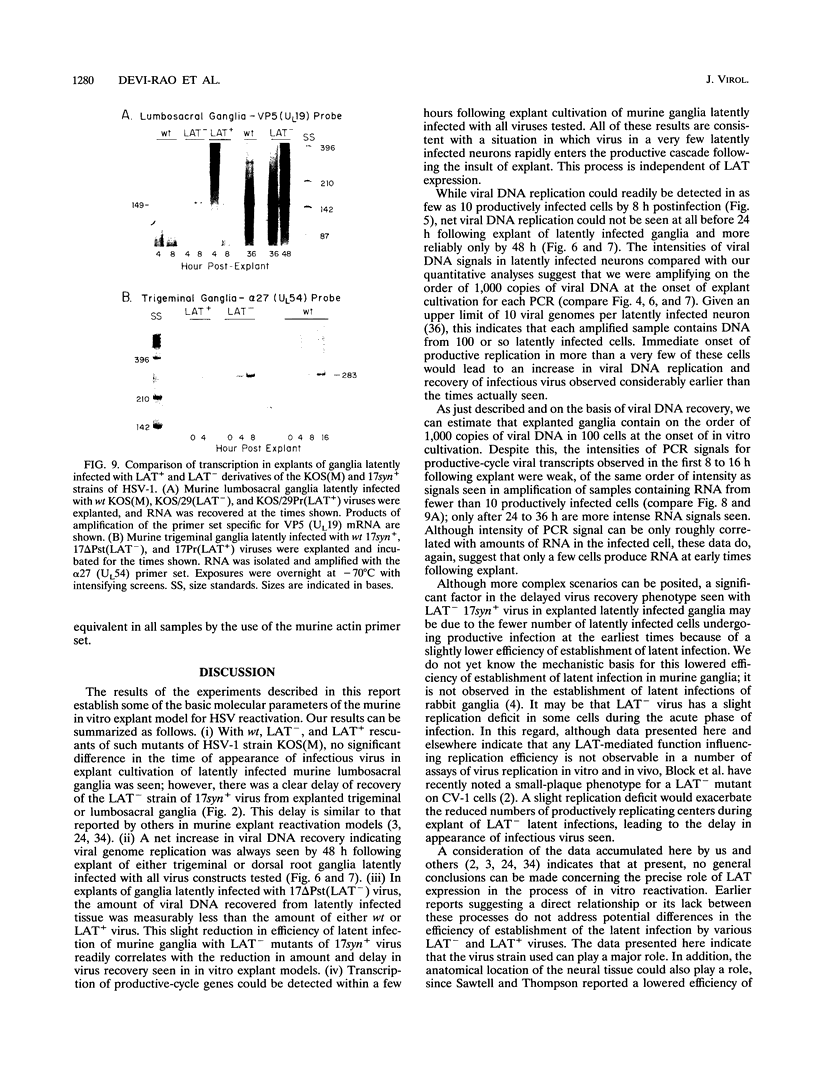
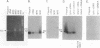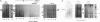Abstract
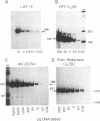
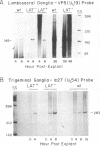
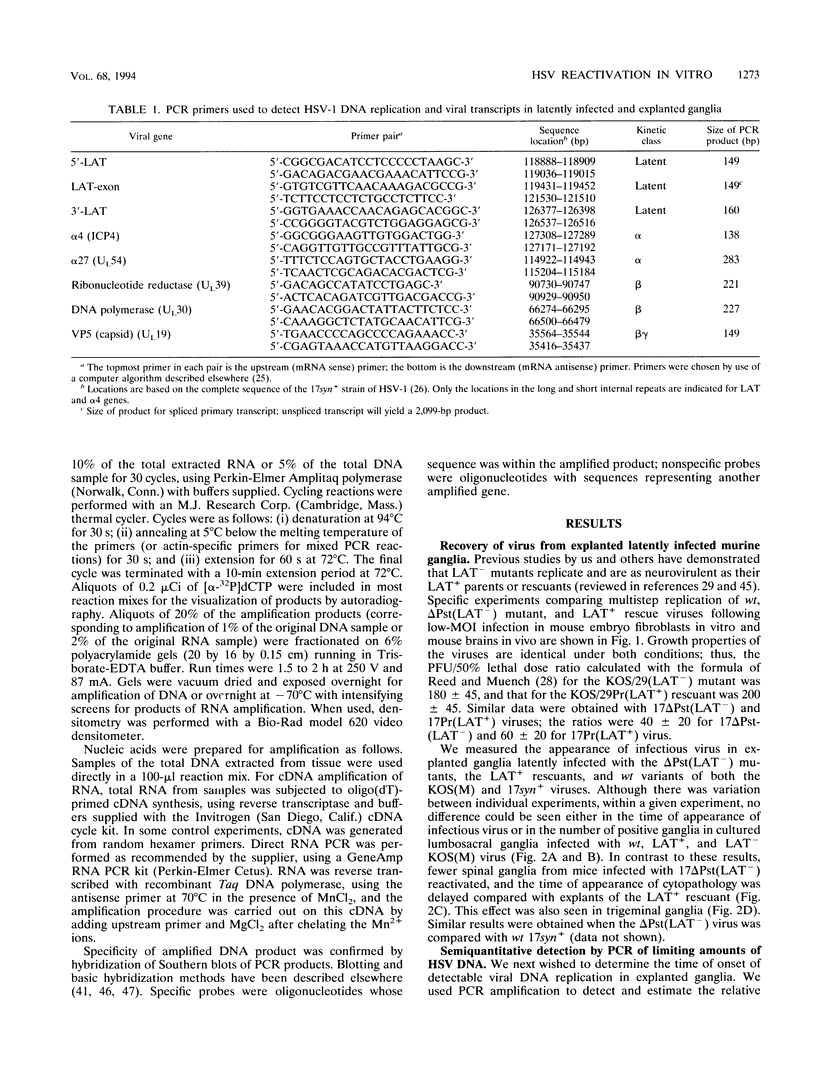
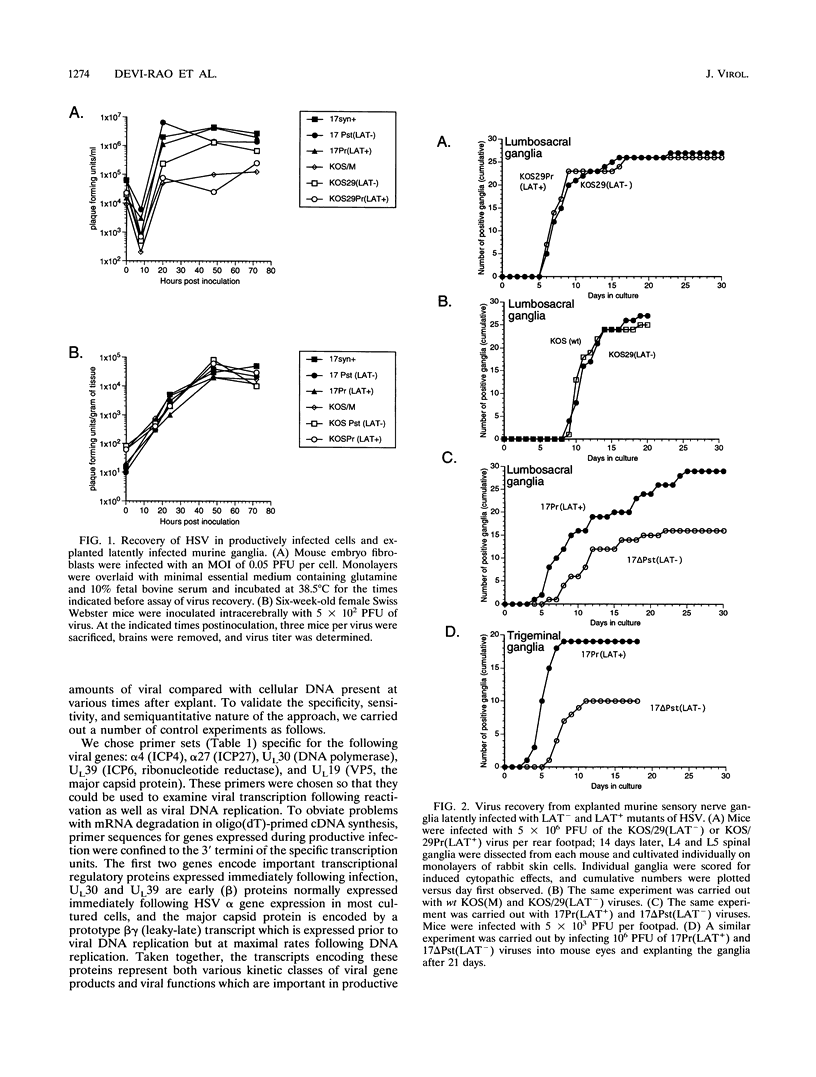
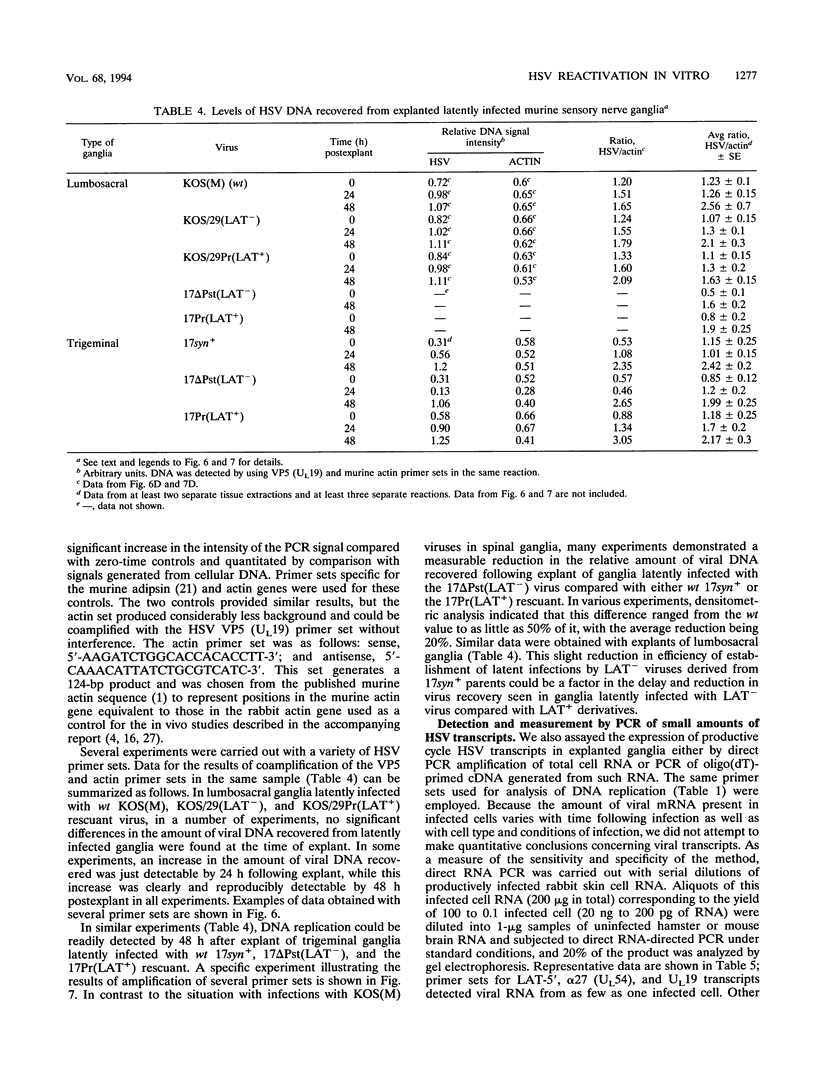
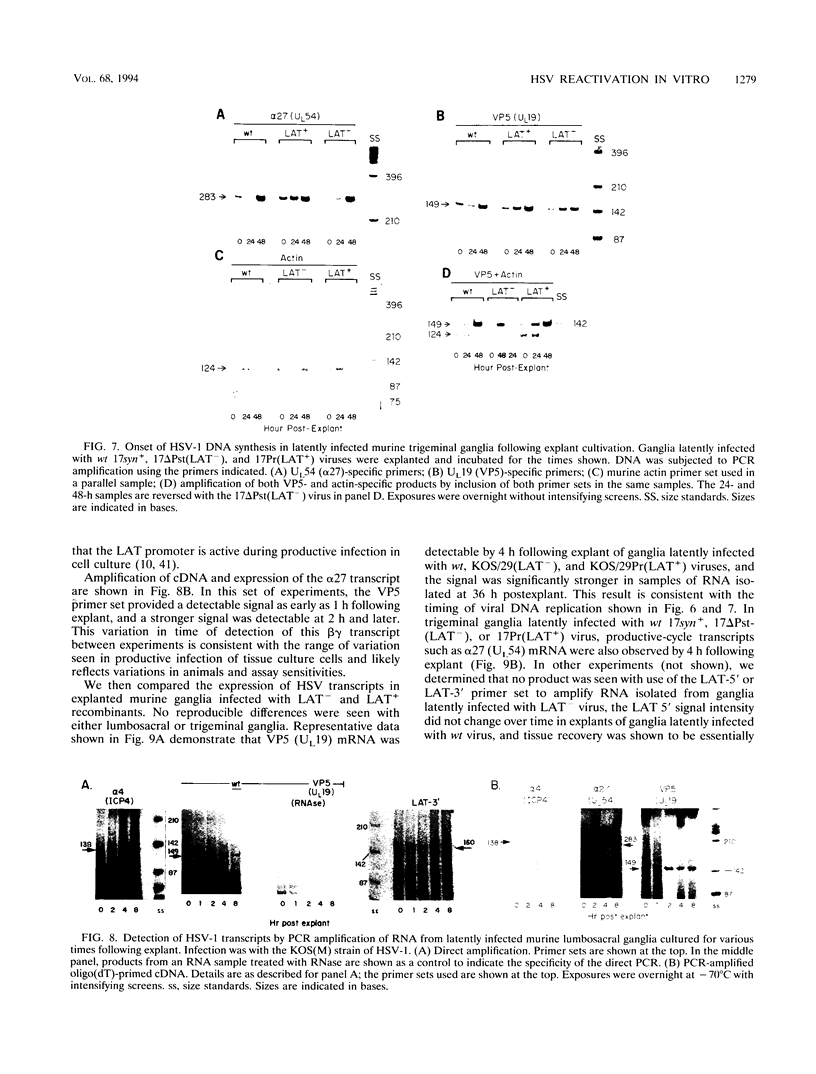
Infectious virus assays and PCR amplification of DNA and RNA were used to investigate herpes simplex virus DNA replication and gene expression in two murine in vitro models for virus reactivation. We examined latent infections with wild-type (wt), precisely defined latency-associated transcript-negative (LAT-) mutants, and LAT+ rescuants of these mutants of the 17syn+ strain of virus in both murine trigeminal and lumbosacral ganglia and of the KOS(M) strain in the latter. In explants of ganglia latently infected with the LAT- mutant of strain 17syn+ virus, a reduction in number of cultures exhibiting cytopathic effects due to virus reactivation and measurable delays in virus recovery were observed compared with wt or the LAT+ rescuant. This LAT-specific effect was not seen in explants of lumbosacral ganglia latently infected with mutants derived from the KOS(M) strain of virus. Although there was appreciable variation between individual animals, no significant difference between LAT+ and LAT- virus in time of onset of viral DNA replication in explanted ganglia was seen with use of either virus strain. There was a slight decrease in the relative amount of viral DNA recovered compared with internal cellular controls in latently infected ganglia harboring the LAT- mutant of 17syn+ compared with the wt virus or the LAT+ rescuant. This reduced relative amount ranged from 0 to as much as 50% but averaged 20%. Such differences were not seen in infections with KOS(M)-derived mutants. In contrast, although expression of productive-cycle transcripts could be detected within 4 h following explant cultivation of latently infected ganglia, no differences between LAT+ and LAT- viruses could be seen. As discussed, these data place specific constraints on possible models for the role of LAT expression in in vitro reactivation systems.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Block T. M., Deshmane S., Masonis J., Maggioncalda J., Valyi-Nagi T., Fraser N. W. An HSV LAT null mutant reactivates slowly from latent infection and makes small plaques on CV-1 monolayers. Virology. 1993 Feb;192(2):618–630. doi: 10.1006/viro.1993.1078. [DOI] [PubMed] [Google Scholar]
- Block T. M., Spivack J. G., Steiner I., Deshmane S., McIntosh M. T., Lirette R. P., Fraser N. W. A herpes simplex virus type 1 latency-associated transcript mutant reactivates with normal kinetics from latent infection. J Virol. 1990 Jul;64(7):3417–3426. doi: 10.1128/jvi.64.7.3417-3426.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom D. C., Devi-Rao G. B., Hill J. M., Stevens J. G., Wagner E. K. Molecular analysis of herpes simplex virus type 1 during epinephrine-induced reactivation of latently infected rabbits in vivo. J Virol. 1994 Mar;68(3):1283–1292. doi: 10.1128/jvi.68.3.1283-1292.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohenzky R. A., Papavassiliou A. G., Gelman I. H., Silverstein S. Identification of a promoter mapping within the reiterated sequences that flank the herpes simplex virus type 1 UL region. J Virol. 1993 Feb;67(2):632–642. doi: 10.1128/jvi.67.2.632-642.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claoué C. M., Hodges T. J., Darville J. M., Hill T. J., Blyth W. A., Easty D. L. Possible latent infection with herpes simplex virus in the mouse eye. J Gen Virol. 1990 Oct;71(Pt 10):2385–2390. doi: 10.1099/0022-1317-71-10-2385. [DOI] [PubMed] [Google Scholar]
- Cook M. L., Bastone V. B., Stevens J. G. Evidence that neurons harbor latent herpes simplex virus. Infect Immun. 1974 May;9(5):946–951. doi: 10.1128/iai.9.5.946-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen K. D. Latency of the human herpesviruses. Annu Rev Med. 1991;42:61–67. doi: 10.1146/annurev.me.42.020191.000425. [DOI] [PubMed] [Google Scholar]
- Deshmane S. L., Nicosia M., Valyi-Nagy T., Feldman L. T., Dillner A., Fraser N. W. An HSV-1 mutant lacking the LAT TATA element reactivates normally in explant cocultivation. Virology. 1993 Oct;196(2):868–872. doi: 10.1006/viro.1993.1548. [DOI] [PubMed] [Google Scholar]
- Devi-Rao G. B., Goodart S. A., Hecht L. M., Rochford R., Rice M. K., Wagner E. K. Relationship between polyadenylated and nonpolyadenylated herpes simplex virus type 1 latency-associated transcripts. J Virol. 1991 May;65(5):2179–2190. doi: 10.1128/jvi.65.5.2179-2190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A. T., Sederati F., Devi-Rao G., Flanagan W. M., Farrell M. J., Stevens J. G., Wagner E. K., Feldman L. T. Identification of the latency-associated transcript promoter by expression of rabbit beta-globin mRNA in mouse sensory nerve ganglia latently infected with a recombinant herpes simplex virus. J Virol. 1989 Sep;63(9):3844–3851. doi: 10.1128/jvi.63.9.3844-3851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M. J., Dobson A. T., Feldman L. T. Herpes simplex virus latency-associated transcript is a stable intron. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):790–794. doi: 10.1073/pnas.88.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M. J., Hill J. M., Margolis T. P., Stevens J. G., Wagner E. K., Feldman L. T. The herpes simplex virus type 1 reactivation function lies outside the latency-associated transcript open reading frame ORF-2. J Virol. 1993 Jun;67(6):3653–3655. doi: 10.1128/jvi.67.6.3653-3655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Y. J., Romanowski E., Harwick J., Berman J., Olsakovsky L., Araullo-Cruz T. The effect of alpha blockade on iontophoresis-induced ocular shedding of latent HSV-1 W in different host animals. Curr Eye Res. 1990 Oct;9(10):1007–1014. doi: 10.3109/02713689009069937. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Dudley J. B., Shimomura Y., Kaufman H. E. Quantitation and kinetics of induced HSV-1 ocular shedding. Curr Eye Res. 1986 Mar;5(3):241–246. doi: 10.3109/02713688609020049. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Haruta Y., Rootman D. S. Adrenergically induced recurrent HSV-1 corneal epithelial lesions. Curr Eye Res. 1987 Aug;6(8):1065–1071. doi: 10.3109/02713688709034878. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Sedarati F., Javier R. T., Wagner E. K., Stevens J. G. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990 Jan;174(1):117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Katz J. P., Bodin E. T., Coen D. M. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J Virol. 1990 Sep;64(9):4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Imamura J., Nagai H., Shimotohno K. Quantification of gene expression over a wide range by the polymerase chain reaction. Anal Biochem. 1992 Nov 1;206(2):231–235. doi: 10.1016/0003-2697(92)90358-e. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Gangarosa L. P., Sr, Green K., Hill J. M. Kinetics of ocular herpes simplex virus shedding induced by epinephrine iontophoresis. Invest Ophthalmol Vis Sci. 1982 Jun;22(6):818–821. [PubMed] [Google Scholar]
- Leib D. A., Bogard C. L., Kosz-Vnenchak M., Hicks K. A., Coen D. M., Knipe D. M., Schaffer P. A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989 Jul;63(7):2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe T., Sharefkin J., Yang S. Q., Dieffenbach C. W. A computer program for selection of oligonucleotide primers for polymerase chain reactions. Nucleic Acids Res. 1990 Apr 11;18(7):1757–1761. doi: 10.1093/nar/18.7.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J. The genome of herpes simplex virus: structure, replication and evolution. J Cell Sci Suppl. 1987;7:67–94. doi: 10.1242/jcs.1987.supplement_7.6. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Herlihy W. C., Schimmel P. A new troponin T and cDNA clones for 13 different muscle proteins, found by shotgun sequencing. Nature. 1983 Apr 21;302(5910):718–721. doi: 10.1038/302718a0. [DOI] [PubMed] [Google Scholar]
- Sawtell N. M., Thompson R. L. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol. 1992 Apr;66(4):2157–2169. doi: 10.1128/jvi.66.4.2157-2169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell N. M., Thompson R. L. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992 Apr;66(4):2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanberry L. R. Pathogenesis of herpes simplex virus infection and animal models for its study. Curr Top Microbiol Immunol. 1992;179:15–30. doi: 10.1007/978-3-642-77247-4_2. [DOI] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., Lirette R. P., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 1989 Feb;8(2):505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Stevens J. G. Human herpesviruses: a consideration of the latent state. Microbiol Rev. 1989 Sep;53(3):318–332. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Stevens J. G. Transcripts associated with herpes simplex virus latency. Adv Exp Med Biol. 1990;278:199–204. doi: 10.1007/978-1-4684-5853-4_20. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Cook M. L., Devi-Rao G. B., Wagner E. K., Stevens J. G. Functional and molecular analyses of the avirulent wild-type herpes simplex virus type 1 strain KOS. J Virol. 1986 Apr;58(1):203–211. doi: 10.1128/jvi.58.1.203-211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. L., Devi-Rao G. V., Stevens J. G., Wagner E. K. Rescue of a herpes simplex virus type 1 neurovirulence function with a cloned DNA fragment. J Virol. 1985 Aug;55(2):504–508. doi: 10.1128/jvi.55.2.504-508.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. L., Wagner E. K., Stevens J. G. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology. 1983 Nov;131(1):180–192. doi: 10.1016/0042-6822(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Steiner I., Spivack J. G., Deshmane S. L., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. In vivo and in vitro reactivation impairment of a herpes simplex virus type 1 latency-associated transcript variant in a rabbit eye model. J Virol. 1991 Dec;65(12):6989–6993. doi: 10.1128/jvi.65.12.6989-6993.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Devi-Rao G., Feldman L. T., Dobson A. T., Zhang Y. F., Flanagan W. M., Stevens J. G. Physical characterization of the herpes simplex virus latency-associated transcript in neurons. J Virol. 1988 Apr;62(4):1194–1202. doi: 10.1128/jvi.62.4.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Flanagan W. M., Devi-Rao G., Zhang Y. F., Hill J. M., Anderson K. P., Stevens J. G. The herpes simplex virus latency-associated transcript is spliced during the latent phase of infection. J Virol. 1988 Dec;62(12):4577–4585. doi: 10.1128/jvi.62.12.4577-4585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh L., Schaffer P. A. A novel class of transcripts expressed with late kinetics in the absence of ICP4 spans the junction between the long and short segments of the herpes simplex virus type 1 genome. J Virol. 1993 Dec;67(12):7373–7382. doi: 10.1128/jvi.67.12.7373-7382.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]