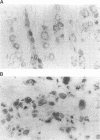
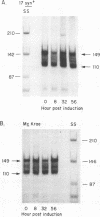
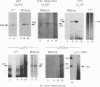
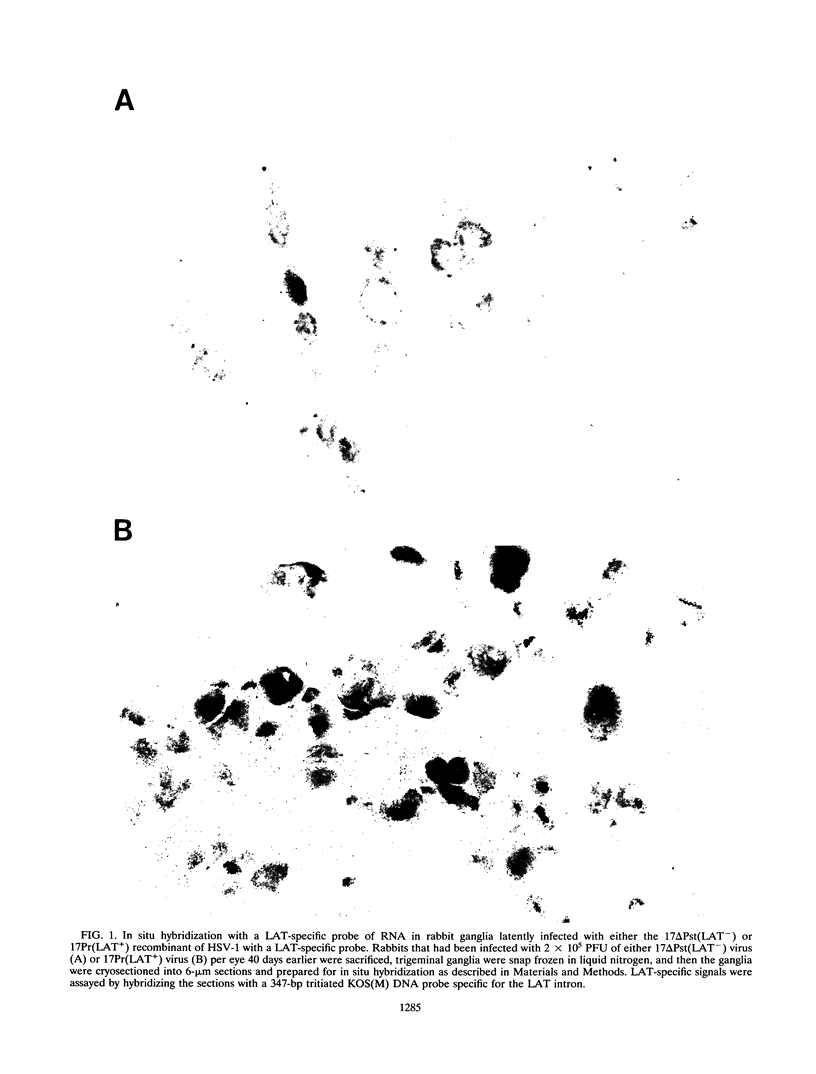
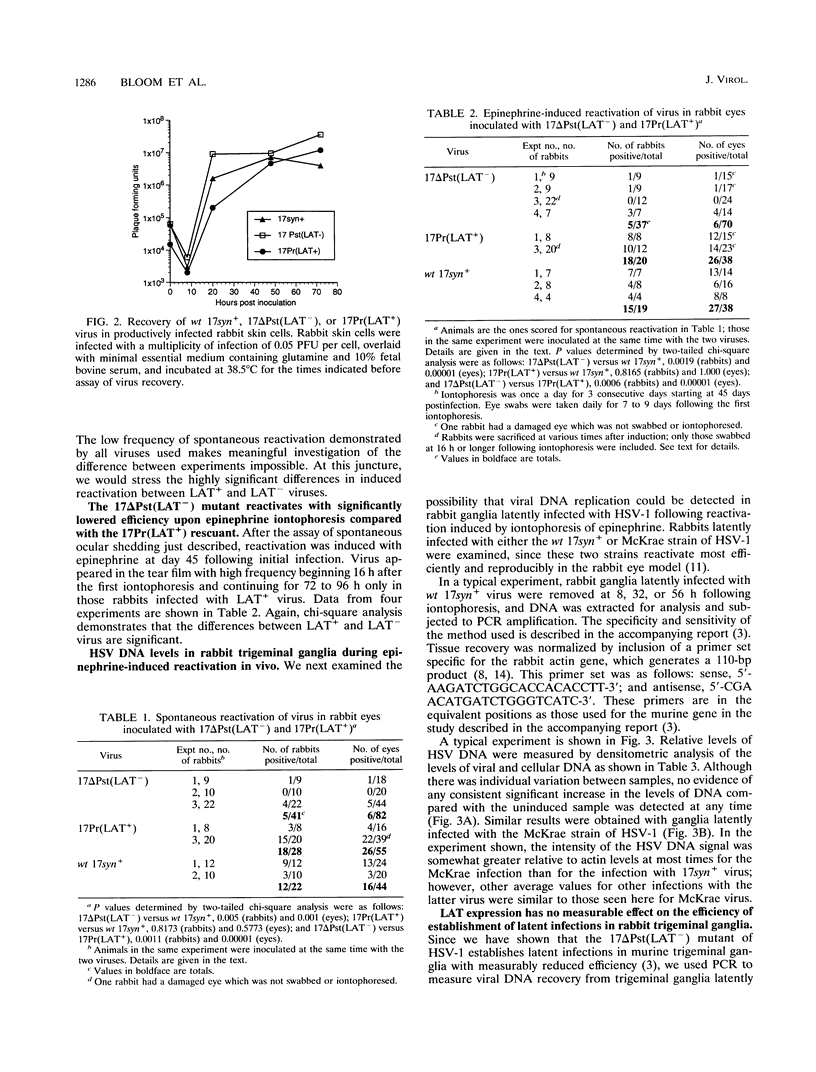
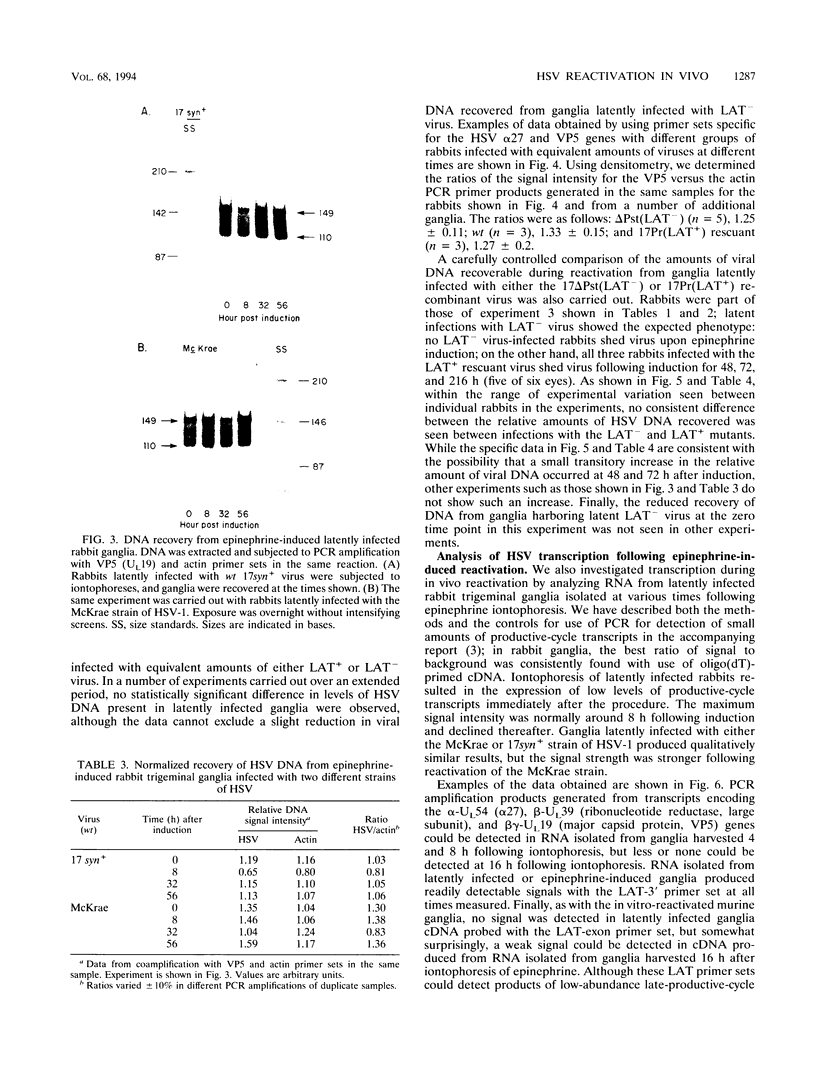
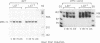Abstract
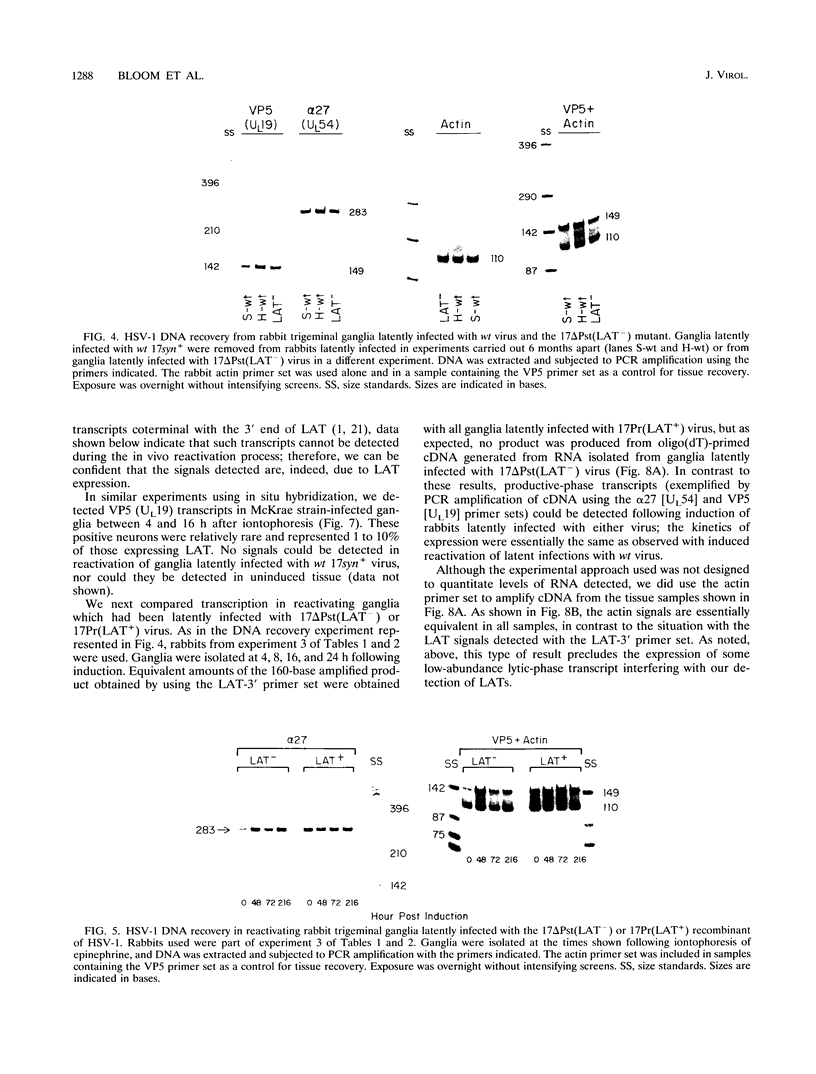
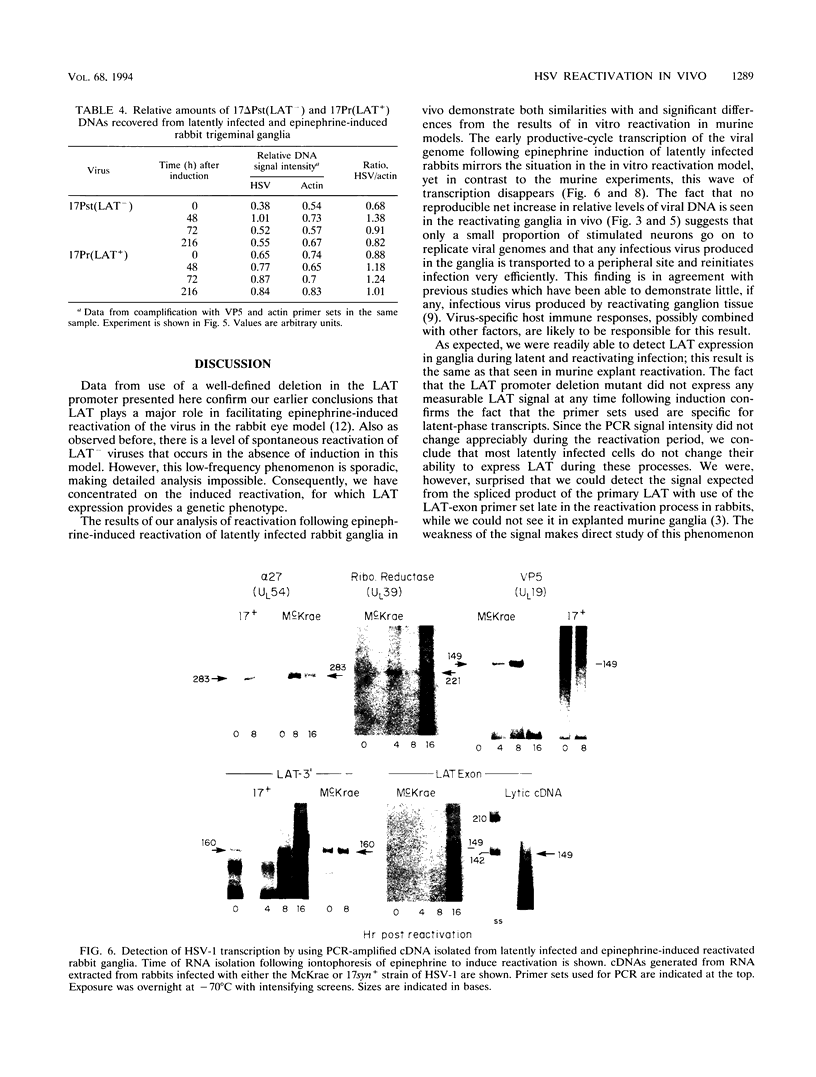
Infectious virus assays and PCR amplification of DNA and RNA were used to investigate herpes simplex virus (HSV) DNA replication and gene expression in the rabbit corneal model for virus reactivation in vivo. We used carefully defined latency-associated transcript-negative (LAT-) and LAT+ promoter mutants of the 17syn+ strain of HSV type 1. In agreement with earlier studies using a more extensive LAT- deletion mutant, the 17 delta Pst(LAT-) virus reactivated with extremely low frequency upon epinephrine induction. In contrast to our findings with murine latency models, amounts of viral DNA recovered from rabbit ganglia latently infected with either LAT+ or LAT- virus were equivalent. Also in contrast with the murine models, no net increase in viral DNA was seen in latently infected rabbit trigeminal ganglia induced to reactivate in vivo by iontophoresis of epinephrine. Despite this, transcription of lytic-phase genes could be detected within 4 h following induction of rabbits latently infected with either LAT+ or LAT- virus; this transcription diminished by 16 h following induction. These results are discussed in relation to models for the mechanism of action of HSV LAT.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohenzky R. A., Papavassiliou A. G., Gelman I. H., Silverstein S. Identification of a promoter mapping within the reiterated sequences that flank the herpes simplex virus type 1 UL region. J Virol. 1993 Feb;67(2):632–642. doi: 10.1128/jvi.67.2.632-642.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. D., Paveloff M. J., Doucet J. J., Cottingham A. J., Sedarati F., Hill J. M. Ocular herpes simplex virus reactivation in mice latently infected with latency-associated transcript mutants. Invest Ophthalmol Vis Sci. 1991 Apr;32(5):1558–1561. [PubMed] [Google Scholar]
- Devi-Rao G. B., Bloom D. C., Stevens J. G., Wagner E. K. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J Virol. 1994 Mar;68(3):1271–1282. doi: 10.1128/jvi.68.3.1271-1282.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi-Rao G. B., Goodart S. A., Hecht L. M., Rochford R., Rice M. K., Wagner E. K. Relationship between polyadenylated and nonpolyadenylated herpes simplex virus type 1 latency-associated transcripts. J Virol. 1991 May;65(5):2179–2190. doi: 10.1128/jvi.65.5.2179-2190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A. T., Sederati F., Devi-Rao G., Flanagan W. M., Farrell M. J., Stevens J. G., Wagner E. K., Feldman L. T. Identification of the latency-associated transcript promoter by expression of rabbit beta-globin mRNA in mouse sensory nerve ganglia latently infected with a recombinant herpes simplex virus. J Virol. 1989 Sep;63(9):3844–3851. doi: 10.1128/jvi.63.9.3844-3851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M. J., Hill J. M., Margolis T. P., Stevens J. G., Wagner E. K., Feldman L. T. The herpes simplex virus type 1 reactivation function lies outside the latency-associated transcript open reading frame ORF-2. J Virol. 1993 Jun;67(6):3653–3655. doi: 10.1128/jvi.67.6.3653-3655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame M. C., McGeoch D. J., Rixon F. J., Orr A. C., Marsden H. S. The 10K virion phosphoprotein encoded by gene US9 from herpes simplex virus type 1. Virology. 1986 Apr 30;150(2):321–332. doi: 10.1016/0042-6822(86)90297-7. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Dudley J. B., Shimomura Y., Kaufman H. E. Quantitation and kinetics of induced HSV-1 ocular shedding. Curr Eye Res. 1986 Mar;5(3):241–246. doi: 10.3109/02713688609020049. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Haruta Y., Rootman D. S. Adrenergically induced recurrent HSV-1 corneal epithelial lesions. Curr Eye Res. 1987 Aug;6(8):1065–1071. doi: 10.3109/02713688709034878. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Rayfield M. A., Haruta Y. Strain specificity of spontaneous and adrenergically induced HSV-1 ocular reactivation in latently infected rabbits. Curr Eye Res. 1987 Jan;6(1):91–97. doi: 10.3109/02713688709020074. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Sedarati F., Javier R. T., Wagner E. K., Stevens J. G. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990 Jan;174(1):117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Herlihy W. C., Schimmel P. A new troponin T and cDNA clones for 13 different muscle proteins, found by shotgun sequencing. Nature. 1983 Apr 21;302(5910):718–721. doi: 10.1038/302718a0. [DOI] [PubMed] [Google Scholar]
- Sedarati F., Margolis T. P., Stevens J. G. Latent infection can be established with drastically restricted transcription and replication of the HSV-1 genome. Virology. 1993 Feb;192(2):687–691. doi: 10.1006/viro.1993.1089. [DOI] [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Steiner I., Spivack J. G., Deshmane S. L., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. In vivo and in vitro reactivation impairment of a herpes simplex virus type 1 latency-associated transcript variant in a rabbit eye model. J Virol. 1991 Dec;65(12):6989–6993. doi: 10.1128/jvi.65.12.6989-6993.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Flanagan W. M., Devi-Rao G., Zhang Y. F., Hill J. M., Anderson K. P., Stevens J. G. The herpes simplex virus latency-associated transcript is spliced during the latent phase of infection. J Virol. 1988 Dec;62(12):4577–4585. doi: 10.1128/jvi.62.12.4577-4585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh L., Schaffer P. A. A novel class of transcripts expressed with late kinetics in the absence of ICP4 spans the junction between the long and short segments of the herpes simplex virus type 1 genome. J Virol. 1993 Dec;67(12):7373–7382. doi: 10.1128/jvi.67.12.7373-7382.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]