Abstract
Although replication-incompetent recombinant adenovirus (rAd) type 5 is a potent vaccine vector for stimulating T and B cell responses, high seroprevalence of adenovirus type 5 (Ad5) within human populations may limit its clinical utility. Therefore, alternative adenovirus serotypes have been studied as vaccine vectors. In this study, we characterized the ability of rAd5 and rAd35 to infect and induce maturation of human CD11c+ myeloid dendritic cells (MDCs) and CD123+ plasmacytoid dendritic cells (PDCs), and their ability to stimulate Ag-specific T cells. Both MDCs and PDCs were found to express the primary receptor for Ad35 (CD46) but not Ad5 (coxsackie-adenovirus receptor; CAR). Both dendritic cell (DC) subsets were also more susceptible to rAd35 than to rAd5. MDCs were more susceptible to both rAd35 and rAd5 than were PDCs. Whereas rAd35 used CD46 for entry into DCs, entry of rAd5 may be through a CAR-independent pathway. Exposure to rAd35 but not rAd5 induced high levels of IFN-αin PDCs and phenotypic differentiation in both DC subsets. MDCs and PDCs exposed to either rAd5 or rAd35 encoding for CMV pp65 were able to present pp65 and activate CMV-specific memory CD8+ and CD4+ T cells in a dose-dependent manner, but MDCs stimulated the highest frequencies of pp65-specific T cells. Responding T cells expressed multiple functions including degranulation (CD107a surface mobilization) and production of IFN-γ, IL-2, TNF-α, and MIP-1β. Thus, the ability of rAd35 to naturally target important DC subsets, induce their maturation, and appropriately present Ag to T cells may herald greater in vivo immunogenicity than has been observed with rAd5.
Dendritic cells (DC)3 play an essential role in bridging the innate and adaptive immune systems and in promoting the Ag-specific activation and expansion of CD8+ and CD4+ T cells (1). Thus, vaccine developers are interested in targeting Ags and viral vectors to DCs. Among viral vectors, recombinant adenoviruses (rAd) have emerged as promising vaccine delivery systems (2). Replication-incompetent rAd5 has the ability to stimulate both the innate and adaptive immune systems (3–6). This platform is being used to produce several HIV vaccine products currently in human testing. However, high seroprevalence of adenovirus (Ad) 5 within human populations, especially in Africa, may limit its clinical utility (7). Also, rAd5 has shown poor transduction efficiency in monocyte-derived DCs (8–10). Therefore, alternative Ad serotypes have been studied as vaccine vectors. Ad35 is of high interest given that it is a serotype with low seroprevalence worldwide.
In this study, we characterized the ability of rAd5 and rAd35 to infect and induce maturation of freshly isolated human CD11c+ myeloid DCs (MDCs) and CD123+ plasmacytoid DCs (PDCs). We found that both DC subsets were more susceptible to rAd35 than to rAd5. In addition, rAd35 but not rAd5 induced high levels of IFN-α in PDCs and phenotypic differentiation in both DC subsets. We constructed rAd5 and rAd35 that encoded for the immunodominant human CMV protein pp65. Both rAd5- and rAd35-pp65-infected DCs were able to stimulate pp65-specific activation of autologous human memory CD8+ and CD4+ T cells. Responding T cells expressed multiple functions including degranulation (CD107a) and production of IFN-γ, IL-2, TNF-α, and MIP-1β. These findings have important implications for the future use of rAd-vectored vaccines in human subjects, and suggest that alternative adenovirus serotypes may offer advantages over existing vectors.
Materials and Methods
DC isolation
This study was approved by the National Institutes of Health Institutional Review Board. Our sorting procedures for direct isolation of subsets of DCs from blood have been described previously (11–13). Briefly, PBMCs were collected from healthy normal CMV-seropositive blood donors by automated leukapheresis. Enriched populations of lymphocytes and monocytes were obtained by counterflow centrifugal elutriation. MDCs and PDCs were isolated from elutriated monocytes using magnetic bead isolation including DC isolation kits (Miltenyi Biotec) followed by sequential separation on AutoMacs (Miltenyi Biotec). The BDCA-4 and the CD1c isolation kits were used for isolation of PDCs and MDCs, respectively. After this purification, the subsets of DCs were highly enriched (on average, >85% PDCs and >85% MDCs) as determined by staining for the lineage markers (CD3, CD14, CD15, CD20, and CD56), HLA-DR, CD123, and CD11c, respectively. Any contaminating cells were CD14+ monocytes. DCs were cultured in complete medium (RPMI 1640 (Bio-Whittaker) supplemented with 2 mmol/l l-glutamine, 1% streptomycin and penicillin, and 10% FCS (Invitrogen Life Technologies). To maintain viability, the media for PDCs and MDCs were supplemented with the recombinant human cytokines IL-3 (1 ng/ml; R&D Systems) and GM-CSF (2 ng/ml; PeproTech), respectively.
Construction of rAds
E1/E3-deleted, replication-incompetent Ad5 or Ad35 vectors were generated in PER.C6/55K cells using pBR322-based adaptor plasmid pAdApt535 together with cosmid pWE.Ad.AfllII-rITRΔE3 or pWE.Ad35.pIX-rITRΔE3 essentially as previously described (14–16). The adaptor plasmids contained the left portion of the Ad genomes (nt 1–454 in Ad5 or nt 1–464 in Ad35), followed by transcriptional control elements and the adaptor Ad DNA region (nt 3511–6095 in Ad5 or nt 3401–4669 in Ad35). The GFP gene and CMV pp65 gene optimized for high levels of expression in mammalian cells were cloned into the expression cassette in the adaptor plasmids. The resulting pAdApt-GFP and pAdApt535-pp65 plasmids expressed the GFP or pp65 genes under transcriptional control of the human, full-length, immediate-early CMV promoter and the SV40 polyadenylation signal. These plasmids were linearized and transfected into PER.C6/55K cells together with the cosmid pWE.Ad.AfllII-rITRΔE3 or pWE.Ad35.pIX-rITRΔE3 containing the right portion of the Ad genomes using Lipofectamine (Invitrogen Life Technologies). Ad vectors in crude lysates were plaque purified using limiting dilutions and agar overlays, and Ad vector clones were analyzed for the presence and expression of the transgene. Positive clones were amplified for large-scale production using PER.C6/55K cells in 24–48 triple-layer 3- × 175-cm2 flasks. Stock viruses were purified by standard two-step CsCl gradient ultracentrifugation and dialyzed three times into PBS containing 5% sucrose. Purified Ad vectors were aliquoted and stored at −80°C. Total virus particle (vp) titers were determined by HPLC. Infectivity was assessed by plaque assays using PER.C6/55K cells.
rAd5 and rAd35 infection and stimulation of DCs
The sorted DC populations were cultured at 1 × 106 cells/ml (with no <0.25 × 106 cells/tube) for 12 h at 37°C in polystyrene round-bottom tubes (BD Biosciences). The DCs were thereafter exposed to either rAd5 or rAd35 encoding for GFP at different doses of ip per cell and cultured for 24 h. To induce maturation, the DCs were stimulated by TLR7/8 ligand provided by 3 M Pharmaceuticals (17) as previously described (11–13).
Phenotypic characterization of DCs
Cells were harvested, washed in PBS supplemented with 0.5% BSA, and surface stained with different combinations of anti-CD11c, -CD123, -CD3, -CD4, -CD14, -CD20, -CD40, -CD80, -CD83, and -CD86 and anti-HLA-DR Ab (BD Pharmingen) for 15 min at 4°C and thereafter washed as previously described (11–13). For characterization of expression of the receptors for rAd5 and rAd35, the cells were stained with anti-CAR (clone RmcB; Upstate) and anti-IgG1-PE (BD Biosciences) or anti-CD46 PE (clone MEM-258; Serotec). The cell lines 293T, Chinese hamster ovary (CHO), and CHO-B cell line (BCL; obtained from American Type Tissue Collection), were used as controls for the staining. Staining of integrins were performed with anti-αVβ5 integrin (clone LM609; Chemicon International) and anti-αVβ5 integrin (clone P1F6; Chemicon International). The cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences) or on a modified LSR II (BD Biosciences). All data were evaluated by FlowJo software (Treestar).
Inhibition assay of rAd infection
The DCs were exposed to rAd5 and rAd35 encoding for GFP at different doses of ip per cell in the presence or absence of neutralizing Ab to CAR (clone RmcB from Upstate or clone E1-1 from Abcam; Refs. 18–20) or to CD46 (clone M177 from Hycult Biotechnology or clone 13/42 from BMA Biomedicals; Ref. 21) and cultured for 24 h. The cell lines 293T, A549, and HeLa (obtained from American Type Tissue Collection) were used as controls for the inhibition assays. The numbers of infected cells were determined by GFP expression using flow cytometry.
Measurement of IFN-α release
The supernatants from rAd-exposed and unexposed DCs were harvested and analyzed by ELISA (Biosource International) according to the manufacturer's instructions (11–13).
Intracellular staining of IFN-α in PDCs
The DCs were exposed to rAd5 or rAd35 encoding for GFP or to TLR7/8 ligand for indicated time points at 37°C in polystyrene round-bottom tubes. The DCs were then fixed and permeabilized with BD Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions and then stained with anti-IFN-α-FITC (clone MMHA-11; PBL Biomedical Laboratories) for 15 min at 4°C, washed, and resuspended in 1% paraformaldehyde. Expression of IFN-α was analyzed by a FACSCalibur flow cytometer.
Isolation of T cells
Enriched populations of autologous negatively selected CD4+ and CD8+ T cells, depleted of CD14+, CD15+, CD19+, CD56+, CD1c+, and BDCA-4+ cells were isolated using microbeads (Miltenyi Biotec) on elutriated lymphocytes and sequential separation on AutoMacs. The purity was >96%. The sorted T cells were incubated for 24 h until cocultured with DCs. Cell viability was determined by trypan blue exclusion before coculture with DCs.
Coculture of rAd-infected DCs with autologous CD4+ T cells
rAd exposed or unexposed DCs were washed in prewarmed media by centrifugation. The DCs were then cocultured with the sorted autologous T cells in a DC:T cell ratio of 1:10 in complete medium in polystyrene round-bottom tubes. The DC-T cell cocultures were conducted for 6 h with addition of monensin (Golgistop; BD Pharmingen; 0.7 μl/ml), brefeldin A (Sigma-Aldrich; 10 μg/ml), and anti-CD107a-Alexa 680 (22). As positive controls for recall responses to CMV, the DCs were pulsed with overlapping peptides to CMV pp65, 15-mers overlapping by 11 (4 μg of peptide per ml).
Abs for polychromatic flow cytometric analysis of T cell responses
The following Ab panel was used to assess the magnitude and quality of induced T cell responses: CD3-Cy7APC, IFN-γ-FITC, MIP-1β-PE, IL-2-APC, TNF-α-Cy7PE (all from BD Pharmingen); CD4-Cy5.5PE (Caltag); CD45RO-ECD (Beckman Coulter); CD107a-Alexa 680, CD14-Cascade blue, CD19-Cascade blue, CD27-Cy5PE (conjugated according to standard protocols; see http://drmr.com/abcon/index.html); and CD8-QD705 and CD57-QD565 (conjugated as described (23). Unconjugated Abs were obtained from BD Pharmingen. Q-Dots, Alexa 680, and Cascade blue were obtained from Invitrogen Life Technologies; Cy5 was obtained from Amersham Biosciences, and PE was from ProZyme. A violet fluorescent-reactive dye, ViViD (Invitrogen Life Technologies) was used as a viability marker to exclude dead cells from the analysis as described (24). All Abs were pretitered for optimal staining.
Polychromatic flow cytometric analysis of DC-mediated activation of CMV-specific T cells
After incubation, the cocultures of DCs and T cells were harvested, washed, and stained with the described surface Abs. Cells were then washed and permeabilized using the Cytofix/Cytoperm kit (BD Pharmingen) according to the manufacturer's instructions. After an additional two washes in the supplied buffer, cells were stained intracellularly with Abs specific for CD3, cytokines, and chemokines. Cells were then washed and fixed in PBS containing 1% paraformaldehyde. Fixed cells were stored at 4°C in the dark for no longer than 24 h. Stained cells were analyzed on a modified LSR II (BD Immunocytometry Systems). At least 700,000 cells were collected. All data were evaluated by FlowJo software. Forward scatter-area vs forward scatter-height parameters were used to gate out cell doublets. Next, dead cells, CD14+ monocytes, and CD19+ B lymphocytes were detected in the same channel and excluded from analysis. A small lymphocyte gate was set based on forward and side scatter properties. A CD3+ gate was created to select T cells, followed by analysis of T cell subsets. CD8+ T cells were selected by first gating out CD4+ cells and subsequently gating on CD8+ cells. Alternatively, CD4+ T cells were selected by first gating out CD8+ cells and then gating on CD4+ cells. All T cell markers were plotted vs IFN-γ to observe and include stimulated cells that had down-regulated surface marker expression. Functional analysis was performed for each T cell subset by gating on cells positive for each of the five functional parameters: CD107a, IFN-γ, IL-2, MIP-1β, and TNF-α. Negative control samples were used to set single function gates, and gates were placed consistently across samples. Boolean combinations of single function gates were then created using FlowJo software to determine the frequency of each response based on all possible combinations of the five functions. Thus, each responding cell is defined by a unique functional response. Background responses detected in negative control tubes were subtracted from those detected in stimulated samples for every functional combination.
Analysis of T cell functional profiles
The data analysis program, Simplified Presentation of Incredibly Complex Evaluations (SPICE; from Mario Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health) was used to analyze and generate graphic representations of T cell responses detected by polychromatic flow cytometry. All data were background subtracted using the unstimulated controls for either MDCs or PDCs cocultured with T cells. A lower threshold was calculated using SPICE, and all data less than this threshold were set to zero.
Statistical analyses
Statistical analyses were performed using Wilcoxon's paired t test or the Mann-Whitney unpaired t test with Graph Pad Prism software.
Results
Human primary DC subsets are more susceptible to rAd35 than to rAd35
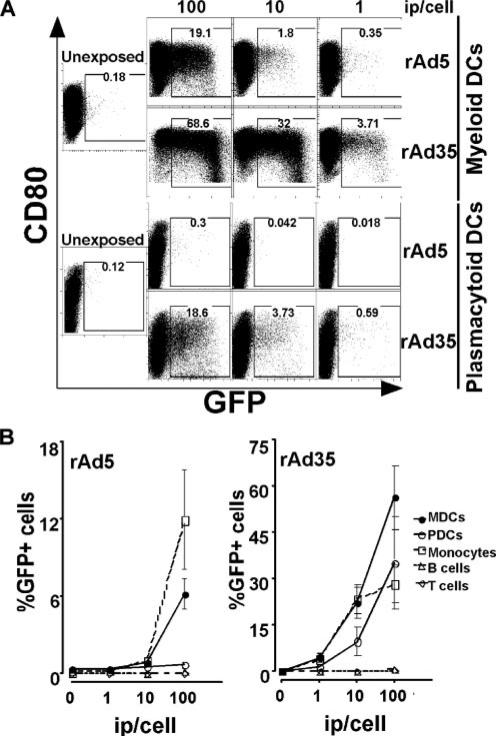
Most studies of DC susceptibility to vaccine vectors have utilized in vitro monocyte-derived DCs to model the DCs that circulate in vivo. To provide a more physiological system, we investigated human CD123+ PDCs and CD11c+ MDCs directly isolated from blood. PDCs and MDCs are both important APCs, but they originate from different lineages and exhibit some distinct differences, which is why both DC subsets are important to investigate for vaccine development. MDCs are superior in priming naive T cell responses, whereas PDCs are unique producers of type I IFNs, and the interactions between the DC subsets may play a significant role in induction of immunity (25–27). We exposed PDCs and MDCs to different amounts of replication-incompetent rAd5 and rAd35 encoding for GFP. Cells infected with these vectors express the encoded insert but will not produce infectious particles that can spread the infection to other cells. The DCs were harvested after 24 h, and the number of infected cells was determined by analyzing expression of GFP using flow cytometry. No stimulation of the DCs was performed to induce expression of the vector inserts. We found that MDCs were significantly more susceptible to both rAd5-GFP and rAd35-GFP than were donor-matched PDCs (Fig. 1A). At 100 ip of rAd35-GFP per cell, GFP was expressed in 56.4 ± 10.4% (mean ± SEM) of MDCs as compared with 35.3 ± 14.9% of donor-matched PDCs (n ≥ 4; p < 0.033). At 100 ip of rAd5-GFP per cell, GFP was expressed in 6.2 ± 1.2% of MDCs as compared with 0.7 ± 0.3% of PDCs (n ≥ 4, p < 0.004). Both DC subsets were more susceptible to rAd35 than to rAd5 (n ≥ 4, p ≤ 0.014; Fig. 1A). When compared with nonexposed DCs, cell viability tended to be lower in the DCs that were exposed to very high inocula (≥100 ip per cell) of rAd35. The cell viability was not affected in the DC cultures exposed to rAd5 at any of the doses tested (1–200 ip per cell).
FIGURE 1.
Susceptibility of MDCs and PDCs to rAd5 and rAd35. A, Directly isolated MDCs and PDCs were exposed for 24 h to different doses of rAd vectors 5 and 35, respectively, both encoding GFP. GFP-expressing cells were analyzed by flow cytometry. Both DC subsets were more susceptible to rAd35-GFP than rAd5-GFP, and MDCs were more susceptible than PDCs. B, Different cell populations were exposed for 24 h to rAd5 and rAd35, respectively, both encoding GFP. Again, both DC subsets were more susceptible to rAd35-GFP than to rAd5-GFP, and MDCs were more susceptible than PDCs. Whereas monocytes were susceptible to both rAd5 and rAd35, B and T cells were not susceptible to either rAd5 or rAd35. One representative donor of at least three is shown.
As mentioned above, no stimulation of the DCs was performed before exposure to the rAds. The DCs were therefore phenotypically immature when they were exposed. We found that the infection rates decreased if we first stimulated the DCs with TLR7/8 ligand for 24 h to induce differentiation and then exposed them to rAd (data not shown). Immature MDCs showed GFP expression in 18.9 ± 4.3 and 29.4 ± 6.2% of the cells after rAd35 and rAd5 exposure, respectively, whereas 7.5 ± 1.3 and 15.0 ± 2.3% of TLR7/8 ligand-stimulated mature MDCs showed infection. Therefore, mature DCs may be less susceptible to both rAd5 and rAd35 than immature DCs. In contrast, if the DCs were exposed to rAd and stimulated with TLR7/8 ligand simultaneously, there was a trend toward more infection. GFP was expressed in 15.0 ± 0.7 and 21.8 ± 6.3% of unstimulated MDCs exposed to rAd5 and rAd35, respectively, as compared with 30.7 ± 3.2 and 26.4 ± 5.6% of donor-matched MDCs that were stimulated with TLR7/8 ligand and exposed to rAd5 or rAd35 at the same time (n = 4). However, defining infection through the detection of GFP reporter gene expression and not PCR for other Ad gene products may underestimate the true infection frequency given that CMV promoter activity can vary in different cell types (28).
To compare the rAd5 and rAd35 susceptibility of DCs to other immune competent cells, we exposed donor-matched sorted populations of CD14+ monocytes, CD3+ T cells, and CD19+ B cells to rAd5 and rAd35 encoding for GFP. Both the DC subsets and monocytes were found to be more susceptible to rAd35 than to rAd5 (Fig. 1B). MDCs were the cell type most susceptible to rAd35, whereas monocytes and PDCs were approximately equal in their capacity to take up rAd35. In contrast, whereas PDCs were found to exhibit a rather low susceptibility to rAd5, in most donors monocytes showed the highest susceptibility to rAd5 (Fig. 1B). Finally, low or undetectable levels of GFP+ cells were observed after T and/or B cells were exposed to rAd5 or rAd35 (Fig. 1B).
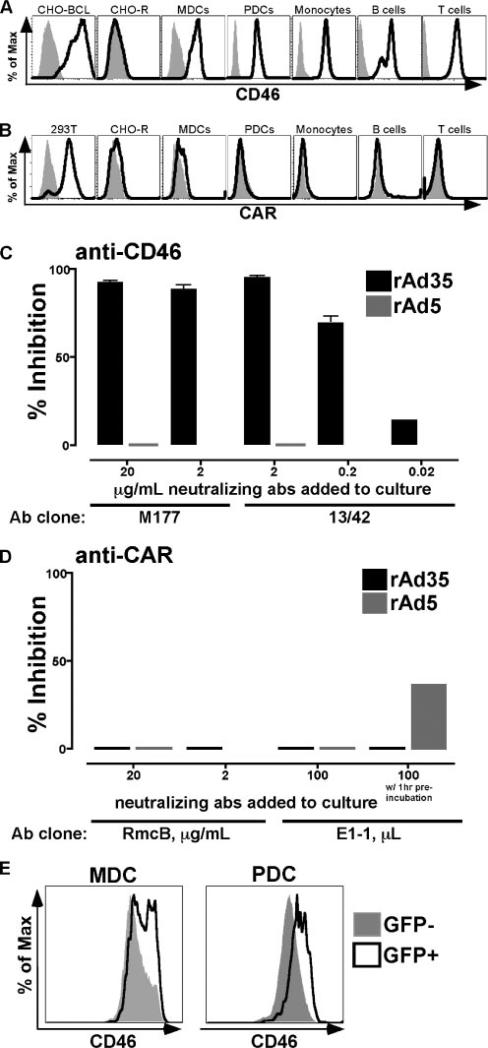
High expression of the receptor for Ad35 but not for Ad5 on DCs
We investigated the expression of the relevant receptors used for Ad5 and Ad35 entry into cells. CAR and CD46 have been identified as the primary receptors for Ad5 and Ad35, respectively (29, 30). MDCs, PDCs, monocytes, T cells, and B cells all expressed CD46 on their cell surface (Fig. 2A). As positive and negative controls (Fig. 2A), we used 293T cells (which express CD46) and CHO-R cells (which do not). In contrast to their uniform expression of CD46, MDCs, PDCs, monocytes, T cells, and B cells did not show detectable levels of CAR on their cell surface (Fig. 2B). CAR-transfected CHO-BCL cells and CAR− CHO-R cells were used as positive and negative controls, respectively, for the CAR staining (18). We did not see significantly different levels of expression of either CD46 or CAR in the various cell populations tested, indicating that the differences in susceptibility found among PDCs, MDCs, monocytes, T cells, and B cells are unlikely related to receptor expression.
FIGURE 2.
rAd35 uses CD46 for infection while rAd5 may use a CAR independent pathway. A and B, CD46 and CAR expression was assessed on various cell populations. The histograms show stained cells (black lines) vs the unstained control (gray shaded histogram) for each cell type. CHO-BCL and CHO-R cell lines were used for CD46 positive and negative controls, respectively, whereas 293T and CHO-R cell lines were used as the CAR-positive and -negative controls, respectively. Monocytes, MDCs, PDCs, B cells, and T cells expressed CD46 (A), whereas CAR expression (B) was undetectable on these cell types. C and D, MDCs were exposed to either rAd5-GFP or rAd35-GFP for 24 h in the presence and absence of neutralizing anti-CD46 and anti-CAR Ab. Values are the percent inhibition (mean ± SEM) of infection of either rAd5 (gray bars) or rAd35 (black bars). C, Infection of MDCs by rAd35, but not rAd5, was effectively blocked in the presence of either of the two clones of anti-CD46-neutralizing Ab. D, Infection of MDCs by rAd5 and rAd35 was not or poorly blocked in the presence of either of the two neutralizing anti-CAR Ab if anti-CAR and rAd5 exposure occurred simultaneously, whereas preincubation with anti-CAR clone E1-1 showed partial blocking of Ad5 infection. E, CD46 expression was found to be higher on rAd35-infected GFP+ DCs than GFP− DCs within the same culture.
Blocking of CD46 inhibits rAd35 infection of DCs
To further examine the pathways used by rAd5 and rAd35 for infection, we exposed DCs to either rAd35-GFP or rAd5-GFP for 24 h in the presence or absence of neutralizing Abs to CD46 or CAR. Using different doses of neutralizing Abs to either the short consensus repeat 2 domain of CD46 (clone M177) or the short consensus repeat 1 and 2 domains combined (clone 13/42), we were able to efficiently block infection of rAd35 in DCs whereas rAd5 infection was not affected (Fig. 2C). The Ab-mediated inhibition of rAd35 infection was dose dependent. In contrast, we were unable to inhibit rAd5 infection in DCs using either of the two Abs to CAR (clones RmcB and E1-1) that decreased but did not completely block rAd5 infection in A549 human lung carcinoma cells (clone E1-1, 4-fold inhibition) and HeLa cells (clone RmcB, <50% inhibition; Refs. 18–20). Preincubation of DCs with anti-CAR Ab for 1 h before addition of rAd5 had a small effect on infection (Fig. 2D). This suggests that rAd5 may also use CAR independent receptor(s) like undefined heparin-sensitive receptors and/or lactoferrin receptor to infect DCs as suggested for other cell types (31, 32). The doses of either anti-CD46 or anti-CAR Ab that were used did not affect the viability of the DCs (data not shown).
Because CD46 clearly was used as the primary receptor for rAd35 entry into the DC subsets, we examined its expression in more detail. We found that phenotypic maturation of DCs induced by either rAd35 exposure or TLR7/8 ligand stimulation led to a notable up-regulation of CD46 (data not shown). In addition, the greatest increase in CD46 expression was found in the GFP+ DCs as compared with the GFP− DCs within the same culture (Fig. 2E). This pattern was evident for both PDCs and MDCs and may be explained by a more mature phenotype in infected cells that is accompanied by higher CD46 expression. Alternatively, this fraction of DCs could have exhibited higher CD46 at the time of exposure to rAd35 and thus were the most susceptible to infection. Up-regulation of CD46 with rAd35 exposure has been demonstrated earlier in CD34+ bone marrow-derived DCs (33). We did not see any alteration of CD46 expression with rAd5 exposure (data not shown).
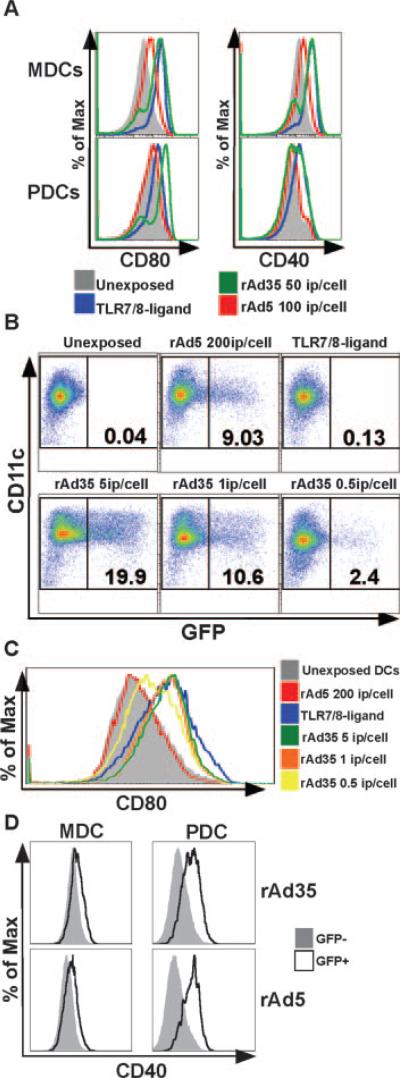
Phenotypic differentiation of DCs occurs after rAd35 exposure
As mentioned earlier, we found that both MDCs and PDCs were susceptible to rAd5 and rAd35 without prior activation or DC maturation (Fig. 1A). We next asked whether rAd exposure leads to phenotypic maturation of DCs, which could facilitate optimal stimulation of T cell responses. Exposure of DCs to rAd5 led to limited or no phenotypic differentiation in either MDCs or PDCs as evidenced by incomplete or no up-regulation of the costimulatory molecules CD40 and CD80 (Fig. 3A). However, exposure of DCs to rAd35 led to full maturation that exceeded the differentiation induced by rAd5. The phenotypic differentiation induced by rAd35 was similar to that induced by TLR7/8 ligation which is known to stimulate full maturation of DCs (11–13). These differential maturation patterns found after rAd5 and rAd35 exposure were further examined after exposure to various doses of rAd5 and rAd35. We found that some maturation was evident after exposure to as little as 0.5 ip/cell of rAd35, whereas no or very low maturation was evident using 200 ip/cell of rAd5 (Fig. 3, B and C). Even if the numbers of infected (GFP+) DCs were equalized by adjusting the doses of rAd5 and rAd35, the DCs exposed to rAd35 showed much more phenotypic differentiation than DCs exposed to rAd5 (Fig. 3, B and C). This suggests that rAd35 more efficiently triggers signaling pathway(s) that leads to phenotypic maturation of DCs than does rAd5. To examine whether this increase in phenotypic maturation was associated with actual infection of the DCs by the rAds, we analyzed the phenotype in the GFP+ DCs vs the GFP− DCs within the same culture. In MDCs, the GFP+ cells were not notably different in their cell surface expression of CD40, CD80, and HLA-DR than the GFP− cells (data not shown). This was found after either rAd5 or rAd35 exposure. In contrast, GFP+ PDCs generally exhibited higher levels of these markers than the GFP− cells, suggesting that the infected cells were more mature than their noninfected counterparts (Fig. 3D).
FIGURE 3.
Phenotypic profile of MDCs and PDCs following rAd5 and rAd35 exposure. DC maturation was assessed after 24 h of exposure to rAd5 and rAd35. A, rAd35 induced full maturation of both DC subsets, whereas rAd5 induced only partial maturation. The histograms show expression levels of CD80 and CD40 after exposure to TLR7/8 ligand (blue), rAd35 (green), or rAd5 (red) compared with an unexposed control (filled gray). B and C, rAd35 doses of 5 (green), 1 (orange), or 0.5 (yellow) i.p./cell induced partial maturation, whereas a rAd5 dose of 200 (red) i.p. per cell induced no maturation. Unexposed MDCs (filled gray) were used as the negative undifferentiated control and TLR7/8 ligand-stimulated MDCs (blue) were used as a positive control for full maturation. D, Actual infection by the rAds of the PDCs but not the MDCs was associated with a more mature phenotype as visualized by higher cell surface expression of CD40 in the GFP+ PDCs vs the GFP− PDCs within the same culture. One representative donor of at least three is shown.
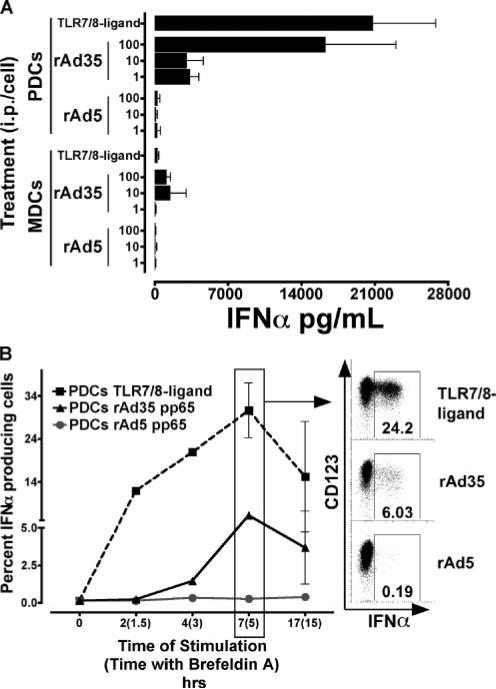
IFN-α production is induced by rAd35 infection
We found that rAd35 but not rAd5 induced production of high amounts of IFN-α (Fig. 4). IFN-α was mainly found in the PDC cultures. As little as 1 ip of rAd35 per PDC induced detectable IFN-α 24 h after exposure (Fig. 4A). The levels of IFN-α produced by PDCs increased with higher doses of rAd35 with the highest dose tested (100 ip/cell) inducing levels of IFN-α similar to those found after TLR7/8 ligand stimulation (Fig. 4A). Exposure to rAd5 induced only minimal (<263 pg/ml) IFN-α production from PDCs, even at the highest inoculum tested. The high secretion of IFN-α by PDCs was very low or undetectable if the rAd35 exposure was conducted in the presence of anti-CD46 Ab, indicating that infection by rAd35, or at least receptor engagement, is required to induce IFN-α production (data not shown). No or low levels of IFN-α were also detected in the supernatants of MDCs exposed to rAd35. However, we were unable to detect IFN-α in MDCs by intracellular staining which may indicate that the IFN-α found in the supernatants from MDC cultures could come from a few contaminating PDCs within the MDC population (data not shown).
FIGURE 4.
Production of IFN-α induced by rAd35 but not rAd5. A, Supernatants were collected after 24 h of exposure to rAd5, rAd35, or TLR7/8 ligand and IFN-α production was analyzed by ELISA. Values reflect total IFN-α production (picograms per milliliter; mean ± SEM) at various doses of rAd5, rAd35 exposure, or TLR7/8 ligand stimulation. Exposure of rAd35, but not rAd5, induced high levels of IFN-α in PDCs and low levels in MDCs. B, PDCs were stained for intracellular IFN-α production after exposure of rAd5 or rAd35 or TLR7/8 ligand stimulation. Values are the percent of IFN-α-producing PDCs (mean ± SEM) over time indicating time including brefeldin A in parentheses. Stimulation of PDCs by TLR7/8 ligand (■) or rAd35 (▲) induced a similar kinetics pattern of rapid IFN-α production that peaks after 7 h, whereas intracellular IFN-α was nearly undetectable in PDCs following rAd5 (●) stimulation (n ≤ 3).
The kinetics of IFN-α production in PDCs was assessed using intracellular detection of IFN-α by flow cytometry. Production of IFN-α occurred rapidly after TLR7/8 ligation; IFN-α-expressing PDCs were found after only 2 h (Fig. 4B). In contrast, IFN-α was first detected after 4 h of rAd35 exposure. IFN-α expression in PDCs (after either TLR7/8 ligation or rAd35 exposure) peaked around 7 h and was declining by 17 h. Very few or no PDCs expressed IFN-α after exposure to rAd5 (Fig. 4B).
DCs use rAd-encoded Ags to activate Ag-specific T cell responses
We next assessed whether the differences found in infectivity and maturation of DCs induced by rAd5 and rAd35 would affect their ability to induce fully functional Ag-specific memory T cells responses. For these experiments, we used healthy CMV-seropositive subjects with documented CMV-specific T cell responses to model Ag presentation by DCs and assess the functionality of the recall responses induced by rAd-infected DCs. We therefore constructed rAd5 and rAd35 that encoded for the CMV protein pp65. MDCs and PDCs were exposed for 24 h to either rAd5-pp65 or rAd35-pp65. They were then washed and cocultured with purified autologous T cells. Polychromatic flow cytometry was used to examine the functional complexity of the induced recall T cell responses. We have developed a flow cytometric assay to measure simultaneously and individually degranulation (CD107a mobilization) and IFN-γ, MIP-1β, TNF-α, and IL-2 production in responding Ag-specific T cells (22). Using this assay, we were able to dissect the quantity and quality (polyfunctionality) of the pp65-specific T cell responses that were stimulated. We compared the total magnitude of CD8+ and CD4+ T cell responses stimulated by different APCs exposed to either the rAd5 or rAd35 (Figs. 5A and 6A) and also the functional profiles generated (Figs. 5B and 6B).
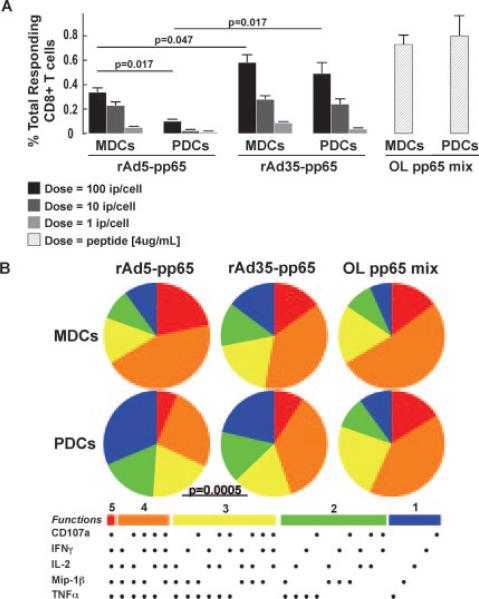
FIGURE 5.
rAd induces polyfunctional memory CD8+ T cell responses. The DC subsets were exposed to rAd5-pp65 and rAd35-pp65 for 24 h at 1, 10, or 100 ip/cell before 6 h of coculture with autologous sorted T cells. A, Bars show total frequencies of responding (IFN-γ, TNF-α, IL-2, MIP-1β, CD107a expressing) pp65-specific CD8+ T cells. rAd35-pp65 exposure of either MDCs or PDCs induced a greater magnitude of pp65 responses than did rAd5-pp65 exposure. After exposure to rAd5-pp65 but not rAd35-pp65, MDCs were superior to PDCs at inducing CD8+ T cell responses. B, The portions of the pies represent the 31 combinations of responses and are shown grouped by number of functions: 5 (red); 4 (orange); 3 (yellow); 2 (green); and 1 (blue). MDCs exposed to either rAd5-pp65 or rAd35-pp65 induced a more polyfunctional CD8+ T cell response than PDCs, and one that resembles the response induced by the overlapping (OL) pp65 peptide stimulation. There was no difference between the qualities of the CD8+ response induced by rAd5-pp65 and rAd35-pp65.
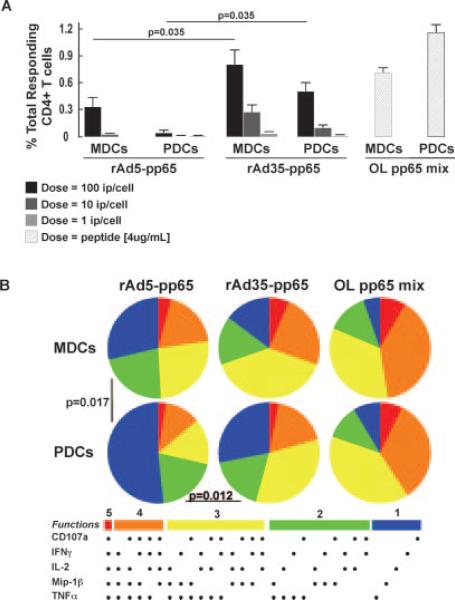
FIGURE 6.
rAd induces polyfunctional memory CD4+ T cell responses. The DC subsets were exposed to different doses of rAd5-pp65 and rAd35-pp65 for 24 h before a 6-h coculture with autologous sorted T cells. A, Bars show the total frequencies of responding (IFN-γ, TNF-α, IL-2, MIP-1β, CD107a expressing) pp65-specific CD4+ T cells. Both DC subsets induced a greater magnitude of pp65 responses after exposure of rAd35-pp65 than to rAd5-pp65. B, The portions of the pies represent the 31 combinations of responses as described earlier and are shown grouped by number of functions: 5 (red); 4 (orange); 3 (yellow); 2 (green); and 1 (blue). The responses stimulated by rAd5 or rAd35 were compared with those found with overlapping (OL) peptide stimulation.
The numbers of responding T cells that were detected with rAd5-pp65 and rAd35-pp65 were compared with the numbers found in cocultures stimulated with exogenously added overlapping peptides for pp65. We found that both MDCs and PDCs were able to activate pp65-specific T cell responses after exposure to either rAd5 or rAd35 encoding for pp65 in a dose-dependent manner (Figs. 5 and 6). DCs exposed to rAd35-pp65 induced more pp65-specific T cells to respond than did rAd5-pp65-exposed DCs.
When exposed to rAd35-pp65, both MDCs and PDCs were able to induce significantly more CD8+ T cells to respond than when they were exposed to the same dose of rAd5-pp65 (p = 0.047 and p = 0.017, respectively) (Fig. 5A). This difference was also shown for stimulation of CD4+ T cell responses (p = 0.035 and p = 0.035, respectively; Fig. 6A). Although the highest dose (100 ip/cell) of rAd35-pp65 did not stimulate as many T cells to respond as was observed using overlapping peptides, rAd35-pp65 was still shown to be a potent source of pp65 Ag through which DCs could present and activate T cell responses. There was no significant difference in the magnitude of either CD8+ or CD4+ T cell responses stimulated by donor-matched rAd35-pp65 exposed MDCs or PDCs (p = 0.422 CD8 and p = 0.148 CD4, respectively), indicating that both the DC subsets were equal in their ability to use rAd35-derived pp65 as a source of Ag. Whereas MDCs were able to activate pp65-specific responses after exposure to rAd5-pp65, PDCs were poor stimulators of T cells after rAd5-pp65 exposure. In some experiments (n = 2), the T cell responses found with rAd5- or rAd35-exposed monocytes were compared with the responses seen with donor-matched MDCs and PDCs. Monocytes induced notably lower CD8+ and CD4+ T cell responses than did either MDCs or PDCs after rAd35 exposure. However, only MDCs and not PDCs were superior to monocytes at activating T cells after rAd5 exposure. Consistent with the lack of expression of adenoviral products by these vectors, low to undetectable T cell responses were observed when MDCs and PDCs exposed to rAd5 or rAd35 not expressing pp65 were used in the assays (data not shown).
Polyfunctional T cell responses are stimulated by DCs using rAd-derived Ags
As mentioned above, we used polychromatic flow cytometry to examine the functional profile of the induced T cell responses by analyzing several effector markers (CD107a mobilization, IFN-γ, MIP-1β, TNF-α, and IL-2) simultaneously (22). By this assay, we can investigate whether the responding T cells are expressing a single or several of the effector markers combined. The responses found in the cultures stimulated by overlapping peptides were used as the highest standard for polyfunctional activation. MDCs exposed to rAd35-pp65 induced a higher proportion of polyfunctional T cells than did PDCs (Figs. 5B and 6B). This was evident for both CD8+ and CD4+ T cell responses ( p = 0.0005 and p = 0.012, respectively). Due to the low frequencies of responding T cells after PDC stimulation, we could not statistically determine whether there was a difference between MDCs and PDCs in stimulating polyfunctional T cell responses after rAd5 exposure.
In summary, the T cell responses generated after rAd35-pp65 exposure of both MDCs and PDCs were of significantly higher magnitude than after similar doses of rAd5-pp65 exposure. Furthermore, MDCs stimulated more polyfunctional T cell responses after both rAd5 and rAd35-pp65 exposure than did PDCs.
Discussion
DCs have a central role in priming immune responses and are therefore of special interest for vaccine targeting. Viral vectors that efficiently infect and cause maturation of DCs in vivo may induce optimal vaccine responses. Currently, vaccines based on a serotype 5 rAd platform are being tested in several clinical trials. However, this serotype is inefficient in its ability to infect in vitro differentiated human DCs (8–10, 34). The reason for this may be the lack of expression of the primary receptor for Ad5, CAR (10). In contrast, monocyte-derived DCs, epidermal DCs (e.g., Langerhans cells), and dermal DCs express CD46, the high-affinity receptor for rAd35, and are therefore more easily infected with rAd35 than with rAd5 (28, 35–38). Here, we show that the distinct DC subsets (primary MDCs and PDCs) isolated directly from blood also lack cell surface CAR expression yet express high levels of CD46. Furthermore, MDCs and PDCs are highly susceptible to infection by rAd35. Exposure of rAd35 but not rAd5 leads to induction of high levels of IFN-α in PDCs and phenotypic differentiation in both DC subsets. In addition, both MDCs and PDCs can efficiently use Ags expressed as inserts by rAd35 vectors to activate high frequencies of Ag-specific polyfunctional CD4+ and CD8+ T cell responses. When one also considers that there is low worldwide seroprevalence to Ad35, our findings strongly suggest that rAd35 may be a superior Ad serotype to use for vaccine development.
The immunogenicity of vaccines based upon rAd5 and rAd35 have been tested in mice and nonhuman primates (37, 39). In general, the primary immune responses generated by immunization with rAd35 have not exceeded the responses seen with rAd5. In the current study, we show that rAd35 uses CD46 to infect human primary PDCs and MDCs whereas rAd5 may enter via a CAR-independent pathway. Although mice are a good animal model for testing the immunogenicity of rAd5-based vaccines, they lack expression of CD46, limiting their use in testing the immunogenicity of rAd35-based vaccines (35, 40). In addition, nonhuman primates (but not humans) express CD46 on erythrocytes that may affect the distribution and access to APCs of rAd35 vectors injected into monkeys (41). For instance, the almost ubiquitous expression of CD46 on monkey hemopoietic cells may misdirect rAd35 into cell types not involved in the elicitation of immune responses. Therefore, rAd35 may perform less well in mice and monkeys than it might in humans due to the differences in CD46 receptor expression in these three species.
However, despite high expression of CD46 on all cells tested (MDCs, PDCs, monocytes, T cells, and B cells), we found that only DCs and monocytes were susceptible to rAd35 infection in vitro. This may mean that only cells with active endocytic pathways are susceptible to Ad35, that other receptors are involved, or that there are postentry blocks to infection in some cells (42). The integrins αVβ5 and αVβ3 are known to be involved in the entry of some adenoviruses (43). We found that all cell populations that we tested expressed these integrins (data not shown), excluding the possibility that the expression level of these receptors would account for the differences in susceptibility. Also, we found that although rAd35 infects the DC subsets much more efficiently than rAd5, high level infection can induce more cell death. This may result in a shorter time exposure to Ag after rAd35 immunization than with rAd5 immunization. Careful dose-response studies in vivo could elucidate the impact of dose and induction of cell death on the immunogenicity of rAd5 and rAd35-based vaccines.
We found that exposure of rAd35 but not rAd5 induced full phenotypic maturation of both MDCs and PDCs. Furthermore, rAd35 but not rAd5 induced high levels of IFN-α by PDCs as has been reported earlier (44–46). Although there may be other yet unstudied activation factors and/or functions that rAd5 induces, our data indicate the rAd35 is superior to rAd5 at inducing DC functions that are associated with the stimulation of immune responses. DC maturation is important for optimal Ag presentation and type I IFNs play a role in the induction of antiviral responses. Although PDCs have a unique capacity to produce high levels of type I IFNs, recent data indicate that PDCs also play an essential conditioning role on MDCs which can be mediated by type I IFNs (25–27). Type I IFNs are potent antiviral cytokines but the production of IFN-α by PDCs in response to rAd35 exposure did not appear to limit the level of rAd35 infection in the cells which is in line with earlier report (45). Despite high IFN-α production, the infection rates found after rAd35 exposure of PDCs were much higher than with rAd5 exposure. The mechanism(s) underlying these differences in the ability of rAd5 and rAd35 to provoke DC functions need further investigation. Because the rAd35-induced IFN-α production was abolished when the infection was blocked by neutralizing anti-CD46 Ab, the effects triggered by rAd35 are likely due to ligation of intracellular pathways rather than receptor binding alone. Intracellular TLR engagement by structures of Ad35 could be responsible for the induction of cell activation. Viral CpG motifs present in large dsDNA viruses such as CMV and HSV have been shown to activate murine PDCs via TLR9-dependent pathways (47–49), and rAd35 has been shown to induce IFN-α in human PDCs presumably via TLR9 ligation, given that IFN-α induction was dependent on the presence of viral DNA and endosomal acidification and was blocked by TLR9-inhibitory oligonucleotides (46, 50). Because human MDCs do not express TLR9, yet we found that they phenotypically matured by rAd35 exposure; other activation pathways must also be operative. Consistent with this idea, murine MDCs (e.g., conventional DCs) can be activated and differentiate in response to rAd in a TLR9-independent manner as demonstrated using DCs from TLR9-deficient mice (51–53), although the mechanism behind TLR-independent recognition of dsDNA is not yet fully understood (54).
Despite the difference in induction DC maturation and IFN-α production between rAd5 and rAd35, we found that the DC subsets were able to use Ags (CMV) encoded by either rAd5 or rAd35 for stimulation of Ag (CMV)-specific T cell responses. These data provide evidence that direct targeting of DCs with either rAd vector leads to Ag presentation. After entry into the cell, endosomal acidification allows the rAd to penetrate the cytoplasm and traffic to the nucleus. Ad-derived proteins are thereafter produced, processed, and presented in association with MHC class I on the cell surface. Efficient MHC class I presentation of Ad-derived Ag is required for CD8+ T cell activation. The pathway for the presentation of endogenously produced Ag and presentation on MHC class II with subsequent stimulation of CD4+ T cells remains unclear. The source of Ag may be from alternative processing of internally produced Ad-derived Ag or uptake and exogenous processing of Ag released from other Ad-infected cells. We used CMV pp65 as a model Ag inserted in the genome of rAd5 and rAd35 to examine Ag presentation by DCs and activation of preexisting Ag-specific T cells. We found that both rAd5-pp65 and rAd35-pp65 exposure of either DC subset activated pp65-specific CD8+ T cells. Exposure of rAd35-pp65 stimulated higher frequencies of pp65-specific T cells than did rAd5-pp65. This may simply be an effect of the fact that more DCs were infected with rAd35 than rAd5 and therefore more DCs presented Ags. It can also relate to the fact that the DCs exposed to rAd35 differentiated to a more mature phenotype and therefore were more efficient at activating T cells. We have found in earlier studies that IFN-α levels produced by PDCs have minimal effects on the activation of pre-existing memory T cell responses in vitro in our coculture model, which is why that is an unlikely reason for the functional differences found between the vectors (11). We can only speculate whether it is the higher maturation or the higher transduction efficiency by rAd35 that is leading to higher number of responding CMV pp65-specific T cells, because the exact numbers of pp65-expressing DCs after Ad-pp65 exposure were not investigated here (28, 35, 51). We used expression of GFP after infection with comparable doses of rAd5 and rAd35-GFP to obtain insight into whether the numbers of Ag-expressing DCs were the primary determinant of T cell stimulation efficiency. Our results suggest that rAd35-infected DCs are not more efficient at stimulating memory T cells than rAd5-infected DCs on a per cell basis. Importantly, because activation of naive rather than memory T cells is more dependent on receptor ligation and the correct cytokine milieu, it is possible that the two rAd vectors would have different efficiencies in generating primary T cell responses. Such information would of course provide highly relevant information for vaccine design.
In conclusion, we show that rAd35 more than rAd5 infects and activates primary DC subsets that in turn stimulate T cell responses. Phase I clinical trials of rAd35-based vaccines will be needed to determine whether these potentially desirable characteristics of rAd35 will translate into better immunogenicity in vivo.
Acknowledgments
We thank Drs. Gary Nabel, Mario Roederer, Robert A. Seder, and Jason M. Brenchley, Vaccine Research Center, National Institutes of Health, for constructive advice on this study.
Footnotes
Abbreviations used in this paper: DC, dendritic cell; MDC, myeloid DC; PDC, plasmacytoid DC; rAd, recombinant adenovirus vector; ip, infectious particle; CAR, coxsackie-adenovirus receptor; CHO, Chinese hamster ovary; BCL, B cell line.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Jenne L, Schuler G, Steinkasserer A. Viral vectors for dendritic cell-based immunotherapy. Trends Immunol. 2001;22:102–107. doi: 10.1016/s1471-4906(00)01813-5. [DOI] [PubMed] [Google Scholar]
- 3.Bowen GP, Borgland SL, Lam M, Libermann TA, Wong NC, Muruve DA. Adenovirus vector-induced inflammation: capsid-dependent induction of the C-C chemokine RANTES requires NF-κB. Hum. Gene Ther. 2002;13:367–379. doi: 10.1089/10430340252792503. [DOI] [PubMed] [Google Scholar]
- 4.Jooss K, Yang Y, Fisher KJ, Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J. Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muruve DA. The innate immune response to adenovirus vectors. Hum. Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- 6.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 7.Kostense S, Koudstaal W, Sprangers M, Weverling GJ, Penders G, Helmus N, Vogels R, Bakker M, Berkhout B, Havenga M, Goudsmit J. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS. 2004;18:1213–1216. doi: 10.1097/00002030-200405210-00019. [DOI] [PubMed] [Google Scholar]
- 8.Maguire CA, Sapinoro R, Girgis N, Rodriguez-Colon SM, Ramirez SH, Williams J, Dewhurst S. Recombinant adenovirus type 5 vectors that target DC-SIGN, ChemR23 and αvβ3 integrin efficiently transduce human dendritic cells and enhance presentation of vectored antigens. Vaccine. 2006;24:671–682. doi: 10.1016/j.vaccine.2005.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Offringa R, Kwappenberg K, Rabelink M, Rea D, Hoeben R. Adenoviral transduction of dendritic cells. Methods Mol. Med. 2005;109:83–96. doi: 10.1385/1-59259-862-5:083. [DOI] [PubMed] [Google Scholar]
- 10.Rea D, Schagen FH, Hoeben RC, Mehtali M, Havenga MJ, Toes RE, Melief CJ, Offringa R. Adenoviruses activate human dendritic cells without polarization toward a T-helper type 1-inducing subset. J. Virol. 1999;73:10245–10253. doi: 10.1128/jvi.73.12.10245-10253.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lore K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, Roederer M, Seder RA, Koup RA. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J. Immunol. 2003;171:4320–4328. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- 12.Lore K, Smed-Sorensen A, Vasudevan J, Mascola JR, Koup RA. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J. Exp. Med. 2005;201:2023–2033. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smed-Sorensen A, Lore K, Vasudevan J, Louder MK, Andersson J, Mascola JR, Spetz AL, Koup RA. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J. Virol. 2005;79:8861–8869. doi: 10.1128/JVI.79.14.8861-8869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 15.Havenga MJ, Lemckert AA, Grimbergen JM, Vogels R, Huisman LG, Valerio D, Bout A, Quax PH. Improved adenovirus vectors for infection of cardiovascular tissues. J. Virol. 2001;75:3335–3342. doi: 10.1128/JVI.75.7.3335-3342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shabram PW, Giroux DD, Goudreau AM, Gregory RJ, Horn MT, Huyghe BG, Liu X, Nunnally MH, Sugarman BJ, Sutjipto S. Analytical anion-exchange HPLC of recombinant type-5 adenoviral particles. Hum. Gene Ther. 1997;8:453–465. doi: 10.1089/hum.1997.8.4-453. [DOI] [PubMed] [Google Scholar]
- 17.Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J. Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 18.Ebbinghaus C, Al-Jaibaji A, Operschall E, Schoffel A, Peter I, Greber UF, Hemmi S. Functional and selective targeting of adenovirus to high-affinity Fcγ receptor I-positive cells by using a bispecific hybrid adapter. J. Virol. 2001;75:480–489. doi: 10.1128/JVI.75.1.480-489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu KH, Lonberg-Holm K, Alstein B, Crowell RL. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J. Virol. 1988;62:1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie J, Chiang L, Contreras J, Wu K, Garner JA, Medina-Kauwe L, Hamm-Alvarez SF. Novel fiber-dependent entry mechanism for Ad5 in lacrimal acini. J. Virol. 2006;80:11833–11851. doi: 10.1128/JVI.00857-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakurai F, Murakami S, Kawabata K, Okada N, Yamamoto A, Seya T, Hayakawa T, Mizuguchi H. The short consensus repeats 1 and 2, not the cytoplasmic domain, of human CD46 are crucial for infection of subgroup B adenovirus serotype 35. J. Control Release. 2006;113:271–278. doi: 10.1016/j.jconrel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chattopadhyay PK, Price DA, Harper TF, Betts MR, Yu J, Gostick E, Perfetto SP, Goepfert P, Koup RA, De Rosa SC, Bruchez MP, Roederer M. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat. Med. 2006;12:972–977. doi: 10.1038/nm1371. [DOI] [PubMed] [Google Scholar]
- 24.Perfetto SP, Chattopadhyay PK, Lamoreaux L, Nguyen R, Ambrozak D, Koup RA, Roederer M. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J. Immunol. Methods. 2006;313:199–208. doi: 10.1016/j.jim.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 26.Yoneyama H, Matsuno K, Toda E, Nishiwaki T, Matsuo N, Nakano A, Narumi S, Lu B, Gerard C, Ishikawa S, Matsushima K. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 2005;202:425–435. doi: 10.1084/jem.20041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palucka K, Banchereau J. Linking innate and adaptive immunity. Nat. Med. 1999;5:868–870. doi: 10.1038/11303. [DOI] [PubMed] [Google Scholar]
- 28.Rea D, Havenga MJ, van Den Assem M, Sutmuller RP, Lemckert A, Hoeben RC, Bout A, Melief CJ, Offringa R. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fibermodified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J. Immunol. 2001;166:5236–5244. doi: 10.4049/jimmunol.166.8.5236. [DOI] [PubMed] [Google Scholar]
- 29.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 30.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 31.Johansson C, Jonsson M, Marttila M, Persson D, Fan XL, Skog J, Frangsmyr L, Wadell G, Arnberg N. Adenoviruses use lactoferrin as a bridge for CAR-independent binding to and infection of epithelial cells. J. Virol. 2007;81:954–963. doi: 10.1128/JVI.01995-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng C, Gall JG, Kong WP, Sheets RL, Gomez PL, King CR, Nabel GJ. Mechanism of ad5 vaccine immunity and toxicity: fiber shaft targeting of dendritic cells. PLoS Pathog. 2007;3:e25. doi: 10.1371/journal.ppat.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakurai F, Akitomo K, Kawabata K, Hayakawa T, Mizuguchi H. Downregulation of human CD46 by adenovirus serotype 35 vectors. Gene Ther. 2007;14:912–919. doi: 10.1038/sj.gt.3302946. [DOI] [PubMed] [Google Scholar]
- 34.Zhong L, Granelli-Piperno A, Choi Y, Steinman RM. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur. J. Immunol. 1999;29:964–972. doi: 10.1002/(SICI)1521-4141(199903)29:03<964::AID-IMMU964>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.Hsu C, Boysen M, Gritton LD, Frosst PD, Nemerow GR, Von Seggern DJ. In vitro dendritic cell infection by pseudotyped adeno viral vectors does not correlate with their in vivo immunogenicity. Virology. 2005;332:1–7. doi: 10.1016/j.virol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 36.de Gruijl TD, Ophorst OJ, Goudsmit J, Verhaagh S, Lougheed SM, Radosevic K, Havenga MJ, Scheper RJ. Intradermal delivery of adenoviral type-35 vectors leads to high efficiency transduction of mature, CD8+ T cell-stimulating skin-emigrated dendritic cells. J. Immunol. 2006;177:2208–2215. doi: 10.4049/jimmunol.177.4.2208. [DOI] [PubMed] [Google Scholar]
- 37.Ophorst OJ, Kostense S, Goudsmit J, De Swart RL, Verhaagh S, Zakhartchouk A, Van Meijer M, Sprangers M, Van Amerongen G, Yuksel S, Osterhaus AD, Havenga MJ. An adenoviral type 5 vector carrying a type 35 fiber as a vaccine vehicle: DC targeting, cross neutralization, and immunogenicity. Vaccine. 2004;22:3035–3044. doi: 10.1016/j.vaccine.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Slager EH, van der Minne CE, Goudsmit J, van Oers JM, Kostense S, Havenga MJ, Osanto S, Griffioen M. Induction of CAMEL/NYESO-ORF2-specific CD8+ T cells upon stimulation with dendritic cells infected with a modified Ad5 vector expressing a chimeric Ad5/35 fiber. Cancer Gene Ther. 2004;11:227–236. doi: 10.1038/sj.cgt.7700674. [DOI] [PubMed] [Google Scholar]
- 39.Nanda A, Lynch DM, Goudsmit J, Lemckert AA, Ewald BA, Sumida SM, Truitt DM, Abbink P, Kishko MG, Gorgone DA, et al. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J. Virol. 2005;79:14161–14168. doi: 10.1128/JVI.79.22.14161-14168.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhaagh S, de Jong E, Goudsmit J, Lecollinet S, Gillissen G, de Vries M, van Leuven K, Que I, Ouwehand K, Mintardjo R, et al. Human CD46-transgenic mice in studies involving replication-incompetent adenoviral type 35 vectors. J. Gen. Virol. 2006;87:255–265. doi: 10.1099/vir.0.81293-0. [DOI] [PubMed] [Google Scholar]
- 41.Ni S, Bernt K, Gaggar A, Li ZY, Kiem HP, Lieber A. Evaluation of biodistribution and safety of adenovirus vectors containing group B fibers after intravenous injection into baboons. Hum. Gene Ther. 2005;16:664–677. doi: 10.1089/hum.2005.16.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meier O, Boucke K, Hammer SV, Keller S, Stidwill RP, Hemmi S, Greber UF. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 2002;158:1119–1131. doi: 10.1083/jcb.200112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins αvβ3 and αvβ35 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 44.Bontkes HJ, Ruizendaal JJ, Schreurs MW, Kramer D, Meijer CJ, Hooijberg E. Antigen gene transfer to human plasmacytoid dendritic cells using recombinant adenovirus and vaccinia virus vectors. Cell. Oncol. 2005;27:175–182. doi: 10.1155/2005/753549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huarte E, Larrea E, Hernandez-Alcoceba R, Alfaro C, Murillo O, Arina A, Tirapu I, Azpilicueta A, Hervas-Stubbs S, Bortolanza S, et al. Recombinant adenoviral vectors turn on the type I interferon system without inhibition of transgene expression and viral replication. Mol. Ther. J. Am. Soc. Gene Ther. 2006;14:129–138. doi: 10.1016/j.ymthe.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Iacobelli-Martinez M, Nemerow GR. Preferential activation of Toll-like receptor nine (TLR9) by CD46-utilizing adenoviruses. J. Virol. 2006;81:1305–1312. doi: 10.1128/JVI.01926-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 49.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou W, Borvak J, Wei S, Isaeva T, Curiel DT, Curiel TJ. Reciprocal regulation of plasmacytoid dendritic cells and monocytes during viral infection. Eur. J. Immunol. 2001;31:3833–3839. doi: 10.1002/1521-4141(200112)31:12<3833::aid-immu3833>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 51.Hensley SE, Giles-Davis W, McCoy KC, Weninger W, Ertl HC. Dendritic cell maturation, but not CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J. Immunol. 2005;175:6032–6041. doi: 10.4049/jimmunol.175.9.6032. [DOI] [PubMed] [Google Scholar]
- 52.Basner-Tschakarjan E, Gaffal E, O'Keeffe M, Tormo D, Limmer A, Wagner H, Hochrein H, Tuting T. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-α production. J. Gene Med. 2006;8:1300–1306. doi: 10.1002/jgm.964. [DOI] [PubMed] [Google Scholar]
- 53.Hochrein H, Schlatter B, O'Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA. 2004;101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, Ludwig H, Sutter G, Suzuki K, Hemmi H, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]