Abstract
We have recently reported that administration of recombinant tumor necrosis factor alpha (TNF-alpha) to hepatitis B virus (HBV) transgenic mice reduces the hepatic steady-state content of HBV-specific mRNA by up to 80% in the absence of liver cell injury. In the current study, we analyzed the regulatory effects of several other inflammatory cytokines in the same transgenic model system. Hepatic HBV mRNA content was reduced by up to 90% following administration of a single noncytopathic dose (100,000 U) of interleukin 2 (IL-2). Comparable effects were produced by administration of alpha and beta interferons (IFN-alpha and IFN-beta), but only after multiple injections of at least 500,000 U per mouse. Importantly, the regulatory effect of IL-2 was completely blocked by the prior administration of antibodies to tumor necrosis factor alpha (TNF-alpha), which did not block the effect of IFN-alpha or IFN-beta. In contrast to these observations, recombinant IFN-gamma, IL-1, IL-3, IL-6, TNF-beta, transforming growth factor beta, and granulocyte-monocyte colony-stimulating factor were inactive in this system. These results suggest that selected inflammatory cytokines can down-regulate HBV gene expression in vivo by at least two pathways, one that is dependent on TNF-alpha and another that is not. These results imply that antigen-nonspecific products of the intrahepatic HBV-specific inflammatory response may contribute to viral clearance or persistence during HBV infection.
Full text
PDF

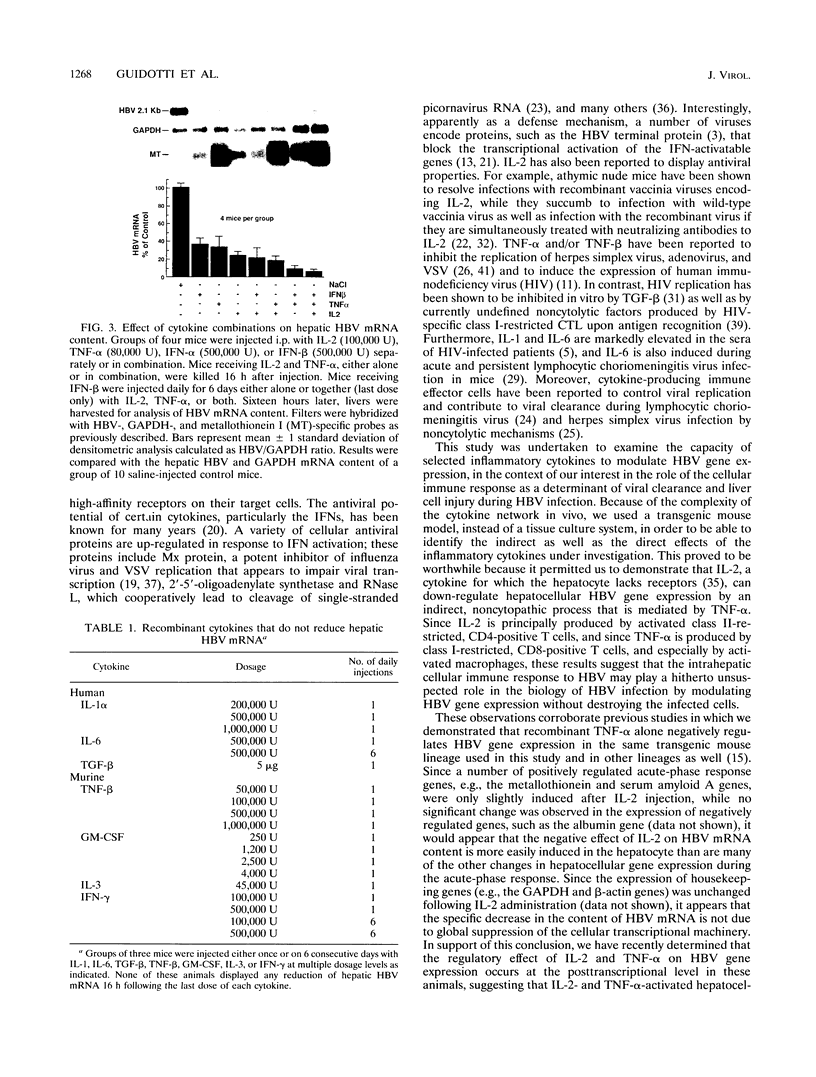


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando K., Moriyama T., Guidotti L. G., Wirth S., Schreiber R. D., Schlicht H. J., Huang S. N., Chisari F. V. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med. 1993 Nov 1;178(5):1541–1554. doi: 10.1084/jem.178.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartenschlager R., Schaller H. The amino-terminal domain of the hepadnaviral P-gene encodes the terminal protein (genome-linked protein) believed to prime reverse transcription. EMBO J. 1988 Dec 20;7(13):4185–4192. doi: 10.1002/j.1460-2075.1988.tb03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A., Ferrari C., Fiaccadori F., Penna A., Margolskee R., Schlicht H. J., Fowler P., Guilhot S., Chisari F. V. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10445–10449. doi: 10.1073/pnas.88.23.10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen E. C., Rezai A. R., Nakajima K., Beall G. N., Mitsuyasu R. T., Hirano T., Kishimoto T., Martinez-Maza O. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990 Jan 15;144(2):480–484. [PubMed] [Google Scholar]
- Charnay P., Pourcel C., Louise A., Fritsch A., Tiollais P. Cloning in Escherichia coli and physical structure of hepatitis B virion DNA. Proc Natl Acad Sci U S A. 1979 May;76(5):2222–2226. doi: 10.1073/pnas.76.5.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., West M. A., Bentz B. G., Simmons R. L. Effect of interleukin 2 on Kupffer cell activation. Interleukin 2 primes and activates Kupffer cells to suppress hepatocyte protein synthesis in vitro. Arch Surg. 1988 Nov;123(11):1373–1378. doi: 10.1001/archsurg.1988.01400350087013. [DOI] [PubMed] [Google Scholar]
- Economou J. S., McBride W. H., Essner R., Rhoades K., Golub S., Holmes E. C., Morton D. L. Tumour necrosis factor production by IL-2-activated macrophages in vitro and in vivo. Immunology. 1989 Aug;67(4):514–519. [PMC free article] [PubMed] [Google Scholar]
- Ferluga J., Allison A. C. Role of mononuclear infiltrating cells in pathogenesis of hepatitis. Lancet. 1978 Sep 16;2(8090):610–611. doi: 10.1016/s0140-6736(78)92828-3. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster G. R., Ackrill A. M., Goldin R. D., Kerr I. M., Thomas H. C., Stark G. R. Expression of the terminal protein region of hepatitis B virus inhibits cellular responses to interferons alpha and gamma and double-stranded RNA. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2888–2892. doi: 10.1073/pnas.88.7.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles P. N., Fey G., Chisari F. V. Tumor necrosis factor alpha negatively regulates hepatitis B virus gene expression in transgenic mice. J Virol. 1992 Jun;66(6):3955–3960. doi: 10.1128/jvi.66.6.3955-3960.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles P. N., Guerrette D. L., Ulevitch R. J., Schreiber R. D., Chisari F. V. HBsAg retention sensitizes the hepatocyte to injury by physiological concentrations of interferon-gamma. Hepatology. 1992 Sep;16(3):655–663. doi: 10.1002/hep.1840160308. [DOI] [PubMed] [Google Scholar]
- Gresser I., Woodrow D., Moss J., Maury C., Tavernier J., Fiers W. Toxic effects of recombinant tumor necrosis factor in suckling mice. Comparisons with interferon alpha/beta. Am J Pathol. 1987 Jul;128(1):13–18. [PMC free article] [PubMed] [Google Scholar]
- Guilhot S., Guidotti L. G., Chisari F. V. Interleukin-2 downregulates hepatitis B virus gene expression in transgenic mice by a posttranscriptional mechanism. J Virol. 1993 Dec;67(12):7444–7449. doi: 10.1128/jvi.67.12.7444-7449.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M. A., Staeheli P., Haller O. Interferon induces a unique protein in mouse cells bearing a gene for resistance to influenza virus. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1910–1914. doi: 10.1073/pnas.80.7.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalvakolanu D. V., Bandyopadhyay S. K., Harter M. L., Sen G. C. Inhibition of interferon-inducible gene expression by adenovirus E1A proteins: block in transcriptional complex formation. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7459–7463. doi: 10.1073/pnas.88.17.7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupiah G., Blanden R. V., Ramshaw I. A. Interferon gamma is involved in the recovery of athymic nude mice from recombinant vaccinia virus/interleukin 2 infection. J Exp Med. 1990 Nov 1;172(5):1495–1503. doi: 10.1084/jem.172.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Choubey D., Lengyel P., Sen G. C. Studies on the role of the 2'-5'-oligoadenylate synthetase-RNase L pathway in beta interferon-mediated inhibition of encephalomyocarditis virus replication. J Virol. 1988 Sep;62(9):3175–3181. doi: 10.1128/jvi.62.9.3175-3181.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Grube F., Assmann U., Löliger C., Moskophidis D., Löhler J. Mechanism of recovery from acute virus infection. I. Role of T lymphocytes in the clearance of lymphocytic choriomeningitis virus from spleens of mice. J Immunol. 1985 Jan;134(1):608–615. [PubMed] [Google Scholar]
- Martz E., Gamble S. R. How do CTL control virus infections? Evidence for prelytic halt of herpes simplex. Viral Immunol. 1992 Spring;5(1):81–91. doi: 10.1089/vim.1992.5.81. [DOI] [PubMed] [Google Scholar]
- Mestan J., Digel W., Mittnacht S., Hillen H., Blohm D., Möller A., Jacobsen H., Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. 1986 Oct 30-Nov 5Nature. 323(6091):816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- Missale G., Redeker A., Person J., Fowler P., Guilhot S., Schlicht H. J., Ferrari C., Chisari F. V. HLA-A31- and HLA-Aw68-restricted cytotoxic T cell responses to a single hepatitis B virus nucleocapsid epitope during acute viral hepatitis. J Exp Med. 1993 Mar 1;177(3):751–762. doi: 10.1084/jem.177.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli M., Vergani G. M., Alberti A., Vergani D., Portmann B., Eddleston A. L., Williams R. Specificity of T lymphocyte cytotoxicity to autologous hepatocytes in chronic hepatitis B virus infection: evidence that T cells are directed against HBV core antigen expressed on hepatocytes. J Immunol. 1982 Dec;129(6):2773–2778. [PubMed] [Google Scholar]
- Moskophidis D., Frei K., Löhler J., Fontana A., Zinkernagel R. M. Production of random classes of immunoglobulins in brain tissue during persistent viral infection paralleled by secretion of interleukin-6 (IL-6) but not IL-4, IL-5, and gamma interferon. J Virol. 1991 Mar;65(3):1364–1369. doi: 10.1128/jvi.65.3.1364-1369.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayersina R., Fowler P., Guilhot S., Missale G., Cerny A., Schlicht H. J., Vitiello A., Chesnut R., Person J. L., Redeker A. G. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J Immunol. 1993 May 15;150(10):4659–4671. [PubMed] [Google Scholar]
- Poli G., Kinter A. L., Justement J. S., Bressler P., Kehrl J. H., Fauci A. S. Transforming growth factor beta suppresses human immunodeficiency virus expression and replication in infected cells of the monocyte/macrophage lineage. J Exp Med. 1991 Mar 1;173(3):589–597. doi: 10.1084/jem.173.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramshaw I. A., Andrew M. E., Phillips S. M., Boyle D. B., Coupar B. E. Recovery of immunodeficient mice from a vaccinia virus/IL-2 recombinant infection. Nature. 1987 Oct 8;329(6139):545–546. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Davison B. L., Stuart G. W., Wilkie T. M., Norstedt G., Palmiter R. D. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984 Jul;4(7):1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Staeheli P. Interferon-induced proteins and the antiviral state. Adv Virus Res. 1990;38:147–200. doi: 10.1016/s0065-3527(08)60862-3. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Pavlovic J. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J Virol. 1991 Aug;65(8):4498–4501. doi: 10.1128/jvi.65.8.4498-4501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs G., Wolter M., Wendel A. Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem Pharmacol. 1989 Feb 15;38(4):627–631. doi: 10.1016/0006-2952(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Wang J. F., Wendel A. Studies on the hepatotoxicity of galactosamine/endotoxin or galactosamine/TNF in the perfused mouse liver. Biochem Pharmacol. 1990 Jan 15;39(2):267–270. doi: 10.1016/0006-2952(90)90025-g. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H., McCauley J. W., Skehel J. J., Kerr I. M. Interferon action--sequence specificity of the ppp(A2'p)nA-dependent ribonuclease. Nature. 1981 Jan 29;289(5796):414–417. doi: 10.1038/289414a0. [DOI] [PubMed] [Google Scholar]