Abstract
Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) shows promise as a chemotherapeutic agent. However, many human cancer cells are resistant to killing by TRAIL. We have previously demonstrated that reovirus infection increases the susceptibility of human lung (H157) and breast (ZR75-1) cancer cell lines to TRAIL-induced apoptosis. We now show that reovirus also increases the susceptibility of human ovarian cancer cell lines (OVCAR3, PA-1 and SKOV-3) to TRAIL-induced apoptosis. Reovirus-induced increases in susceptibility of OVCAR3 cells to TRAIL require virus uncoating and involve increased activation of caspases 3 and 8. Reovirus infection results in the down-regulation of cFLIP (cellular FLICE inhibitory protein) in OVCAR3 cells. Down-regulation of cFLIP following treatment of OVCAR3 cells with antisense cFLIP oligonucleotides or PI3 kinase inhibition also increases the susceptibility of OVCAR3 cells to TRAIL-induced apoptosis. Finally, over-expression of cFLIP blocks reovirus-induced sensitization of OVCAR3 cells to TRAIL-induced apoptosis. The combination of reovirus and TRAIL thus represents a promising new therapeutic approach for the treatment of ovarian cancer.
Keywords: Reovirus, TRAIL, cFLIP, OVCAR3, Apoptosis
Introduction
Ovarian cancer is a major gynecological malignancy and ranks fifth as the cause of cancer deaths in women [1]. Most patients present with late stage disease and the long term survival for patients with advanced high-grade ovarian cancer remains less than 5% [2]. The major factor that limits the effectiveness of chemotherapy in patients with advanced ovarian cancer is the acquisition of resistance [2]. New treatment strategies that overcome intrinsic and acquired resistance are therefore needed.
Tumor necrosis factor (TNF)-related apoptosis inducing ligand (TRAIL) induces apoptosis in a wide variety of tumor cells, both in vivo and in vitro, whilst being relatively nontoxic to normal cells [3-7]. TRAIL binds to cell surface death receptors TRAIL-R1 and TRAIL-R2 [8, 9]. TRAIL-R1 and TRAIL-R2 have a cytoplasmic death domain (DD) which recruits the adaptor molecule FADD (Fas associated death domain) [10]. FADD then recruits pro-caspase 8 via its death effector domain (DED) to form the death inducing signaling complex (DISC) where activation of caspase 8 (also called FLICE) appears to involve two subsequent cleavage events [11-13].
TRAIL can also bind to two cell surface decoy receptors (TRAIL-R3 and TRAIL-R4) which are unable to transduce death signals due to a truncated or absent intracellular domain [14-16]. In addition, soluble death receptors, such as osteoprotegerin (OPG) [17, 18], are produced by some cancer cells and can act as survival factors for human cancer cells in vitro [19-21].
Preclinical studies using recombinant TRAIL in animal models have demonstrated a potent anti-tumor effect [22]. However, not all tumor cell lines respond to TRAIL. The lack of response to TRAIL has been associated with multiple factors including loss of caspase 8 [23, 24], activation of NF-κB [25], alteration in the expression of genes encoding Bcl-2 family members [26-28] and over-expression of decoy receptors [29] or cFLIP [5, 30].
Previous studies have demonstrated that ovarian cancer cell lines show variable sensitivity to TRAIL-induced apoptosis that can be enhanced by chemotherapy [31-35]. The expression of cFLIP may be especially important in determining TRAIL sensitivity in ovarian cancer cell lines [35]. cFLIP is differentially expressed in 40% of epithelial ovarian tumors compared with normal ovarian epithelium [36]. In addition, a significant enhancement in cell death was seen in ovarian cancer cells when c-FLIP levels were down-regulated by RNA interference [33].
Active cFLIP proteins exist as long (cFLIPL, 55 kDa) and short (cFLIPS, 26 kDa) splice variants, both of which contain two DEDs and compete with caspase 8 for recruitment to FADD. cFLIPL closely resembles the overall structure of pro-caspase 8 allowing the formation of caspase 8/cFLIPL heterodimers. The C-terminus of cFLIPL contains an activation loop that overlaps and exposes the enzymatic pocket of caspase 8 to allow partial processing and release of the p10 fragment but not further cleavage to fully active caspase 8 [37]. Interestingly, when cellular levels of cFLIPL are high it can also act as an activator of caspase 8 [38-40].
Reoviruses are commonly isolated from human respiratory and gastrointestinal tracts and are considered “orphan” viruses because they are not associated with clinical illness in humans. Reoviruses preferentially replicate in cells with an activated Ras pathway [41-43], a feature of many human cancers. Reovirus infection results in oncolysis of numerous human cancer cells in vitro [44-48], including ovarian cancer cells [44] and in the significant regression of a variety of tumors established in mice from human cancer cells [43-48]. In mouse models both intratumoral and intravenous inoculation of reovirus induce the significant regression of ovarian tumors implanted in the hind flank [44]. It has also been shown that reovirus infection is restricted to ovarian cancer cells, both in vitro and in vivo, whilst normal ovarian cell lines and normal tissue surrounding the implanted tumor remain uninfected [44].
In this study we demonstrate that reovirus increases the susceptibility of human ovarian cancer cells (OVCAR3, PA1 and SKOV-3) to TRAIL. The reovirus-induced increase in susceptibility of OVCAR3 cells to TRAIL is dependent on caspase 8 and is associated with the increased activation of caspases 8 and 3 and the down-regulation of cFLIP. Down-regulation of cFLIP expression following treatment with antisense cFLIP oligonucleotides or inhibition of PI3 kinase signaling also increases the susceptibility of OVCAR3 cells to TRAIL. Our results have implications for the use of reovirus infection or cFLIP down-regulation in combination with TRAIL for the treatment of ovarian cancer.
Materials and methods
Cells and virus
The human ovarian cancer cell line, OVCAR3, was obtained from the University of Colorado Cancer Center. OVCAR3 cells were grown in Optimem (Invitrogen, Carlsbad, CA) supplemented with 2.4 mM L-glutamine and containing 10% heat inactivated fetal bovine serum. HeLa cells (ATCC CCL2) were grown in Eagle’s minimal essential medium (MEM) supplemented with 2.4 mM L-glutamine, nonessential amino acids, 60 U/ml each of penicillin and streptomycin and containing 10% fetal bovine serum (Gibco/BRL). The ovarian cancer cell line PA-1 (ATCC CRL-1572) was grown in minimal essential medium with 2 mM L-glutamine and Eales BSS adjusted to contain 1.5 g/L sodium bicarbonate, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate and 10% heat-inactivated fetal bovine serum. SKOV-3 cells (ATCC HTB-77) were a kind gift from Dr. Kian Behbakht (University of Colorado Health Sciences Center. SKOV-3 cells were grown in McCoys 5a medium with 1.5 mM L-glutamine adjusted to contain 2.2 g/L sodium bicarbonate and 10% fetal bovine serum. Reovirus (Type 3 Abney, T3A) is a laboratory stock, which has been plaque purified and passaged (twice) in L929 (ATCC CCL1) cells to generate working stocks [49]. Infections were carried out at a multiplicity of infection (MOI) of 10. For infections, growth media was removed from cells and was replaced with virus in 200 μl gelatin saline. Cells and virus were incubated at 37°C for 1 h with rocking every 15 mins. After this time fresh growth media was added to the cells. Mock infections were performed as above except without virus. To prevent viral uncoating infections were carried out at 4°C for 1 h with rocking every 15 mins (to allow viral adsorption but not internalization). Growth media containing ammonium chloride (10 mM) was then added back to the cells to prevent viral uncoating [50].
Reagents
TRAIL was obtained from Sigma-Aldrich (St. Louis, MO, K4761). This preparation of TRAIL consists of the extracellular domain of human TRAIL with a histidine tag at the amino terminus. The cell permeable caspase 8 inhibitor IETD-fmk was obtained from Clontech (Mountain View, CA) and was used at a concentration of 20 μM. Wortmannin was obtained from Calbiochem (San Diego, CA) and was used at a concentration of 2 μM. Cells were pre-treated with IETD-fmk or wortmannin for 1 h prior to virus infection or TRAIL-treatment. Antisense and sense oligonucleotides were prepared by Integrated DNA Technologies (IDT, Skokie, IL) at a concentration of 1 μM following HPLC purification. Phosphothionate bonds present between nucleotides were included to enhance cell permeability. Antisense cFLIP:5′-gatttcagcagacatcctac-3′and sense cFLIP:5′-catcctacagacgacttcag-3′sequences were used [51, 52]. Oligonucleotides were added directly to the media and cells were incubated for 18-24 h prior to TRAIL treatment. The plasmid pEGFP-N1, which was designed to over-express cFLIP was a gift from Dr. Gregory Gores, Mayo Clinic.
Western blotting
Following treatment, cells were pelleted by centrifugation, washed twice with ice-cold phosphate-buffered saline and lysed by sonication in 200 μl of a buffer containing 15 mM Tris, pH 7.5, 2 mM EDTA, 10 mM EGTA, 20% glycerol, 0.1% NP-40, 50 mM β-mercaptoethanol, 100 μg/ml leupeptin, 2 μg/ml aprotinin, 40 μM Z-D-DCB, and 1 mM PMSF. The lysates were then cleared by centrifugation at 16,000 × g for 5 min, normalized for protein amount, mixed 1:1 with SDS sample buffer (100 mM Tris, pH 6.8, 2% SDS, 300 mM β-mercaptoethanol, 30% glycerol, and 5% pyronine Y), boiled for 5 min and stored at -70°C. Proteins were electrophoresed by SDS-PAGE (13% gels) and probed with antibodies directed against cleaved PARP (Cell Signaling Technology, Danvers MA, #9541), caspase 8 (Cell Signaling Technology #9746) and cFLIP (Axxora, San Diego CA, #PSC-1159 and Abcam, Cambridge, MA #ab10864 and #ab21486). All lysates were standardized for protein concentration with antibodies directed against actin (Oncogene, Cambridge, MA #CP01). Autoradiographs were quantitated by densitometric analysis using a Fluor-S MultiImager (Bio-Rad Laboratories, Hercules, CA).
Apoptosis assays
Apoptotic nuclear morphology and cell viability were determined by staining with acridine orange and ethidium bromide at a final concentration of 1 μg/ml each. Following staining, cells were examined by epifluorescence microscopy (Nikon Labophot-2: B-2A filter, excitation, 450-490 nm; barrier, 520 nm; dichroic mirror, 505 nm). The percentage of cells containing condensed nuclei and/or marginated chromatin in a population of 100 cells was recorded. Cells which contained condensed nuclei that stained green (acridine orange) were considered to be early apoptotic whereas cells which contained condensed nuclei that stained red (ethidium bromide) were considered to be late apoptotic. The specificity of this assay has been previously established in reovirus-infected cells using DNA laddering techniques and electron microscopy [53, 54]. Cell populations were also analyzed by flow cytometry to determine intracellular levels of active caspase 8, using a fluorochrome inhibitor of caspases (FLICA, Immunochemistry Technologies, Bloomington, MN) and to determine phosphatidyl serine exposure on the outer cell membrane by annexin assay (Trevigen).
Immunocytochemistry
Cells were grown on 8-well chamber slides coated with rat-tail collagen (Becton Dickenson, Germantown, WI, 354630). Following treatment cells were fixed with 3.7% formaldehyde/phosphate-buffered saline (PBS) for 15 min at room temperature. Following 3 washes in PBS cells were permeablized overnight at 4°C in PBS containing 0.1% TritonX (PBSX). Cells were then blocked in PBSX with 5% BSA for 2-4 h at room temperature before being incubated overnight at 4°C with antibodies directed against cleaved (active) caspase 3 (Cell Signaling Technologies #9661) at a 1:100 dilution in PBSX with 3% BSA. After washing (×3) in PBSX cells were incubated with secondary anti-rabbit IgG conjugated to FITC (Vector Laboratories, Burlingame, CA, FI 1200) for 1 h at room temperature and were washed again (×3) in PBSX, before being counterstained with Hoechst 33342 (Invitrogen/Molecular Probes, H 3570) for 10 min at room temperature. Cells were then washed again (×3) in PBSX before being mounted with vectashield (Vector Laboratories, H1000) and digitally imaged using a Zeiss Axioplan2 epifluorescence microscope.
Results
Reovirus increases the susceptibility of OVCAR3 cells to TRAIL-induced apoptosis in a caspase 8 dependent manner
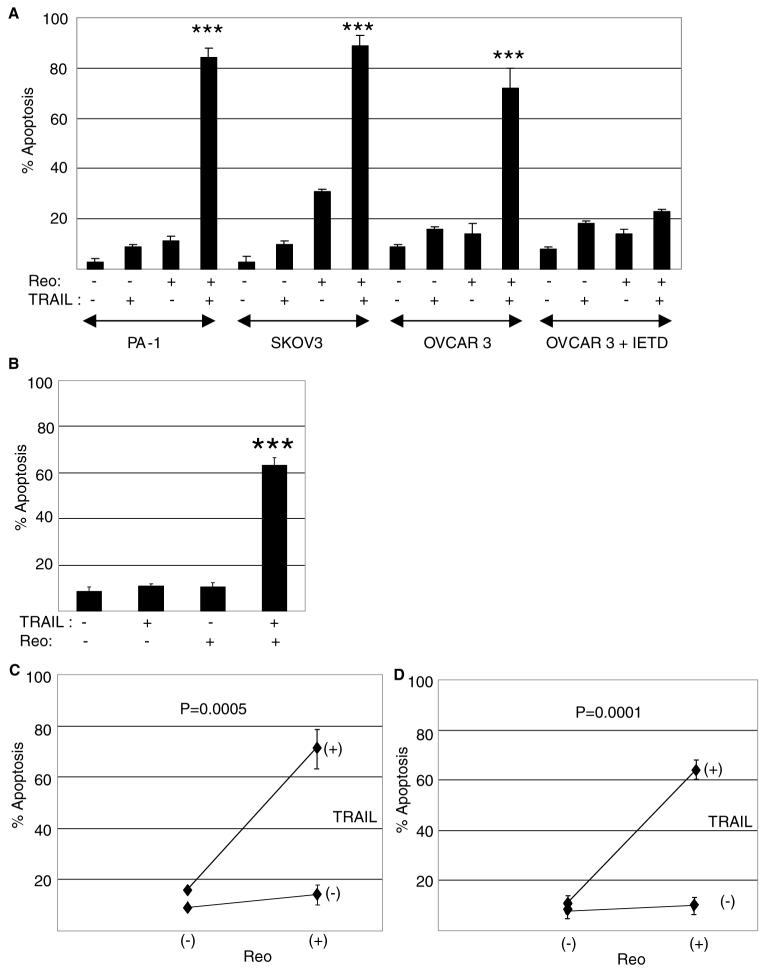
We have previously shown that reovirus increases the susceptibility of human breast (ZR75-1) and lung (H157) cancer cells to TRAIL-induced apoptosis [54]. We next wanted to discover whether reovirus could also increase the susceptibility of human ovarian cancer cells to TRAIL-induced apoptosis and to determine the mechanism by which this occurs. The human ovarian cancer cell lines OVCAR3, PA-1 and SKOV-3 were infected with reovirus (multiplicity of infection, MOI, 10). After 24 (OVCAR3, PA-1) or 8 (SKOV-3) h cells were then treated with TRAIL (20 ng/ml) and apoptosis was determined after a further 18 h by determination of nuclear morphology using acridine orange and ethidium bromide stains. TRAIL susceptibility was significantly (P < 0.001) enhanced in all three ovarian cancer cells lines (Fig. 1). A shorter infection time was used for SKOV-3 cells since reovirus infection alone induced around 80% apoptosis after 42 h in these cells. Even with the shorter viral infection period these cells showed the highest levels of apoptosis in cells treated with virus alone. However it should be noted that even under the extended (42 h) infection times reovirus infection produced an early apoptotic phenotype whereas in the population of cells treated with both reovirus and TRAIL the majority of cells had a late apoptotic phenotype (not shown).
Fig. 1.
Reovirus increases the susceptibility of human ovarian cancer cells to TRAIL-induced apoptosis in a caspase 8-dependent manner. (A) The ovarian cancer cell lines PA-1, SKOV-3 and OVCAR3 were infected with reovirus (MOI 10) in the presence or absence of IETD-fmk (20 μM). Twenty four (OVCAR3 and PA-1) or 8 (SKOV3) h later the cells were treated with TRAIL (20 ng/ml) and apoptosis was determined after a further 18-24 h. Apoptosis was significantly (P < 0.001,***) enhanced in PA-1 and SKOV-3 and OVCAR3 cells treated with reovirus and TRAIL compared to cells treated with TRAIL or reovirus alone. Apoptosis was also significantly (P < 0.001,***) enhanced in OVCAR3 cells treated with reovirus and TRAIL compared to OVCAR3 cells treated with TRAIL and reovirus in the presence of IETD-fmk. (B) OVCAR3 cells were treated with TRAIL (20 ng/ml) for 1 h prior to being infected with reovirus (MOI 10). Apoptosis was determined after a further 35 h. Apoptosis was significantly (P < 0.001,***) enhanced in cells treated with reovirus and TRAIL compared to cells treated with TRAIL or reovirus alone. The graphs (A and B) show the percentage of cells with apoptotic nuclear morphology from at least 3 independent experiments for OVCAR3 and PA-1 cells and 2 independent experiments for SKOV-3 cells. Error bars represent standard errors of the mean. The effect of reovirus and TRAIL on apoptosis in OVCAR3 cells was found to be synergistic as determined by a 2×2 factorial ANOVA both when reovirus was added before TRAIL (C, see above for details) and when TRAIL was added before reovirus (D, see above for details)
Significant (P < 0.001) increases in apoptosis were also observed in OVCAR3 cells in which TRAIL treatment preceded reovirus infection by 1 h (Fig. 1(B)). Under these conditions cells were harvested 36 h following TRAIL treatment.
The effects of TRAIL and reovirus on the induction of apoptosis in OVCAR3 cells resulted in statistically significant synergy as determined by a 2 × 2 factorial ANOVA as described by Slinker (Fig. 1(C, D)) [55].
The reovirus-induced increase in the susceptibility of OVCAR3 cells to TRAIL was significantly (P < 0.001) reduced in the presence of IETD-fmk, demonstrating that this effect requires caspase 8 activity (Fig. 1(A)). These results are similar to what we had previously observed following reovirus infection of ZR75-1 cells [54].
The reovirus-induced increase in susceptibility of human ovarian cancer cells to TRAIL was also examined by annexin assay. PA-1 and SKOV-3 cells were infected with reovirus (MOI 10). Eighteen h following infection cells were treated with TRAIL (20 ng/ml) and were then harvested and stained with a combination of FITC-labelled annexin and propidium iodide after a further 4-6 h. A significant (P < 0.01) increase in annexin positive, propidium iodide negative (early apoptotic) cells in PA-1 and SKOV-3 cells treated with TRAIL and reovirus compared to cells treated with TRAIL or reovirus alone was demonstrated (Fig. 2). OVCAR3 cells showed high background staining and were not used for the experiment.
Fig. 2.
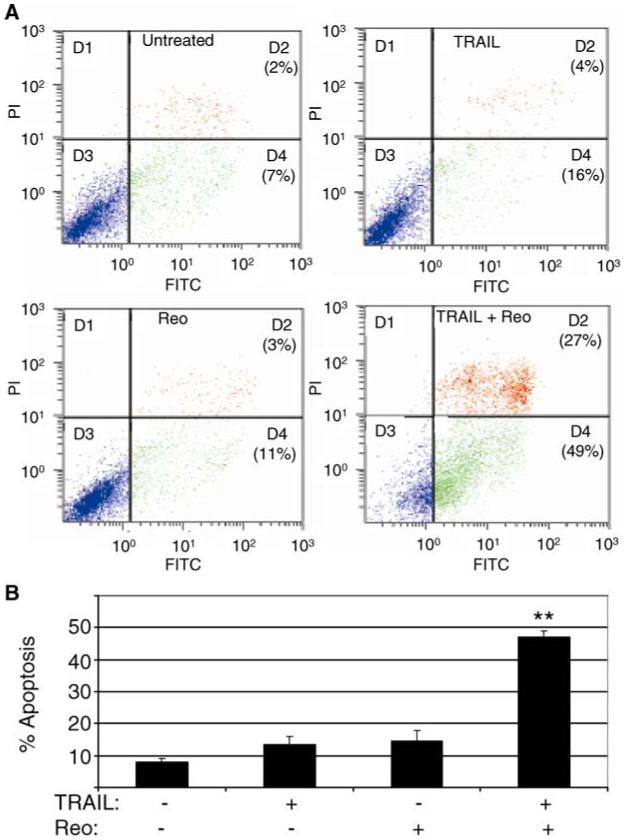
Reovirus increases the susceptibility of human ovarian cancer cells to TRAIL-induced apoptosis. The ovarian cancer cell lines PA-1 and SKOV-3 were infected with reovirus (MOI 10) and were treated with TRAIL (20 ng/ml) for a further 4-6 h. Cells were then harvested, stained with FITC-labelled annexin V and propidium iodide and analyzed by flow cytometry. The number of cells that stained positive for annexin V but negative for propidium iodide (early apoptotic) is shown. Data obtained from PA-1 cells (A) and a graphical representation of results from PA-1 and SKOV-3 cell (B) is shown. The graph shows the percentage of early cells from 2 independent experiments. Error bars represent standard errors of the mean. The percentage of early apoptotic cells was significantly (P < 0.1,**) enhanced in PA-1 and SKOV-3 cells treated with reovirus and TRAIL compared to cells treated with TRAIL or reovirus alone
These experiments indicated that reovirus increases the susceptibility of a variety of human ovarian cancer cells to TRAIL-induced apoptosis. We chose OVCAR3 cells to further explore the mechanism by which reovirus increases the susceptibility of human ovarian cancer cells to TRAIL-induced apoptosis.
Reovirus increases the susceptibility of OVCAR3 cells to TRAIL-induced activation of caspase 3 in a caspase 8-dependent mannner
We next showed that reovirus increases the activation of caspase 3 in TRAIL-treated OVCAR3 cells. OVCAR3 cells were infected with reovirus (MOI 10) for 18 h prior to TRAIL-treatment (20 ng/ml) and were incubated with TRAIL for 6 h before being harvested for western blot analysis and immunocytochemistry (ICC). Six h was chosen as an appropriate time to determine caspase 3 activity since we expected this to occur prior to the appearance of apoptotic nuclear morphology. To determine the activity of caspase 3 in reovirus-infected, TRAIL-treated cells western blot analysis was performed using an antibody directed against cleaved PARP, a product of caspase 3 activity. Six h following TRAIL treatment increased PARP cleavage was evident in cells treated with reovirus and TRAIL, compared to cells treated with TRAIL alone (Fig. 3(A)). In addition, ICC was performed using an antibody directed against active caspase 3. Six h following TRAIL treatment 32% of TRAIL-treated, mock-infected OVCAR3 cells were found to contain active caspase 3, compared to 92% of TRAIL-treated, reovirus-infected cells (P < 0.001, Fig. 3(B)). No activated caspase 3 was seen in cells treated with reovirus alone.
Fig. 3.
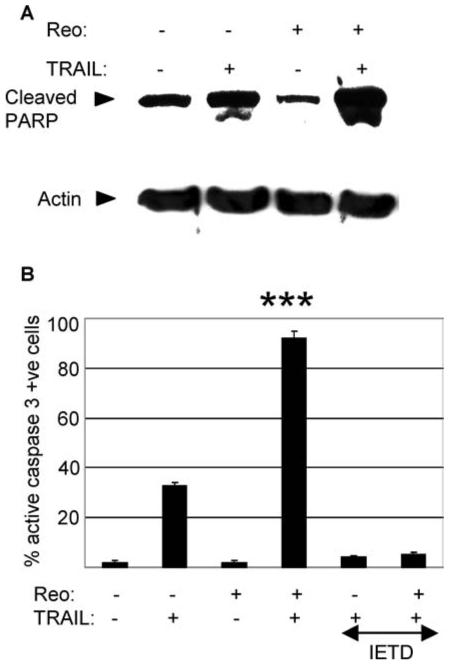
Reovirus sensitizes OVCAR3 cells to TRAIL-induced activation of caspase 3 in a caspase 8 dependent manner. OVCAR 3 cells were infected with reovirus (MOI 10) in the presence or absence of IETD-fmk (20 μM). After 18 h cells were treated with TRAIL (20 ng/ml). After a further 6 h cells containing cleaved PARP were determined by western blot analysis (A) and cells expressing activated caspase 3 were determined by immunocytochemistry (ICC) (B). Anti-actin antibodies were used to control for protein loading. The graph shows the percentage of cells containing activated caspase 3 from three independent experiments. Error bars indicate the standard error of the mean. Caspase 3 activation was significantly (P < 0.001,***) enhanced in cells treated with reovirus and TRAIL compared to cells treated with TRAIL or reovirus alone and compared to cells treated with TRAIL and reovirus in the presence of IETD-fmk
Activation of caspase 3 was significantly blocked by inhibition of caspase 8 activity in both cells treated with reovirus and TRAIL (P < 0.001) and in cells treated with TRAIL alone (P < 0.001, Fig. 3(B)), again demonstrating that the reovirus-induced increase in the susceptibility of OVCAR3 cells to TRAIL requires caspase 8 activity.
Reovirus increases caspase 8 activation in TRAIL-treated OVCAR3 cells
Having shown that caspase 8 activity was required for sensitization of OVCAR3 cells to TRAIL-induced apoptosis and caspase 3 activation we next wanted to determine whether reovirus induced increased levels of caspase 8 activity in TRAIL-treated cells. OVCAR3 cells were infected with reovirus or were mock-infected for 18 h prior to TRAIL treatment and were incubated with TRAIL for 4 or 6 h before being harvested for FLICA assays or western blot analysis respectively using probes or antibodies specific for activated caspase 8 (Fig. 4(A)). Four hours was chosen as an appropriate time to determine caspase 8 activity by FLICA analysis since we expected caspase 8 activation to precede caspase 3 activation. Four h following TRAIL treatment 22% of reovirus-infected OVCAR3 cells contained active caspase 8 by FLICA assay, compared to 7% of TRAIL-treated mock-infected cells (P < 0.05) and 4% of cells infected with reovirus alone (P < 0.01).
Fig. 4.
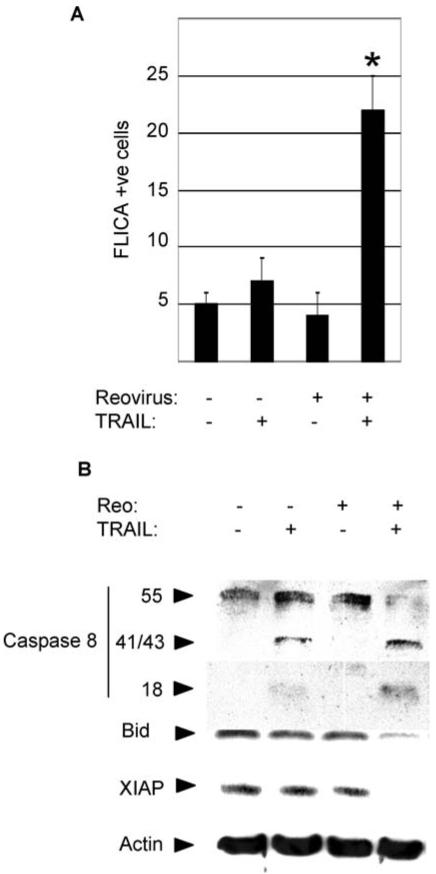
Reovirus increases caspase 8 activation in TRAIL-treated cells. OVCAR3 cells were infected with reovirus (MOI 10) or were mockinfected. Eighteen h post infection cells were treated with TRAIL (20 ng/ml). Cells were harvested after a further 4 h to determine the percentage of cells containing activated caspase 8 by FLICA analysis (A) or a further 6 h for western blot analysis using antibodies directed against caspase 8, Bid and XIAP (B). The graph shows the percentage of cells containing activated caspase 8 from three independent experiments. Error bars indicate the standard error of the mean. Caspase 8 activation was significantly enhanced in cells treated with reovirus and TRAIL compared to cells treated with TRAIL (P < 0.05, *)or reovirus (P < 0.01) alone. Anti-actin antibodies were used to control for protein loading
Increased activation of caspase 8 in reovirus-infected TRAIL-treated OVCAR3 cells compared to cells treated with TRAIL alone was also demonstrated by western blot analysis (Fig. 4(B)). Six h following TRAIL treatment western blot assays demonstrated the presence of the active (p18) fragment of caspase 8 only in reovirus-infected, TRAIL-treated cells and not in cells treated with TRAIL-alone. Cleavage of full-length pro-caspase 8 was also seen under these conditions. The appearance of a p41/43 band (representing partial cleavage of pro-caspase 8), but no detectable decrease in levels of pro-caspase 8, were observed in TRAIL-treated OVCAR3 cells. No evidence of caspase 8 activation was seen in mock infected cells or in cells infected with reovirus alone.
Reovirus enhances mitochondrial apoptotic signaling in TRAIL-treated cells
We next determined whether mitochondrial apoptotic signaling was enhanced in reovirus-infected, TRAIL treated cells compared to cells treated with TRAIL alone. The BH3 only Bcl-2 family member Bid promotes the release of proapoptotic mitochondria factors, including the second mitochondrial activator of caspases (smac), following caspase 8-dependent cleavage. Released smac contributes to apoptosis by reducing levels of cellular IAPs (cellular inhibitors of apoptosis proteins), including XIAP, which has the effect of increasing caspase 3 activity. OVCAR3 cells were infected with reovirus or were mock-infected for 18 h prior to TRAIL treatment and were incubated with TRAIL for 6 h before being harvested for western blot analysis antibodies specific for Bid and XIAP (Fig. 4(B)). Decreased levels of Bid and XIAP were seen in reovirus-infected, TRAIL-treated cells compared to cells treated with TRAIL or reovirus alone indicating that reovirus enhances mitochondrial signaling in TRAIL-treated cells.
The reovirus-induced increase in the susceptibility of OVCAR3 cells to TRAIL requires viral uncoating
We used ammonium chloride (AC), which inhibits endosomal acidification, viral uncoating and viral release into the cytoplasm [56] to determine whether viral uncoating and release into the cytoplasm was required for reovirus-induced sensitization of OVCAR3 cells to TRAIL. In the presence of AC reovirus no longer increased the susceptibility of OVCAR3 cells to TRAIL-induced apoptosis (Fig. 5(A)). Similarly, AC inhibited the ability of reovirus to induce increased PARP cleavage and caspase 8 activation in TRAIL-treated OVCAR3 cells (Fig. 5(B)). As expected, AC had no effect on apoptosis seen in OVCAR3 cells treated with TRAIL alone (Fig. 5(A)). These results indicate that, in addition to being required for reovirus-induced cancer cell death, virus uncoating is also required for the ability of reovirus to increase the susceptibility of OVCAR3 cells to TRAIL-induced apoptosis.
Fig. 5.
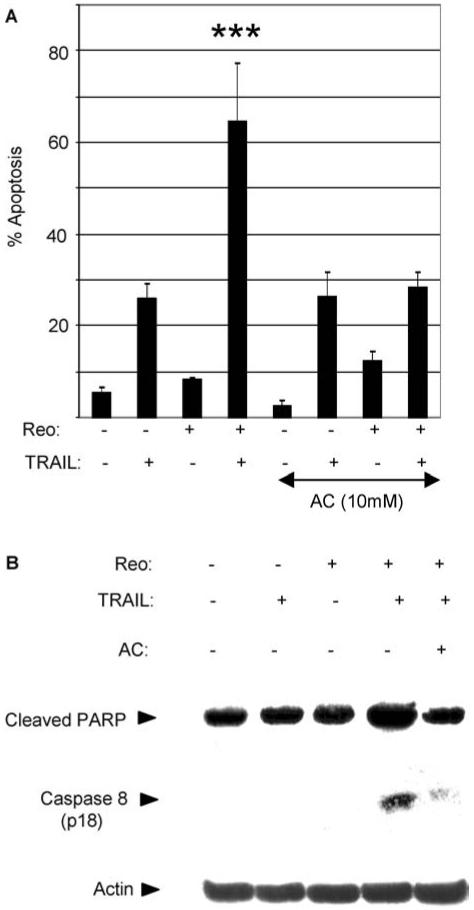
Reovirus induced sensitization of OVCAR3 cells to TRAIL requires viral uncoating. OVCAR3 cells were infected with reovirus (MOI 10) in the presence or absence of ammonium chloride (AC, 10 mM). Twenty four h later the cells were treated with TRAIL (20 ng/ml). (A) 24 h following TRAIL treatment the percentage of cells with apoptotic nuclear morphology was determined. The graph shows the mean percentage of apoptotic cells from three independent experiments. Error bars indicate the standard error of the mean. Apoptosis was significantly reduced (P < 0.001,***) in reovirus-infected, TRAIL-treated cells in the presence of AC compared to that seen in reovirus-infected, TRAIL-treated cells without AC. (B) 6 h following TRAIL treatment cells were analyzed by western blot using antibodies directed against cleaved PARP and caspase 8. Anti-actin antibodies were used to control for protein loading
Reovirus down-regulates cFLIP in infected OVCAR3 cells
FLICE inhibitory protein (cFLIP) is a cellular inhibitor of caspase 8 activation. Having shown that reovirus increases caspase 8 activation in TRAIL-treated cells and that this leads to increased activation of caspase 3 and apoptosis we next investigated levels of cFLIP in OVCAR3 cells. TRAIL treatment of OVCAR3 cells was not found to result in changes in the expression of either of the anti-apoptotic isoforms of cFLIP, cFLIP long (cFLIPL, 55 kDa) or cFLIP short (cFLIPS, 25 kDa) (Fig. 6). In contrast, TRAIL treatment of the TRAIL-sensitive HeLa cell line [54] resulted in a marked decrease in the expression of cFLIPL and a less dramatic but consistent decrease in cFLIPS. These results suggest that decreased levels of cFLIP expression are associated with apoptosis in TRAIL-treated cells.
Fig. 6.
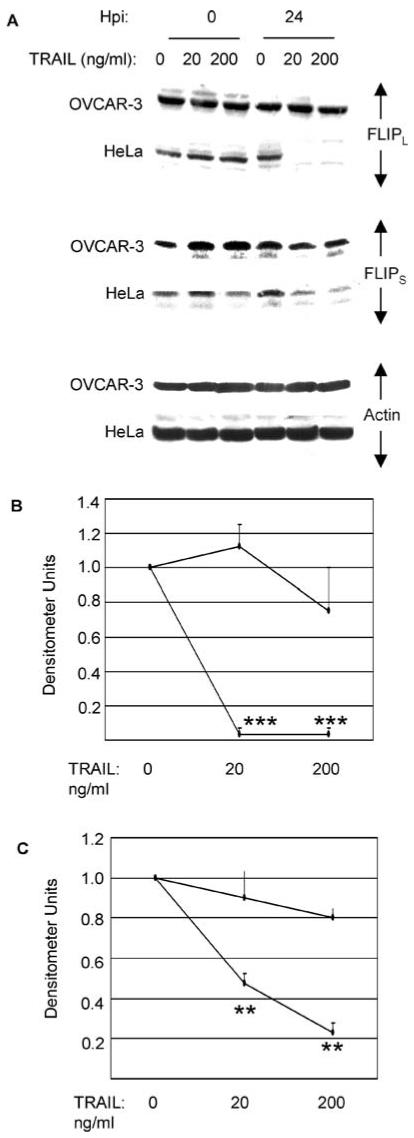
Decreased levels of cFLIP correlate with apoptosis in TRAIL treated cancer cells. (A) OVCAR3 and HeLa (TRAIL sensitive) cells were treated with 20 or 200 ng/ml TRAIL. After 0 and 24 h cell lysates were analyzed by western blotting using antibodies directed against cFLIPL and cFLIPS. Anti-actin antibodies were used to control for protein loading. Densitometric analyses of the blots for cFLIPL (B) and cFLIPS (C) are shown. At 18 or 24 h following TRAIL treatment levels of both cFLIPL (P < 0.001,***) and cFLIPS (P < 0.01,**) were significantly reduced in HeLa cells compared to levels in untreated cells. No significant decreases in cFLIP levels were observed in TRAILtreated OVCAR3 cells
Levels of cFLIP were then investigated in reovirusin-fected OVCAR3 cells. Cells were infected with reovirus (MOI 10) for 24 h before being treated with TRAIL for a further 6 or 24 h. Reovirus infection resulted in the down-regulation of both cFLIPL and cFLIPS in OVCAR3 cells in both the presence and absence of TRAIL treatment (Fig. 7). Since cFLIP is an inhibitor of caspase 8 activation its down-regulation following reovirus infection would be expected to enhance caspase 8 activation and potentiate apoptosis in TRAIL-treated cells.
Fig. 7.
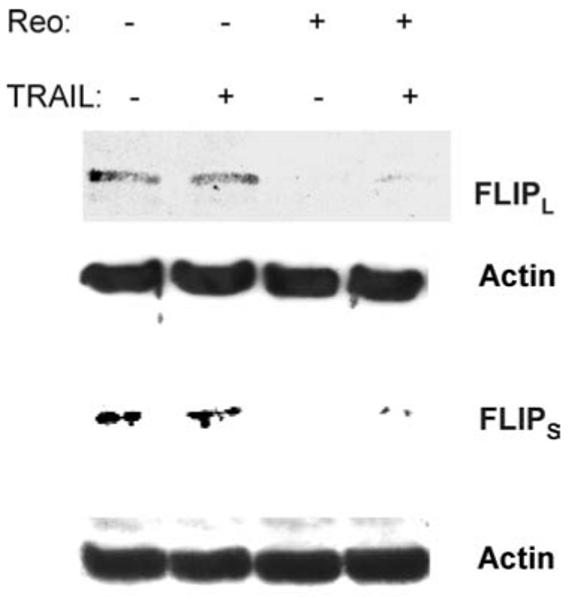
Reovirus down-regulates cFLIP in infected OVCAR3 cells. OVCAR3 cells were infected with reovirus (MOI 10). After 18 h cells were then treated with TRAIL (20 ng/ml). Cells were harvested after a further 24 or 6 h and were analyzed by western blotting using antibodies directed against cFLIPL or cFLIPS, respectively. Anti-actin antibodies were used to control for protein loading
Inhibition of cFLIP expression increases the susceptibility of OVCAR3 cells to TRAIL-induced apoptosis
Having shown that reovirus down-regulates cFLIP in OVCAR3 cells we wanted to determine whether down-regulation of cFLIP expression using antisense (AS) oligonucleotides or by inhibition of PI3 kinase also increased the susceptibility of OVCAR3 cells to TRAIL-induced apoptosis. Cells were incubated with AS oligonucleotides for 24 h prior to TRAIL treatment and were assayed for apoptosis after a further 18 h. Figure 8(A) shows that the presence of 1 μM AS cFLIP significantly (P < 0.05) increased the susceptibility of OVCAR3 cells to TRAIL resulting in 50% apoptosis compared to 20% seen in cells treated with TRAIL alone. The AS cFLIP oligonucleotides down-regulated the expression of both forms of cFLIP in OVCAR3 cells, although not as efficiently as reovirus infection (not shown). In contrast an oligonuceotide containing scrambled cFLIP sequences did not increase the susceptibility of OVCAR3 cells to TRAIL-induced apoptosis (Fig. 8(A)).
Fig. 8.
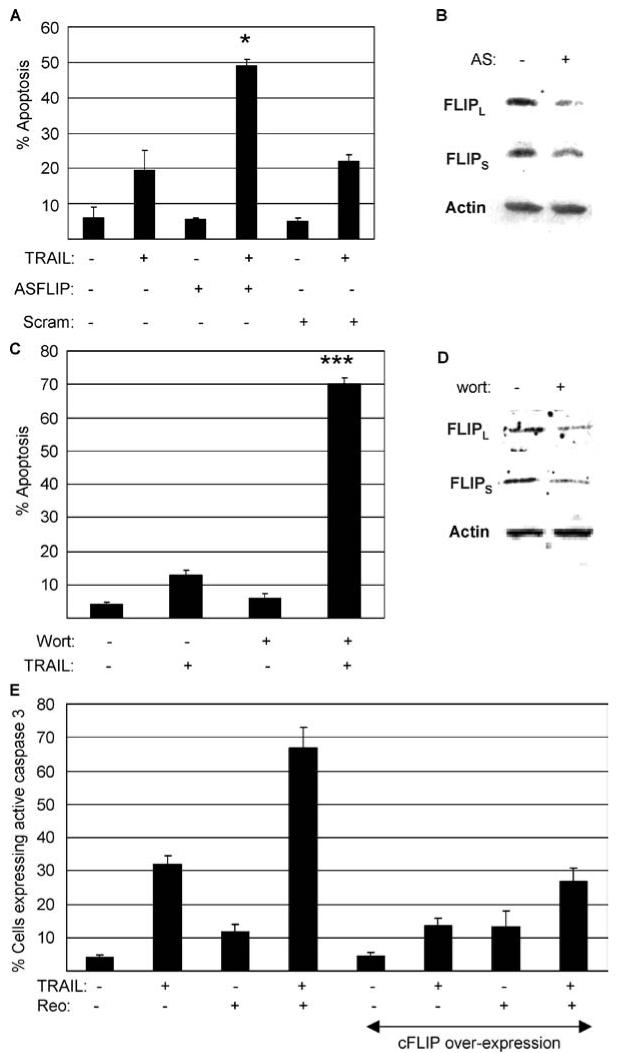
Inhibition of cFLIP expression by antisense (AS) oligonucleotides sensitizes OVCAR3 cells to TRAIL-induced apoptosis and over-expression of cFLIP blocks reovirus-induced sensitization of OVCAR3 cells to TRAIL. (A) OVCAR 3 cells were treated with AS cFLIP or scrambled cFLIP oligonucleotides (1 μM). Twenty four hours later cells were treated with TRAIL (10 ng/ml). The percentage of cells containing apoptotic nuclear morphology was determined after a further 18 h. Apoptosis in cells treated with the AS oligonucleotide and TRAIL was significantly (P < 0.05, *) greater than that seen in cells treated with TRAIL alone. (B) Twenty four hours following treatment of OVCAR3 cells with AS oligonucleotides (1 μM) levels of cFLIPL and cFLIPS were decreased as determined by western blot. Levels of actin were used to control for protein loading. (C) OVCAR 3 cells were treated with wortmannin (2 μM). One hour later cells were treated with TRAIL (20 ng/ml). The percentage of cells containing apoptotic nuclear morphology was determined after a further 18 h. Apoptosis in cells treated with wortmannin and TRAIL was significantly (P < 0.001,***) greater than that seen in cells treated with TRAIL alone. (D) Eighteen h following treatment of OVCAR3 cells with wortmannin (2 μM) levels of cFLIPL and cFLIPS were decreased as determined by western blot. Levels of actin were used to control for protein loading. (E) Wild-type OVCAR3 cells or OVCAR3 cells over-expressing cFLIP nwere infected with reovirus (MOI 20). Eighteen h following infection cells were treated with TRAIL (20 ng/ml) and were harvested after a further 5 h for immunocytochemistry using an antibody directed against active caspase 3. The graphs in A and C show the mean apoptosis from three independent experiments. The graph in E shows the mean number of cells expressing active caspase 3 from three independent fields. Error bars represent standard errors of the mean
Inhibition of PI3 kinase has been shown to down-regulate cFLIP expression in some cancer cells [51, 57, 58]. After finding that inhibition of PI3 kinase using wortmannin decreased cFLIP expression in OVCAR3 cells (not shown) we investigated whether inhibition of PI3 kinase could also enhance TRAIL-induced apoptosis in OVCAR3 cells. OVCAR3 cells were treated with wortmannin (2 μM) for 1 h prior to TRAIL treatment and were assayed for apoptosis after a further 24 h. Figure 8(B) shows that wortmannin significantly (P < 0.001) increased the susceptibility of OVCAR3 cells to TRAIL resulting in 70 % apoptosis, compared to 13% seen in cells treated with TRAIL alone.
These results support our hypothesis that the downregulation of cFLIP increases the susceptibility of OVCAR3 to TRAIL-induced apoptosis in reovirus-infected cells.
Over-expression of cFLIP blocks reovirus-induced sensitization of OVCAR3 cells to TRAIL-induced apoptosis
To test our hypothesis that the down-regulation of cFLIP increases the susceptibility of OVCAR3 to TRAIL-induced apoptosis in reovirus-infected cells we next showed that this increase was blocked in the presence of over-expression of cFLIP. OVCAR3 cells, or OVCAR3 cells that had been transfected with a plasmid designed to over-express cFLIP, were infected with reovirus (MOI 10). Eighteen h following infection cells were treated with TRAIL (20 ng/ml) and were harvested after a further 5 h for immunocytochemistry using an antibody directed against active caspase 3 (Fig. 8(E)). Overexpression of cFLIP was found to significantly inhibit apoptosis in OVCAR3 cells treated with either TRAIL (P < 0.05) alone or TRAIL and reovirus together (P < 0.001).
Discussion
Like other human cancer cell lines, human ovarian cancer cells show different sensitivities to TRAIL-induced apoptosis which limits the clinical application of this reagent for the treatment of ovarian cancer. In this paper we show that reovirus infection synergistically increases the susceptibility of human ovarian cancer cell lines OVCAR3, SKOV-3 and PA-1 to TRAIL-induced apoptosis. Enhanced apoptosis in reovirus-infected, TRAIL treated OVCAR3 cells, compared to cell treated with TRAIL alone, was associated with increased activation of caspase 3. Both enhanced apoptosis and caspase 3 activation were dependent on caspase 8 activity and were blocked by a cell permeable peptide inhibitor of caspase 8 activity (z-IETD-fmk). Caspase 8 activation was also enhanced in reovirus-infected, TRAIL-treated OVCAR3 cells compared to cells treated with TRAIL alone. We further showed that reovirus infection decreased the levels of both cFLIP l and cFLIPS in OVCAR3 cells. In contrast to enhanced caspase 8 and caspase 3 activation decreased levels of cFLIP did not require TRAIL treatment and occurred in the presence of reovirus infection alone. This suggests that the increased susceptibility of OVCAR3 cells to TRAIL-induced activation of caspase 8, caspase 3 and apoptosis results from decreased levels of cFLIP in reovirus-infected OVCAR3 cells. This model of enhanced TRAIL-induced apoptosis following reovirus infection is supported by our demonstrations that; (1) over-expression of cFLIP blocks the ability of reovirus to sensitize OVCAR3 cells to TRAIL-induced apoptosis and (2) decreased cFLIP expression, following treatment with AS cFLIP oligonucleotides or inhibition of PI3 kinase activity, also enhances TRAIL susceptibility in OVCAR3 cells. It should be noted however that the PI3K inhibitor wortmannin is likely to induce additional effects in OVCAR3 cells that may influence the sensitivity of OVCAR3 cells to TRAIL. This may explain why wortmannin was better than AS cFLIP at sensitizing OVCAR3 cells to TRAIL-induced apoptosis even though their effects on cFLIP down-regulation were similar.
Our previous studies have demonstrated that reovirus does not alter the cell surface expression of TRAIL receptors in breast cancer cells [54]. Similarly we did not detect any changes in the expression of TRAIL-R1 or TRAIL-R2 receptor expression in reovirus-infected OVCAR3 cells (not shown). It is still possible, however that reovirus down-regulates the expression of TRAIL decoy receptors in OVCAR3 cells.
An increasing number of reports have indicated a role for cFLIP expression in determining TRAIL sensitivity in a variety of human cancer cells [29, 51, 59-61], including ovarian cancer cell lines [29, 34, 35] and epithelial ovarian cancer tissues [36]. Decreased cFLIP expression following RNA interference has also been shown to significantly enhance cell death in TRAIL-treated cancer cells [30, 62], including ovarian cancer cells [33]. Decreased cFLIP expression following treatment with other agents, including the chemotherapeutic agents actinomycin D, doxorubicin and CDDP [63], histone deacetylase inhibitors [64], and trichostatin A [65] may also contribute to increased TRAIL sensitivity, although in many cases these agents also induce pro-apoptotic changes in the expression of other molecules associated with apoptosis. Decreased cFLIP expression following reovirus infection in combination with TRAIL thus represents a new treatment strategy which has potential for the treatment of many human cancers.
The mechanism by which reovirus down-reglates cFLIP expression in OVCAR3 cells is not known. Previous studies have shown that cFLIP expression is influenced by the PI3 kinase [51, 57, 58] and NF-κB [51, 57, 66, 67] signaling pathways, neither of which were altered in OVCAR3 cells following reovirus infection (not shown). However, a recent report found that lymphoblastic Jurkat cells show increased TRAIL sensitivity following transfection with the prostate-apoptosis-response gene (par 4) [68]. Enhanced apoptosis in the TRAIL-treated Jurkat cells was brought about by enforced cleavage of cFLIP together with increased activation of the initiator caspases 8 and 10. Microarray analysis indicates that par-4 expression is increased in HEK293 cells following reovirus infection [69] representing a potential mechanism for the down-regulation of cFLIP in OVCAR3 cells.
Reovirus infection is being currently being developed as a therapeutic strategy for the treatment of human cancer. Results from phase 1 clinical trails have shown that direct injection of reovirus into subcutaneous tumors has no toxic effects and phase 1 trials for intravenous inoculation and phase II trials for recurrent malignant gliomas are currently underway. Our results indicate that reovirus infection may also have potential for use in combination with TRAIL.
Acknowledgments
This work was supported by Public Health Service grants 1RO1NS051403 and 5R01NS050138 from the National Institute of Health (KLT), a VA Merit grant (KLT), the Reuler-Lewin Family Professorship of Neurology (KLT) and the Ovarian Cancer Research Fund (PC). Microscope assistance was provided by Ron Bouchard. Assistance using the Flow Cytometer was provided by the University of Colorado Cancer Center.
Contributor Information
P. Clarke, Departments of Neurology, University of Colorado Health Sciences Center, 4200 East 9th Avenue, Box B182, Denver, Colorado, 80262 USA.
K. L. Tyler, Departments of Neurology, University of Colorado Health Sciences Center, 4200 East 9th Avenue, Box B182, Denver, Colorado, 80262 USA; Departments of Medicine, Microbiology and Immunology, Denver Veterans Affairs Medical Center, Denver, Colorado, 80220 USA
References
- 1.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Partridge EE, Barnes MN. Epithelial ovarian cancer: Prevention, diagnosis, and treatment. CA Cancer J Clin. 1999;49:297–320. doi: 10.3322/canjclin.49.5.297. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 4.Hao C, Beguinot F, Condorelli G, et al. Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apotosis in human malignant glioma cells. Cancer Res. 2001;61:1162–1170. [PubMed] [Google Scholar]
- 5.Chang DW, Xing Z, Pan Y, et al. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21:3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 8.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 9.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 10.Bodmer JL, Holler N, Reynard S, et al. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol. 2000;2:241–243. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- 11.Kischkel FC, Hellbardt S, Behrmann I, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM. An induced proximity model for caspase-8 activation. J Biol Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 13.Boatright KM, Renatus M, Scott FL, et al. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 14.Degli-Esposti MA, Smolak PJ, Walczak H, et al. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997;186:1165–1170. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 16.Marsters SA, Sheridan JP, Pitti RM, et al. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol. 1997;7:1003–1006. doi: 10.1016/s0960-9822(06)00422-2. [DOI] [PubMed] [Google Scholar]
- 17.Sheridan JP, Marsters SA, Pitti RM, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 18.Emery JG, McDonnell P, Burke MB, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 19.Holen I, Croucher PI, Hamdy FC, Eaton CL. Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res. 2002;62:1619–1623. [PubMed] [Google Scholar]
- 20.Neville-Webbe HL, Cross NA, Eaton CL, et al. Osteoprotegerin (OPG) produced by bone marrow stromal cells protects breast cancer cells from TRAIL-induced apoptosis. Breast Cancer Res Treat. 2004;86:269–279. doi: 10.1023/b:brea.0000036900.48763.b3. [DOI] [PubMed] [Google Scholar]
- 21.Shipman CM, Croucher PI. Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res. 2003;63:912–916. [PubMed] [Google Scholar]
- 22.Bonavida B, Ng CP, Jazirehi A, Schiller G, Mizutani Y. Selectivity of TRAIL-mediated apoptosis of cancer cells and synergy with drugs: The trail to non-toxic cancer therapeutics (review) Int J Oncol. 1999;15:793–802. doi: 10.3892/ijo.15.4.793. [DOI] [PubMed] [Google Scholar]
- 23.Eggert A, Grotzer MA, Zuzak TJ, Wiewrodt BR, Ikegaki N, Brodeur GM. Resistance to TRAIL-induced apoptosis in neuroblastoma cells correlates with a loss of caspase-8 expression. Med Pediatr Oncol. 2000;35:603–607. doi: 10.1002/1096-911x(20001201)35:6<603::aid-mpo24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Grotzer MA, Eggert A, Zuzak TJ, et al. Resistance to TRAIL-induced apoptosis in primitive neuroectodermal brain tumor cells correlates with a loss of caspase-8 expression. Oncogene. 2000;19:4604–4610. doi: 10.1038/sj.onc.1203816. [DOI] [PubMed] [Google Scholar]
- 25.Hu WH, Johnson H, Shu HB. Activation of NF-kappaB by FADD, Casper, and caspase-8. J Biol Chem. 2000;275:10838–10844. doi: 10.1074/jbc.275.15.10838. [DOI] [PubMed] [Google Scholar]
- 26.Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002;21:2283–2294. doi: 10.1038/sj.onc.1205258. [DOI] [PubMed] [Google Scholar]
- 28.Hinz S, Trauzold A, Boenicke L, et al. Bcl-XL protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated apoptosis. Oncogene. 2000;19:5477–5486. doi: 10.1038/sj.onc.1203936. [DOI] [PubMed] [Google Scholar]
- 29.Kim K, Fisher MJ, Xu SQ, el Deiry WS. Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res. 2000;6:335–346. [PubMed] [Google Scholar]
- 30.Siegmund D, Hadwiger P, Pfizenmaier K, Vornlocher HP, Wajant H. Selective inhibition of FLICE-like inhibitory protein expression with small interfering RNA oligonucleotides is sufficient to sensitize tumor cells for TRAIL-induced apoptosis. Mol Med. 2002;8:725–732. [PMC free article] [PubMed] [Google Scholar]
- 31.Cuello M, Ettenberg SA, Nau MM, Lipkowitz S. Synergistic induction of apoptosis by the combination of trail and chemotherapy in chemoresistant ovarian cancer cells. Gynecol Oncol. 2001;81:380–390. doi: 10.1006/gyno.2001.6194. [DOI] [PubMed] [Google Scholar]
- 32.Siervo-Sassi RR, Marrangoni AM, Feng X, et al. Physiological and molecular effects of Apo2L/TRAIL and cisplatin in ovarian carcinoma cell lines. Cancer Lett. 2003;190:61–72. doi: 10.1016/s0304-3835(02)00579-7. [DOI] [PubMed] [Google Scholar]
- 33.Lane D, Cartier A, L’Esperance S, Cote M, Rancourt C, Piche A. Differential induction of apoptosis by tumor necrosis factor-related apoptosis-inducing ligand in human ovarian carcinoma cells. Gynecol Oncol. 2004;93:594–604. doi: 10.1016/j.ygyno.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Vignati S, Codegoni A, Polato F, Broggini M. Trail activity in human ovarian cancer cells: Potentiation of the action of cytotoxic drugs. Eur J Cancer. 2002;38:177–183. doi: 10.1016/s0959-8049(01)00345-8. [DOI] [PubMed] [Google Scholar]
- 35.Tomek S, Horak P, Pribill I, et al. Resistance to TRAIL-induced apoptosis in ovarian cancer cell lines is overcome by co-treatment with cytotoxic drugs. Gynecol Oncol. 2004;94:107–114. doi: 10.1016/j.ygyno.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Horak P, Pils D, Kaider A, et al. Perturbation of the tumor necrosis factor-related apoptosis-inducing ligand cascade in ovarian cancer: Overexpression of FLIPL and deregulation of the functional receptors DR4 and DR5. Clin Cancer Res. 2005;11:8585–8591. doi: 10.1158/1078-0432.CCR-05-1276. [DOI] [PubMed] [Google Scholar]
- 37.Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276:20633–20640. doi: 10.1074/jbc.M101780200. [DOI] [PubMed] [Google Scholar]
- 38.Chang DW, Xing Z, Pan Y, et al. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21:3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Micheau O, Thome M, Schneider P, et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 40.Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS. Activation of caspases-8 and -10 by FLIP(L) Biochem J. 2004;382:651–657. doi: 10.1042/BJ20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strong JE, Lee PW. The v-erbB oncogene confers enhanced cellular susceptibility to reovirus infection. J Virol. 1996;70:612–616. doi: 10.1128/jvi.70.1.612-616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: Usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 44.Hirasawa K, Nishikawa SG, Norman KL, Alain T, Kossakowska A, Lee PW. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002;62:1696–1701. [PubMed] [Google Scholar]
- 45.Alain T, Hirasawa K, Pon KJ, et al. Reovirus therapy of lymphoid malignancies. Blood. 2002;100:4146–4153. doi: 10.1182/blood-2002-02-0503. [DOI] [PubMed] [Google Scholar]
- 46.Norman KL, Coffey MC, Hirasawa K, et al. Reovirus oncolysis of human breast cancer. Hum Gene Ther. 2002;13:641–652. doi: 10.1089/10430340252837233. [DOI] [PubMed] [Google Scholar]
- 47.Wilcox ME, Yang W, Senger D, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93:903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- 48.Ikeda Y, Nishimura G, Yanoma S, Kubota A, Furukawa M, Tsukuda M. Reovirus oncolysis in human head and neck squamous carcinoma cells. Auris Nasus Larynx. 2004;31:407–412. doi: 10.1016/j.anl.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Tyler KL, Squier MK, Brown AL, et al. Linkage between reovirus-induced apoptosis and inhibition of cellular DNA synthesis: Role of the S1 and M2 genes. J Virol. 1996;70:7984–7991. doi: 10.1128/jvi.70.11.7984-7991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connolly JL, Dermody TS. Virion disassembly is required for apoptosis induced by reovirus. J Virol. 2002;76:1632–1641. doi: 10.1128/JVI.76.4.1632-1641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okano H, Shiraki K, Inoue H, et al. Cellular FLICE/caspase-8-inhibitory protein as a principal regulator of cell death and survival in human hepatocellular carcinoma. Lab Invest. 2003;83:1033–1043. doi: 10.1097/01.lab.0000079328.76631.28. [DOI] [PubMed] [Google Scholar]
- 52.Perlman H, Pagliari LJ, Georganas C, Mano T, Walsh K, Pope RM. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J Exp Med. 1999;190:1679–1688. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyler KL, Squier MK, Rodgers SE, et al. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein sigma 1. J Virol. 1995;69:6972–6979. doi: 10.1128/jvi.69.11.6972-6979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clarke P, Meintzer SM, Spalding AC, Johnson GL, Tyler KL. Caspase 8-dependent sensitization of cancer cells to TRAIL-induced apoptosis following reovirus-infection. Oncogene. 2001;20:6910–6919. doi: 10.1038/sj.onc.1204842. [DOI] [PubMed] [Google Scholar]
- 55.Slinker BK. The statistics of synergism. J Mol Cell Cardiol. 1998;30:723–731. doi: 10.1006/jmcc.1998.0655. [DOI] [PubMed] [Google Scholar]
- 56.Sturzenbecker LJ, Nibert M, Furlong D, Fields BN. Intra-cellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J Virol. 1987;61:2351–2361. doi: 10.1128/jvi.61.8.2351-2361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bortul R, Tazzari PL, Cappellini A, et al. Constitutively active Akt1 protects HL60 leukemia cells from TRAIL-induced apoptosis through a mechanism involving NF-kappaB activation and cFLIP(L) up-regulation. Leukemia. 2003;17:379–389. doi: 10.1038/sj.leu.2402793. [DOI] [PubMed] [Google Scholar]
- 58.Asakuma J, Sumitomo M, Asano T, Asano T, Hayakawa M. Selective Akt inactivation and tumor necrosis actorrelated apoptosis-inducing ligand sensitization of renal cancer cells by low concentrations of paclitaxel. Cancer Res. 2003;63:1365–1370. [PubMed] [Google Scholar]
- 59.Ryu BK, Lee MG, Chi SG, Kim YW, Park JH. Increased expression of cFLIP(L) in colonic adenocarcinoma. J Pathol. 2001;194:15–19. doi: 10.1002/path.835. [DOI] [PubMed] [Google Scholar]
- 60.Jonsson G, Paulie S, Grandien A. High level of cFLIP correlates with resistance to death receptor-induced apoptosis in bladder carcinoma cells. Anticancer Res. 2003;23:1213–1218. [PubMed] [Google Scholar]
- 61.Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- 62.Brooks AD, Sayers TJ. Reduction of the antiapoptotic protein cFLIP enhances the susceptibility of human renal cancer cells to TRAIL apoptosis. Cancer Immunol Immunother. 2005;54:499–505. doi: 10.1007/s00262-004-0595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Valen F, Fulda S, Schafer KL, et al. Selective and non-selective toxicity of TRAIL/Apo2L combined with chemotherapy in human bone tumour cells vs. normal human cells. Int J Cancer. 2003;107:929–940. doi: 10.1002/ijc.11503. [DOI] [PubMed] [Google Scholar]
- 64.Shankar S, Singh TR, Fandy TE, Luetrakul T, Ross DD, Srivastava RK. Interactive effects of histone deacetylase inhibitors and TRAIL on apoptosis in human leukemia cells: Involvement of both death receptor and mitochondrial pathways. Int J Mol Med. 2005;16:1125–1138. [PubMed] [Google Scholar]
- 65.El Zawahry A, Lu P, White SJ, Voelkel-Johnson C. in vitro efficacy of AdTRAIL gene therapy of bladder cancer is enhanced by trichostatin A-mediated restoration of CAR expression and downregulation of cFLIP and Bcl-XL. Cancer Gene Ther. 2006;13:281–289. doi: 10.1038/sj.cgt.7700905. [DOI] [PubMed] [Google Scholar]
- 66.Shishodia S, Aggarwal BB. Guggulsterone inhibits NF-kappaB and IkappaBalpha kinase activation, suppresses expression of anti-apoptotic gene products, and enhances apoptosis. J Biol Chem. 2004;279:47148–47158. doi: 10.1074/jbc.M408093200. [DOI] [PubMed] [Google Scholar]
- 67.Piva R, Gianferretti P, Ciucci A, Taulli R, Belardo G, Santoro MG. 15-Deoxy-delta 12,14-prostaglandin J2 induces apoptosis in human malignant B cells: An effect associated with inhibition of NF-kappa B activity and down-regulation of antiapoptotic proteins. Blood. 2005;105:1750–1758. doi: 10.1182/blood-2004-04-1360. [DOI] [PubMed] [Google Scholar]
- 68.Boehrer S, Nowak D, Puccetti E, et al. Prostate-apoptosis-response-gene-4 increases sensitivity to TRAIL-induced apoptosis. Leuk Res. 2006;30:597–605. doi: 10.1016/j.leukres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 69.DeBiasi RL, Clarke P, Meintzer S, et al. Reovirus-induced alteration in expression of apoptosis and DNA repair genes with potential roles in viral pathogenesis. J Virol. 2003;77:8934–8947. doi: 10.1128/JVI.77.16.8934-8947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]