dominant progressive syndrome of motor-speech loss without dementia. Neurology. 1997;49(5):1298–306. doi: 10.1212/wnl.49.5.1298. [DOI] [PubMed] [Google Scholar]
- Cohen L, Benoit N, Van Eeckhout P, Ducarne B, Brunet P. Pure progressive aphemia. Journal Neurology, Neurosurgery and Psychiatry. 1993;56(8):923–4. doi: 10.1136/jnnp.56.8.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. Journal of Neuropathology and Experimental Neurology. 2002;61(11):935–46. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- Delis DC, Lucas JA, Kopelman MD. Memory. In: Barry S, Fogel E, Randolph B, Schiffer E, et al., editors. Synopsis of neuropsychiatry. Philadelphia: 2000a. pp. 169–91. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, TX: The Psychological Corporation; 2000b. [Google Scholar]
- Delis D, Kaplan EB, Kramer J. The Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–61. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Dhawan V, Moeller JR, Sidtis JJ, Ginos JZ, Strother SC, et al. The metabolic landscape of cortico-basal ganglionic degeneration: regional asymmetries studied with positron emission tomography. Journal of Neurology, Neurosurgery and Psychiatry. 1991;54(10):856–62. doi: 10.1136/jnnp.54.10.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the mental state of patients for the clinician. Journal of Psychiatry Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N, et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;56:216–25. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Garraux G, Salmon E, Peigneux P, Kreisler A, Degueldre C, Lemaire C, et al. Voxel-based distribution of metabolic impairment in corticobasal degeneration. Movement Disorders. 2000;15(5):894–904. doi: 10.1002/1531-8257(200009)15:5<894::aid-mds1021>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Willis CS, Baddeley AD, Emslie H. The Children’s Test of Nonword Repetition: A test of phonological working memory. Memory. 1994;2(2):103–27. doi: 10.1080/09658219408258940. [DOI] [PubMed] [Google Scholar]
- Gibb WRG, Luthert PJ, Marsden CD. Corticobasal degeneration. Brain. 1989;112:1171–92. doi: 10.1093/brain/112.5.1171. [DOI] [PubMed] [Google Scholar]
- Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, Chan D, et al. Automatic differentiation of anatomical patterns in the human brain: Validation with studies of degenerative dementias. Neuroimage. 2002;17(1):29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination (BDAE) Philadelphia: Lea and Febiger; 1983. Distributed by Psychological Assessment Resources, Odessa, FL. [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55(3):335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford NR, Damasio AR, Hyman BT, Hart MN, Tranel D, Damasio H, et al. Progressive aphasia in a patient with Pick’s disease: A neuropsychological, radiologic, and anatomic study. Neurology. 1990;40:620–26. doi: 10.1212/wnl.40.4.620. [DOI] [PubMed] [Google Scholar]
- Graham NL, Bak T, Patterson K, Hodges JR. Language function and dysfunction in corticobasal degeneration. Neurology. 2003;61(4):493–9. doi: 10.1212/01.wnl.0000081230.09863.ed. [DOI] [PubMed] [Google Scholar]
- Grimes DA, Lang AE, Bergeron CB. Dementia as the most common presentation of cortical-basal ganglionic degeneration. Neurology. 1999;53(9):1969–74. doi: 10.1212/wnl.53.9.1969. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and Palm Trees: A test of semantic Access from Pictures and Words. Suffolk; UK: 1992. [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Mauer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479–87. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Ishii K, Sakamoto S, Sasaki M, Kitagaki H, Yamaji S, Hashimoto M, et al. Cerebral glucose metabolism in patients with frontotemporal dementia. Journal of Nuclear Medicine. 1998;39(11):1875–8. [PubMed] [Google Scholar]
- Jackson M, Lennox G, Lowe J. Motor neurone disease-inclusion dementia. Neurodegeneration. 1996;5(4):339–50. doi: 10.1006/neur.1996.0046. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Holton JL, Rossor MN, Godbolt AK, Ozawa T, Strand K, et al. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathology and Applied Neurobiology. 2004;30(4):369–73. doi: 10.1111/j.1365-2990.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- Jaros E, Perry RH, Ince PG, Lowe JS. A new form of dementia: inclusion body cortico-striatal-nigral degeneration. Neuropathology and Applied Neurobiology. 2000;26:189–90. [Google Scholar]
- Kertesz A, Hudson L, Mackenzie IR, Munoz DG. The pathology and nosology of primary progressive aphasia. Neurology. 1994;44(11):2065–72. doi: 10.1212/wnl.44.11.2065. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Mastri AR, Frey WHd, Sung JH, Rustan T. Dementia lacking distinctive histologic features: A common non-Alzheimer degenerative dementia. Neurology. 1990;40(2):251–6. doi: 10.1212/wnl.40.2.251. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Martinez-Lage P, Davidson W, Munoz DG. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology. 2000;55(9):1368–75. doi: 10.1212/wnl.55.9.1368. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, Munoz D. Clinical and pathological overlap between frontotemporal dementia, primary progressive aphasia and corticobasal degeneration: The Pick complex. Dementia and Geriatric Cognitive Disorders. 1999;10(Suppl 1):46–9. doi: 10.1159/000051212. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Munoz DG. Primary progressive aphasia and Pick complex. Journal of Neurological Science. 2003;206(1):97–107. doi: 10.1016/s0022-510x(02)00345-3. [DOI] [PubMed] [Google Scholar]
- Kitagaki H, Hirono N, Ishii K, Mori E. Corticobasal degeneration: Evaluation of cortical atrophy by means of hemispheric surface display generated with MR images. Radiology. 2000;216(1):31–8. doi: 10.1148/radiology.216.1.r00ma0531. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery. Ontario: University of Western Ontario Press; 1980. [Google Scholar]
- Kay J, Lesser R, Coltheart M. Psycholinguistic assessment of language processing in aphasia. Hove, UK: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- Lippa CF, Cohen R, Smith TW, Drachman DA. Primary progressive aphasia with focal neuronal achromasia. Neurology. 1991;41:882–6. doi: 10.1212/wnl.41.6.882. [DOI] [PubMed] [Google Scholar]
- Lang AE. Cortical basal ganglionic degeneration presenting with progressive loss of speech output and orofacial dyspraxia. Journal of Neurology Neurosurgery and Psychiatry. 1992;55(11):1101. doi: 10.1136/jnnp.55.11.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Goetz C, Jankovic J, Wenning GK, Brandel JP, et al. Accuracy of the clinical diagnosis of corticobasal degeneration: A clinicopathologic study. Neurology. 1997;48(1):119–25. doi: 10.1212/wnl.48.1.119. [DOI] [PubMed] [Google Scholar]
- Lendon CL, Lynch T, Norton J, McKeel DW, Jr, Busfield F, Craddock N, et al. Hereditary dysphasic disinhibition dementia: A frontotemporal dementia linked to 17q21–22. Neurology. 1998;50(6):1546–55. doi: 10.1212/wnl.50.6.1546. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Psychological Aging. 1991;6(1):28–35. [Google Scholar]
- Mummery C, Patterson K, Price C, Ashburner J, Frackowiak R, Hodges J. A voxel-based morphometry study of semantic demntia: relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47(1):36–45. [PubMed] [Google Scholar]
- Mimura M, Oda T, Tsuchiya K, Kato M, Ikeda K, Hori K, et al. Corticobasal degeneration presenting with nonfluent primary progressive aphasia: A clinicopathological study. Journal of Neurological Science. 2001;183(1):19–26. doi: 10.1016/s0022-510x(00)00470-6. [DOI] [PubMed] [Google Scholar]
- Mathuranath PS, Xuereb JH, Bak T, Hodges JR. Corticobasal ganglionic degeneration and/or frontotemporal dementia? A report of two overlap cases and review of literature. Journal of Neurology Neurosurgery and Psychiatry. 2000;68(3):304–12. doi: 10.1136/jnnp.68.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43(11):2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Fronto-temporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126(Pt 11):2406–18. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Ozsancak C, Auzou P, Hannequin D. Dysarthria and orofacial apraxia in corticobasal degeneration. Mov Disord. 2000;15(5):905–10. doi: 10.1002/1531-8257(200009)15:5<905::aid-mds1022>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Pick A. Uber die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager Medizinische Wochenschrift. 1892;17:165–167. [Google Scholar]
- Peigneux P, Salmon E, Garraux G, Laureys S, Willems S, Dujardin K, et al. Neural and cognitive bases of upper limb apraxia in corticobasal degeneration. Neurology. 2001;57(7):1259–68. doi: 10.1212/wnl.57.7.1259. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rossor MN, Revesz T, Lantos PL, Warrington EK. Semantic dementia with ubiquitin-positive tau-negative inclusion bodies. Brain. 2000;123(Pt 2):267–76. doi: 10.1093/brain/123.2.267. [DOI] [PubMed] [Google Scholar]
- Rebeiz JJ, Kolodny EH, Richardson EP., Jr Corticodentatonigral degeneration with neuronal achromasia. Archires of Neurology. 1968;18(1):20–33. doi: 10.1001/archneur.1968.00470310034003. [DOI] [PubMed] [Google Scholar]
- Riley DE, Lang AE, Lewis A, Resch L, Ashby P, Hornykiewicz O, et al. Cortical-basal ganglionic degeneration. Neurology. 1990;40(8):1203–12. doi: 10.1212/wnl.40.8.1203. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Lee MS, Thompson PD, Marsden CD. Corticobasal degeneration. A clinical study of 36 cases. Brain. 1994;117(Pt 5):1183–96. doi: 10.1093/brain/117.5.1183. [DOI] [PubMed] [Google Scholar]
- Reed LA, Schmidt ML, Wszolek ZK, Balin BJ, Soontornniyomkij V, Lee VM, et al. The neuropathology of a chromosome 17-linked autosomal dominant Parkinsonism and dementia (“pallido-ponto-nigral degeneration”) Journal of Neuropathology and Experimental Neurology. 1998;57(6):588–601. doi: 10.1097/00005072-199806000-00006. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Neary D, Mann DM, Goulding PJ, Testa HJ. Progressive language disorder due to lobar atrophy. Annals of Neurology. 1992;31(2):174–83. doi: 10.1002/ana.410310208. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Hashida H, Uesugi H, Arima K, Murayama S, Bando M, et al. A clinical profile of corticobasal degeneration presenting as primary progressive aphasia. European Neurology. 1996;36(3):134–7. doi: 10.1159/000117229. [DOI] [PubMed] [Google Scholar]
- Sawle GV, Brooks DJ, Marsden CD, Frackowiak RS. Corticobasal degeneration. A unique pattern of regional cortical oxygen hypometabolism and striatal fluorodopa uptake demonstrated by positron emission tomography. Brain. 1991;114(Pt 1B):541–56. doi: 10.1093/brain/114.1.541. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Ashburner J, Vargha-Khadem F, Connelly A, Gadian DG, Friston KJ. Distributional assumptions in voxel-based morphometry. Neuro-image. 2002;17(2):1027–30. [PubMed] [Google Scholar]
- Silveri MC, Cappa A, Salvigni BL. Speech and language in primary progressive anarthria. Neurocase. 2003;9(3):213–20. doi: 10.1076/neur.9.3.213.15555. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Van Swieten JC, Goedert M. Tau gene mutations in frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) Neurogenetics. 2000;2(4):193–205. doi: 10.1007/pl00022972. [DOI] [PubMed] [Google Scholar]
- Sima AA, Defendini R, Keohane C, D’Amato C, Foster NL, Parchi P, et al. The neuropathology of chromosome 17-linked dementia. Annals of Neurology. 1996;39(6):734–43. doi: 10.1002/ana.410390609. [DOI] [PubMed] [Google Scholar]
- Turner RS, Kenyon LC, Trojanowski JQ, Gonatas N, Grossman M. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Annals of Neurology. 1996;39(2):166–73. doi: 10.1002/ana.410390205. [DOI] [PubMed] [Google Scholar]
- Tang-Wai DF, Josephs KA, Boeve BF, Dickson DW, Parisi JE, Petersen RC. Pathologically confirmed corticobasal degeneration presenting with visuo-spatial dysfunction. Neurology. 2003;61(8):1134–5. doi: 10.1212/01.wnl.0000086814.35352.b3. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Alkadhi H, Moro A, Perani D, Kollias S, Weniger D. Neural correlates for the acquisition of natural language syntax. Neuroimage. 2002;17(2):700–9. [PubMed] [Google Scholar]
- Tyrrell PJ, Kartsounis LD, Frackowiak RS, Findley LJ, Rossor MN. Progressive loss of speech output and orofacial dyspraxia associated with frontal lobe hypometabolism. Journal of Neurology Neurosurgery and Psychiatry. 1991;54(4):351–7. doi: 10.1136/jnnp.54.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) San Antonio, TX: The Psychological Corporation; 1997a. [Google Scholar]
- Warrington EK, James M. Visual Object and Space Perception Battery. St. Edmunds, UK: Thames Valley Test Co; 1991. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Third Edition (WMS-III) San Antonio, TX: The Psychological Corporation; 1997b. [Google Scholar]
- Wertz RT, LaPointe LL, Rosenbek JC. Apraxia of speech: The disorders and its management. New York: Grune and Stratton; 1984. [Google Scholar]
- Wilhelmsen KC, Clark LN, Miller BL, Geschwind DH. Tau mutations in fronto-temporal dementia. Dementia and Geriatric Cognitive Disorders. 1999;10(Suppl 1):88–92. doi: 10.1159/000051221. [DOI] [PubMed] [Google Scholar]


