Abstract
Rats were trained on a delayed matching to position (DMTP) task that embedded either a differential outcomes procedure (DOP) or a non-differential outcomes procedure (NOP). The DOP, via Pavlovian conditioning (stimulus–outcome associations), results in the use of unique reward expectancies that facilitate learning and memory performance above subjects trained with a NOP that requires subjects to retain cue information for accurate choice behavior (stimulus–response associations). This enhancement in learning and/or memory produced by the DOP is called the differential outcomes effect (DOE). After being trained on the DMTP task, rats were implanted with two cannulae aimed at the basolateral amygdala (BLA) nuclei. Rats trained with the DOP, relative to those trained with the NOP, displayed enhanced short-term memory (STM) performance under vehicle conditions (i.e. the DOE). However, injections of the γ-aminobutyric acid (GABA)A agonist muscimol into the BLA dose-dependently (0.0625 and 0.125 μg) impaired STM performance only in DOP-trained rats. These results support the role of the BLA in the use of established reward expectancies during a short-term working memory task. Despite the fact that extracellular signal-regulated kinase/mitogen-activated protein kinases (ERK/MAPK) have been shown to be necessary for amygdala-dependent long-term potentiation and some forms of long-term and STM, inhibition of the ERK/MAPK signaling cascade by U0126 (2.0 or 4.0 μg) in the BLA was not critical for updating the STM of either spatial information or reward expectation.
Keywords: amygdala, differential outcomes, rat, reward
Introduction
Learning to anticipate a reward can occur by associating a discrete stimulus (stimulus–outcome, S–O; Pavlovian conditioning) and/or an action (response–outcome, R–O; instrumental conditioning) with an outcome. The basolateral nuclei of the amygdala (BLA) have been reported to be critical for the establishment of associations between current reward value and cues that predict changes in reward (S–O). Lesions of the BLA produce deficits in Pavlovian second-order conditioning, reinforcer devaluation, conditional discrimination involving reward expectancies and reward-specific Pavlovian-to-instrumental transfer (Hatfield et al., 1996; Blundell et al., 2001; Ramirez & Savage, 2007). Such data suggest that the BLA is critical for cue-evoked sensory/hedonic representations of outcomes, but not the more general ‘reinforcing aspects’ of outcomes (Blundell et al., 2003).
The differential outcomes procedure (DOP) contrasted with the non-differential outcomes procedure (NOP) directly allows for the assessment of how features of unique rewards can be compared with general reward during instrumental learning (Trapold, 1970; Urcuioli, 2005). In the DOP, each to-be-remembered event is consistently paired with a specific outcome, thus a subject eventually develops a specific reward expectation when cues that predict that reward are presented. This expectation has both affective and discriminative properties that enhance instrumental goal-directed behavior (Trapold & Overmier, 1972; Overmier & Linwick, 2001; Savage, 2001; Urcuioli, 2005). In the NOP condition, a single reward or multiple unpredictable rewards is/are contingent upon correct discrimination performance. Thus, under the NOP condition, the subject relies on sample stimulus information, rather than specific reward expectation, for appropriate choice behavior (stimulus–response, S–R).
Some data suggest that once reward expectancies are formed, the BLA is no longer important for maintaining these expectancies in memory or updating them with new information (Pickens et al., 2003). However, such studies did not use paradigms with multiple distinct S–O associations that could be used to guide behavior across delay intervals. In delayed discrimination tasks the cue information for an appropriate behavioral response changes from trial to trial. When the DOP is embedded into a working memory task, the unique reward expectancies also change from trial to trial and this requires continually updating the representations of reward. Under such circumstances, the BLA is likely to be critical.
Using a working memory task, we test this hypothesis by microinfusing two drugs into the BLA that have been shown to disrupt behavior dependent on reward expectancies and/or amygdala-dependent neural plasticity. Muscimol, a γ-aminobutyric acid (GABA)A agonist, impairs delayed matching performance in rats trained with the DOP (Ramirez et al., 2005), and in monkeys it impairs the expression of reinforcer devaluation (Wellman et al., 2005). One of the signaling cascades that have been implicated in neural plasticity and memory processing within the amygdala is the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) signaling pathway. ERK/MAPK inhibition with U0126 impairs long-term potentiation, Pavlovian fear conditioning and short-term memory (STM) formation (Schafe et al., 2000; Igaz et al., 2006), as well as the acquisition and retention of spatial memories (Zhang et al., 2004; Alvarez-Jaimes et al., 2005). Thus, we expected the muscimol, and potentially U0126, to selectively disrupt spatial STM performance in subjects trained with the DOP.
Materials and methods
Subjects
In this experiment, 22 adult male Sprague–Dawley rats (Harlan, Indianapolis, IN, USA) aged 3−4 months were utilized. Subjects weighed approximately 280−320 g at the time of surgery. All subjects were housed in a temperature-controlled vivarium (20 °C) that had a 12 h light : dark cycle (lights on 07.30 h, off 19.30 h). Prior to cannulation surgery, subjects were pair-housed in plastic cages. Post-operatively, subjects were individually housed in plastic cages for the duration of recovery, behavioral training and testing. All subjects had unrestricted access to water. One week prior to the start of behavioral training all subjects were placed on food restriction to decrease their weights to approximately 85% of their free feeding weights.
All experimental protocols were conducted in accordance with the ‘Guide for the Care and Use of Laboratory Rats’ (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996), and were approved by the State University of New York at Binghamton Institutional Animal Care and Use Committee.
Behavioral apparatus
Behavioral training and testing was conducted in operant chambers (Med. Associates Inc., St Albans, VT, USA). The chamber's front and back walls are made of Plexiglas, whereas the chamber's floor, ceiling and sidewalls are made of aluminum. An aperture (5 × 5 cm) in the right side wall allowed for access to a pellet and liquid dispensers. The reward aperture, 1.6 cm above the floor, had a photo beam to detect head insertion. The retractable response levers (5 cm wide) were located to either side of the reward aperture and were 6.3 cm above the floor. There was also a panel light 6.3 cm above the reward aperture and a house-light on the back wall, 24 cm above the floor. Each operant chamber (29 × 24 × 30 cm) was enclosed in a sound-attenuating chamber. This external chamber had a built in ventilation fan that provides background noise (60 dB) during training and testing. Behavioral programs and data collection were conducted with a PC interface and software (Med. Associates Inc.) on a Compaq PC computer.
Habituation and lever press training
Subjects were semi-randomly assigned to either the DOP or NOP group. All subjects first completed two 45-min sessions of habituation, with the house-light and fan on. The next six sessions of autoshaping began the differentiation between the DOP and NOP groups. During each trial of autoshaping, a right or left lever was extended semi-randomly: Each lever was chosen for 50% of trials, but did not extend on the same side for more than three consecutive trials to control for side-bias behavior. After being extended for 8 s, the lever was retracted and a reinforcer was immediately presented, non-contingent on responses. Though, if a subject pressed the lever before the 8 s elapsed, the lever was immediately retracted and the reinforcer was presented. After right lever retraction, subjects trained with the DOP received a 45 mg food pellet (BioServe, Frenchtown, NJ, USA). After left lever retraction, DOP subjects received 0.1 mL of a 20% sucrose solution. Subjects trained with the NOP received a random reinforcer (either 45 mg pellet or sucrose solution) after either left or right lever retraction. The average intertrial interval (ITI) for autoshaping sessions was 65 s (range: 35−90 s). All subjects were required to complete 50 trials in each autoshaping session. The next two sessions fixed ratio (FR)1 and FR2 schedules were used to demonstrate reliable lever pressing: Each FR session consisted of 48 trials. For each trial, one lever was extended. Subjects in the DOP group received 0.1 mL of the sucrose solution after pressing the left lever and the 45 mg pellet after pressing the right lever. Subjects in the NOP group received a randomly chosen reinforcer after a left or right lever press. The average ITI for the FR1 and FR2 sessions was 32.5 s (range: 5−60 s).
Matching to position (MTP) training
In the sample phase of a MTP trial, the subjects were required to press a semi-randomized presented right or left lever. If the subjects did not lever press within 15 s (limited hold), the lever was retracted, a response omission was recorded and a 30-s ITI was initiated. Following the ITI, the same lever was extended for another 15 s. This process was repeated until the subject pressed the lever. After the sample lever was pressed, the light above the reward aperture was activated and the lever retracted. The subjects were then required to nose-poke into the reward aperture. The goal of the nose-poke response was to reduce response-mediated behaviors. This procedure has been demonstrated to keep the rat continuously making nose-poke responses to the food/water aperture through the delay interval (Dunnett & Martel, 1990). Once the photo beam was interrupted, the light was inactivated and both levers extended. In the choice phase, the subjects were given 15 s to press the lever that was extended in the sample phase. If the subject did not press a lever within 15 s, a response omission was recorded and the trial was restarted from the beginning of the sample phase. If a lever was pressed, regardless of which lever, both levers were retracted. If the subject made the correct choice, the light above the reward aperture was activated for the duration of the presentation of the reinforcer. Subjects in the DOP group received 0.1 mL of the sucrose solution if they correctly chose the left lever and a 45 mg pellet if they correctly chose the right lever. Subjects in the NOP group received a randomly chosen reinforcer (pellet or sucrose solution) after correctly choosing the left or right lever. However, if subjects chose the wrong lever, the house-light was turned off for 10 s. This was followed by a repetition of the previous trial in which the same sample stimulus was presented to control for side-biased responding. Trials in which the subject chose the wrong lever were scored as incorrect. There were 60 trials in each MTP session with an average ITI of 32.5 s (range: 20−45 s). MTP training finished when a subject reached at least 90% accuracy for three consecutive sessions.
Delayed matching to position (DMTP) training
During DMTP sessions, various delay intervals were introduced between the sample and choice phases in each trial. The delay intervals (0, 4, 8, 16, 32 and 48 s) were randomly administered across trials. Delay intervals immediately followed a lever press during the sample phase. The choice phase was not initiated until the first nose-poke into the reward aperture after the delay interval elapsed. (This interval is called true delay time.) This nose-poke requirement was to decrease response mediation. Similar to the pretraining and MTP sessions, subjects in the DOP group received 0.1 mL of the sucrose solution if they correctly chose the left lever and a 45 mg pellet if they correctly chose the right lever during the choice phase. Subjects in the NOP group received a randomly chosen reinforcer after correctly choosing the left or right lever. For an incorrect choice, the house-light was inactivated for 10 s followed by a repetition of the previous trial to control for side-bias responding. Each DMTP session consisted of 12 trials at each delay interval, for a total of 72 trials per session. If subjects did not complete the 72 trials within 75 min the session was terminated. The average ITI for DMTP sessions was 14 s (range: 10−18 s). Each subject completed six DMTP training sessions prior to surgery.
Surgery
After behavioral training, all subjects were implanted with bilateral guide cannulae (22-gauge; Plastics One, Roanoke, VA, USA) within the BLA for drug infusions. Subjects were anesthetized with a ketamine (8.25 mL)/xylazine (1.75 mL) mixture (50 mg/kg i.p., Fort Dodge Animal Health, Fort Dodge, IA, USA) and placed in a stereotaxic instrument (David Knopf Instruments, USA). Guide cannulae were implanted bilaterally into the BLA using the following coordinates: anterior–posterior (AP), −3.0 mm from bregma; medial-lateral (ML), ±4.8 mm from the midline from the central suture of the skull (Paxinos & Watson, 1998). The cannulae (7 mm in length) were held in place with three stainless steel skull screws and cranioplastic cement (Plastics One). Stylets (Plastics One) were inserted into the cannulae until drug infusions were made. Subcutaneous injections of the opioid-based analgesic buprenorphine (0.05 mg/kg, Reckitt Benckiser Pharmaceuticals, Richmond, VA, USA) were administered to each subject directly after surgery and again the following day. After surgery, all subjects were given 1 week to recover before resuming behavioral training. To demonstrate post-surgery behavioral recovery, all subjects adequately completed another six DMTP sessions before any drug infusions.
Microinjections and behavioral testing
Fifteen minutes prior to behavioral testing, 0.5 μL of vehicle [saline 0.9%, Sigma-Aldrich, St Louis, MO, USA, or a 50−50 solution of saline/dimethylsulfoxide (DMSO) solution], muscimol (0.125 or 0.0625 μg/μL in saline, Sigma-Aldrich) or U0126 (2.0 and 4.0 μg/μL in 50/50 DMSO vehicle, Sigma-Aldrich) was manually infused into each cannula via microinjection needles (28-gage, Plastics One) that extended 0.5 mm beyond the tip of the cannula. A 5.0-μL glass syringe (Hamilton, Reno, NV, USA) was attached to the injection needle by a polyethylene tube (PE-50, Plastics One). The injection process was timed to take 2 min and the needles were left in the cannulae for an additional 2 min following each infusion to allow for adequate diffusion. Each subject was behaviorally tested under all dose conditions with the order of drug delivery counterbalanced: Half of subjects received injections of muscimol first, whereas the other half received U0126 first. The order of drug delivery was semi-randomly as subjects across groups were matched for dose schedule. All behavioral testing followed the DMTP parameters previously described. Recovery days, on which no injections occurred, but on which behavioral sessions were conducted, intervened between drug testing days until performance recovered. This recovery period was between 24 and 48 h, and within that period of time all subjects returned to pre-drug baseline performance.
Histology
After all behavioral testing, all subjects were deeply anesthetized with Sleepaway (50 mg sodium pentobarbital/kg i.p., Fort Dodge Animal Health, Fort Dodge, IA, USA) and killed. Brains were extracted and placed in a 10% buffered formalin solution (Sigma-Aldrich) and stored in a refrigerator (4 °C). One week prior to slicing, brains were placed in a 30% sucrose solution for dehydration. To examine cannulae placement, 60-μm coronal sections were taken through the injection sites with a sliding microtome (Sm2000r; Lecia Instruments, Germany), mounted on gelatin-coated slides and stained with Cresyl violet. Randomly chosen subjects were infused with India ink prior to brain extraction to examine the region of fluid diffusion. A light microscope (Nikon Eclipse E400, Melville, NY, USA) with an attached digital camera (Scion Corporation; Frederick, MD, USA) at 4−20 × magnification was used to observe selected sections from each subject to verify correct BLA cannulae placement.
Results
Neural histopathology
Only the data from subjects with cannulae placement within the lateral amygdala were used in the statistical analysis (Fig. 1A). India ink was used in a subset of animals to mark diffusion patterns (Fig. 1B). The ink spread into the lateral and basolateral nuclei; however, there also appeared to be some drift of solution encroaching into the central nucleus. Upon histopathological evaluation of cannulae placement, the data from three of the subjects were excluded. An additional two subjects died shortly after surgery, and one subject removed the cannulae and cement base and was killed. The final number of subjects was 16: eight rats remained in each of the groups (DOP, NOP).
Fig. 1.
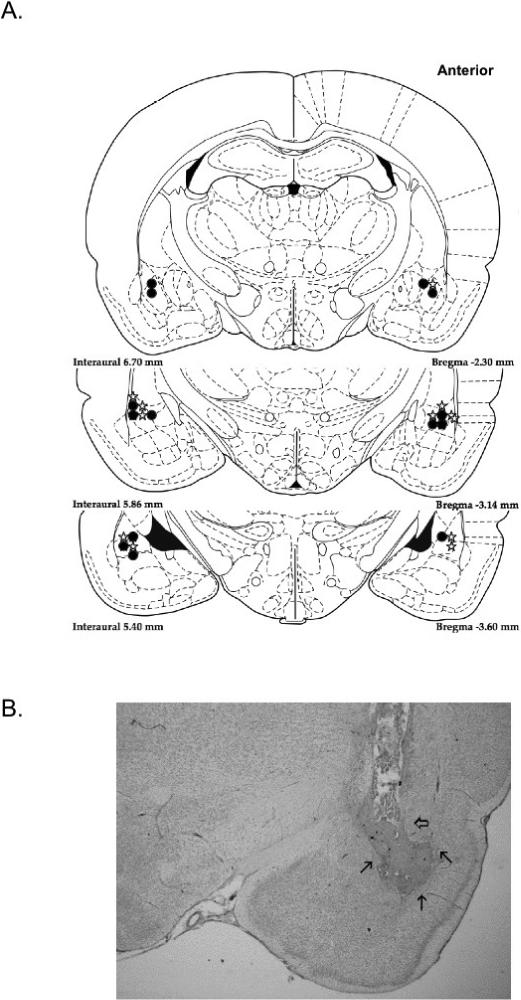
Cannulae placement schematic for all included subjects (A). Filled circles represent the end of cannulae tracts for NOP subjects, while open stars represent the end of the cannulae tracts in the DOP subjects. (Note: the injection needle protruded 0.5 mm further than the cannulae.) In the cannula placement photograph (B) the clear arrow signifies the end of the cannula tract. India ink was used to visualize the drug diffusion area, indicated by the thin arrows.
Statistical analysis
A reinforcement contingency (DOP, NOP) × drug dose (vehicle, low, high) × delay interval (0, 4, 8, 16, 32, 48 s) repeated-measures anova was used to initially screen for group differences in both choice accuracy and true delay time data for both drug conditions (muscimol, U0126). A reinforcement contingency × drug dose repeated-measures anova was used to assess the number of response omissions and trials completed under each drug condition.
Muscimol
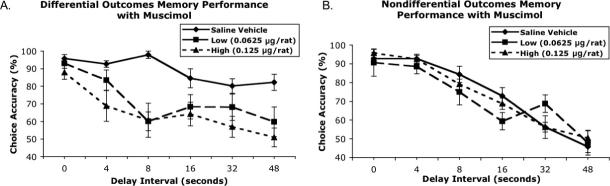
Contrasting the choice accuracy data in Fig. 2A and B, one can see, as hypothesized, that infusing muscimol into the BLA only affected STM performance in the DOP-trained rats. The overall anova revealed significant interaction between reinforcement contingency × drug dose (F2,28 = 11.13, P < 0.001). Furthermore, planned contrasts were conducted to analyse choice accuracy scores of the DOP and the NOP groups separately as a function of drug dose and delay interval. This analysis revealed that only the performance of the DOP-trained subjects was affected by BLA muscimol inactivation (drug main effect: F2,14 = 18.51, P < 0.0001). Muscimol-induced impairment increased as the drug dose increased and produced a delay-dependent effect in the DOP group (drug dose–delay interval interaction: F10,70 = 2.22, P < 0.05). Although there was a reduction in accuracy at all delay intervals with both doses of muscimol, the greatest impairment of the DOP-trained rats was at the longer delay intervals. This was not seen in the NOP-trained rats: there was no effect of the drug dose on choice accuracy performance (all F < 1.50, P > 0.15).
Fig. 2.
Mean choice accuracy (± SEM) of rats when microinfused with saline and two doses of muscimol as a function of delay intervals. The left graph depicts subjects trained with the DOP (A), whereas the right subjects were trained with the NOP (B).
The omnibus F-test also revealed a significant interaction between reinforcement contingency × delay interval (F5,70 = 5.88, P ≤ 0.001). To further understand the differences between the DOP- and NOP-trained rats as a function of delay interval, we contrasted the differences between the two groups separately for each drug condition. When saline was infused into the BLA there was a significant differential outcomes effect (DOE): the main effects of reinforcement contingency (F1,14 = 45.92, P ≤ 0.0001) and delay (F5,70 = 28.23, P ≤ 0.0001) were significant, as was the interaction between those variables (F5,70 = 7.46, P ≤ 0.0001). However, when the low dose of muscimol (0.065 μg) was infused into the BLA, the STM retention curves of the two groups looked the same: there were no differences as a function of reinforcement contingency (F ≤ 1) and no reinforcement contingency–delay interaction (F5,70 = 1.78, P = 0.127), but there was a significant main effect of delay interval (F5,70 15.37, P ≤ 0.0001). When the high dose (0.125 μg) was infused into the BLA there was a trend in the direction of the DOP-trained rats performing worse than the NOP-trained rats, but this effect was not significant (reinforcement contingency–delay interaction, F5,70 = 2.196, P = 0.064).
It should be noted that the in the omnibus F-test the reinforcement contingency × delay interval–drug dose interaction was not significant. However, such an unfocused overall anova can diffuse the impact of more focused F-tests (see Wilcox, 1987; Wilkinson et al., 1999; Howell, 2002), and this is clearly seen in our data.
The second analysis revealed that the number of trials completed was not a function of reinforcement contingency (DOP = 68.9 ± 1.8; NOP = 71.9 ± 0.2) or dose of muscimol (saline = 71.8 ± 0.2; low = 70.5 ± 0.8; high = 69.1 ± 1.7) nor the interaction of those variables. However, the number of response omissions did differ as a function of reinforcement contingency: the DOP-trained rats had more response omissions (DOP = 8.04 ± 1.97; NOP 2.63 ± 0.88) than NOP-trained rats (F1,14 = 5.26, P < 0.05), but the response omissions in either group were not affected by increasing the dose of muscimol. True delay interval times were not affected by reinforcement contingency, drug dose or the interaction of those variables. It was observed that as delay interval increased the response time also increased (F5,70 = 4.33, P < 0.01). Thus, the average true delay times across groups and doses were the following: 0.31, 4.35, 8.63, 17.21, 33.45, 50.32 s. In summary, only STM choice accuracy in DOP-trained subjects was affected by intra-amygdala administration of muscimol, the drug did not impair gross motor performance as measured by response omissions, true delay time or number of trials completed.
ERK/MAPK inhibitor U0126
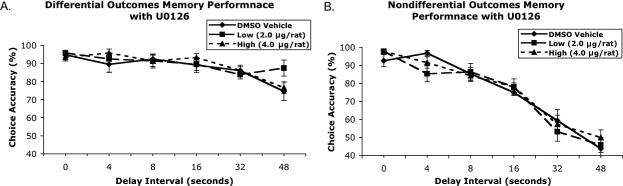
As shown in Fig. 3A and B, infusion of U0126 into the BLA had no effect on choice accuracy in either the DOP- or NOP-trained rats (dose effect: F2,28 ≤ 1). However, the DOP group had dramatically better STM performance than the NOP group, illustrating the DOE. The reinforcement contingency (F1,14 = 99.94, P < 0.001) and the delay interval (F5,70 = 84.77, P < 0.001) main effects were significant. The significant reinforcement contingency–delay interval interaction (F5,70 = 27.25, P < 0.001) revealed how the DOE particularly manifested itself at the longer delay intervals. The reinforcement contingency × drug dose, drug dose × delay interval and reinforcement contingency × delay interval–drug dose interactions were nonsignificant. Therefore, as shown in Fig. 3, ERK/MAPK inhibitor administered into the lateral amygdala had no effect on STM choice accuracy performance in either the DOP or NOP group.
Fig. 3.
Mean choice accuracy (± SEM) of rats when microinfused with vehicle [50 : 50 dimethylsulfoxide (DSMO) : saline] and two doses of U0126 as a function of delay intervals. The left graph depicts subjects trained with the DOP (A), whereas the right subjects were trained with the NOP (B).
Furthermore, neither dose of U0126 increased response omissions (DOP = 7.0 ± 2.08; NOP = 1.96 ± 0.41), increased delay times (0.52, 4.21, 8.24, 16.56, 33.39, 49.07) or decreased the number of trials completed in either group (DOP = 70.2 ± 0.86, NOP = 72 ± 0) beyond vehicle condition. Overall, U0126 did not significantly impact any of the behavioral measures in either reinforcement condition.
Discussion
Without pharmacological manipulations, as with previous studies, a significant memory enhancement was observed in the DOP-trained rats compared with NOP-trained rats, illustrating the DOE. The reliance on reward-related processes activates a memory system that is different, structurally and associatively, from the system commonly used to solve conditional discrimination tasks (Blundell et al., 2001 Ramirez et al., 2005; Savage, 2001; Savage et al., 2004; Ramirez & Savage, 2007). Prior studies have demonstrated that lesions of the BLA impair the expression of the DOE during the acquisition of a conditional discrimination task in rats (Blundell et al., 2001; Ramirez & Savage, in press). However, no one has previously looked at what inactivation or removal of the BLA does to the DOE that is seen after learning when retention intervals intervene between the stimulus and response (see Urcuioli, 2005). There have been some data to suggest that post-training lesions of the BLA after a S–O association is formed, but prior to devaluation of the outcome, do not disrupt re-evaluation of outcome expectancies ) as do lesions made prior to the S–O learning (Pickens et al., 2003). This observation led those authors to conclude that the BLA is not critical for maintaining or updating reward representations. On the contrary, using a working memory task, the current study demonstrates that the BLA is critical to maintain multiple reward expectations even when the S–O associations were formed prior to alterations of the BLA. However, our study involved more than one S–O association. Thus, the reward expectancy used to modulate choice behavior across the delay intervals changes with each trial, and the reward expectancy must then be updated on a momentary scale. These factors make this a complex cognitive task that appears to require the BLA even after S–O associations are well learned. Thus, under situations when there is more than one reward expectancy and/or the reward expectancy that guides behavior can change from trial to trial and rather quickly (moments), the BLA is important in modulating learning and memory performance.
There is another issue that could also contribute to differences in findings between the present study and that of Picken et al. (2003), and that is permanent lesion vs temporary inactivation of the BLA. There are data showing that temporary inactivation of the BLA can cause a larger retention deficit in inhibitory avoidance with previous shock training than observed when the BLA is lesioned (Berlau & McGaugh, 2003). Although this may appear counterintuitive, during temporary inactivation the development of compensatory mechanisms is likely to be minimal, and therefore more impairment could be observed in such a temporary state vs a permanent chronic lesion condition. In addition, the diffusion of drug in our study could have spread over a larger extent of the amygdala than that damaged by a chemical lesion.
GABAA agonists like muscimol have previously been used to functionally inactivate neural structures associated with learning, producing various memory performance impairments (Holt & Maren, 1999; Spanis et al., 1999; Ramirez et al., 2005). However, in this study there was no effect of muscimol infusion into the BLA on the STM performance in the NOP group. This demonstrates that the BLA is not needed for STM of the spatial cues to guide the choice response (S–R), and supports the notion of the differential memory systems in use between the DOP and NOP paradigms. As previously stated, evidence suggests that in the NOP condition, the hippocampus is critical to successfully completing the spatial STM/working memory task of DMTP (Savage et al., 2004). Conversely, the current study demonstrates that in the DOP group, intra-amygdala muscimol infusions dose-dependently impaired STM performance on the DMTP learning task. Furthermore, this impairment by muscimol was larger at delay intervals greater than 8 s. These results demonstrate that by modifying reward contingencies, so that different reward expectations can be used to solve the task (S–O), the BLA is then critical for STM performance. Currently, we do not know what the critical factor(s) is/are for evoking the BLA in a working memory task when reward expectancies are embedded: It could be the delay interval itself or that there are multiple reward expectancies that can be used to guide different choice behaviors – or the interaction between those two psychological factors.
In a previous study, with the same task parameters, intracerebroventricular (i.c.v.) infusions of muscimol impaired performance at all delay intervals (0−48 s) in rats trained with the DOP (Ramirez et al., 2005). With the i.c.v. infusions, it is likely that muscimol penetrated multiple neural structures to exert its general amnestic effect. The amygdala was only one structure that would have been affected with such an infusion procedure. That data combined with the site-specific results of the present study suggest that other neural structures also play a role in the use of reward expectancies. One neural candidate is the orbital frontal cortex (OFC). The OFC has been shown to be important for the later maintenance of reward expectancies and the flexible regulation of goal-directed behavior (McDannald et al., 2005; van Duuren et al., 2007; Ramirez & Savage, 2007). Lesions to this structure produce non-memory-specific deficits, such as impairments in encoding reward expectancy information from the BLA and the loss of cognitive flexibility in switching responses to changes in reward (Schoenbaum et al., 2003; Wallis, 2007).
To better understand the role of protein cascades in the amygdala during STM that changes from moment to moment, the ERK/MAPK pathway inhibitor U0126 was investigated. The ERK/MAPK cascade is an important cell signal system for the regulation and activation of various activities like gene expression, apoptosis, differentiation, proliferation, and has also been implicated in STM, long-term memory (LTM) and synaptic plasticity (Seger & Krebs, 1995; Thomas & Huganir, 2004). Using the inhibitor U0126, the ERK/MAPK signaling cascade in the rat amygdala has been shown to be crucial for synaptic plasticity and LTM formation of fear conditioning (Schafe et al., 2000). Moreover, inhibition with U0126 causes deficits in spatial and hippocampal-dependent LTM formation in the rat nucleus accumbens and area CA1 of the hippocampus, respectively (Zhang et al., 2004; Alvarez-Jaimes et al., 2005). Although this molecular signaling pathway appears to be necessary for long-term potentiation and for some forms of LTM, to date there is conflicting evidence about the role of the ERK/MAPK cascade in STM. Using fear conditioning, some studies have demonstrated that U0126 infused into the hippocampus disrupts short-term recall of non-cued avoidance learning (Igaz et al., 2006), whereas other studies have shown lateral amygdala infusions of U0126 have no effect on STM (Schafe et al., 2000) or reactivation of STM of Pavlovian fear conditioning (Duvarci et al., 2005). These contradictions could be due to the fact that different brain structures were inactivated or that different S–R parameters were used or required. However, no other study to date has examined whether the inhibition of the ERK/MAPK cascade disrupts the flexible form of STM called working memory. Working memory commonly refers to processes in which information in STM requires continually updating in the realm of seconds or minutes (Baddeley & Hitch, 1974). In our study, inhibition of the ERK/MAPK signaling cascade with intra-amygdala U0126 infusions did not have any effect on DMTP memory performance in either the DOP or NOP group. This indicates the ERK/MAPK signaling cascade in the amygdala is not an important molecular component for previously learned spatial–response associations or the use of established reward expectancies.
These findings provide some support for the hypothesis that STM and LTM utilize related, yet separate, molecular substrates. Previous studies have observed that STM processing involves only the modification of existing proteins, and is independent of protein synthesis and gene transcription as is seen in LTM (Kandel, 2001; Schafe et al., 2001). However, very little is known about the molecular substrates of short-term working memory in which critical information changes from trial to trial in the range of seconds to minutes. A recent study (Touzani et al., 2007) did demonstrate that acquisition of a working memory task (delayed non-matching to position in the radial arm maze with 1−2 s delay intervals) is affected by the infusion of the protein synthesis inhibitor anisomycin into the medial frontal cortex.
In summary, our data demonstrate that BLA inactivation by muscimol selectively disrupts working memory performance only in rats trained with the DOP. This demonstrates that the BLA is a critical neural structure for the use of previously learned unique reward expectations. Given that rats trained with the NOP were not affected by intra-BLA infusions of muscimol, it supports the hypothesis that different brain regions are evoked as a function of the type of associative structure (DOP: S–O vs NOP: S–R) used by the organism. It should be noted that in the present paradigm the impact of R–O associations cannot be ruled out as key associative mechanisms in the DOP condition. However, given our training procedures, S–O association may maintain more control than R–O association: what may seem like minor differences in training protocols (such as whether differentially reinforced responses are trained in different sessions or discrete SD are used to signal behavioral choice) can change the type of outcome expectancy (S–O or R–O) that gains stimulus control (Urcuioli, 2005). Most of the studies that have examined R–O associations in rats trained with a DOP-like procedure (different outcomes for different instrumental responses) have not employed discrete SD, and/or each differentially reinforced response was trained separately in different sessions (see Urcuioli, 2005). The acquisition of such R–O associations is not affected by lesions to the BLA; however, lesions to the BLA do attenuate the impact of outcome devaluation and impair the ability of rats to use the different outcomes as cues in a free operant discrimination (Balleine et al., 2003).
These results also further our understanding of behavioral plasticity and recovery of function after brain damage. The DOP eliminates the behavioral impairments seen in rats with amnesia induced by cholinergic antagonism (Savage et al., 1999b), advanced ageing (Savage et al., 1999a), diencephalic pathology caused by thiamine deficiency (Savage & Langlais, 1995) or hippocampal lesions (Savage et al., 2004). When such brain-altered subjects are trained with the NOP there is significant impairment in learning and memory performance: however, no impairment is observed when these brain-damaged subjects are trained with the DOP. The recovery of function observed under DOP is likely because DOP-trained subjects are primarily using amygdala-based strategies to solve the task, which are left intact and functional after those manipulations.
Acknowledgement
A portion of this research was funded by grant NINDS 054272 to L.M.S.
Abbreviations
- BLA
basolateral nuclei of the amygdala
- DMSO
dimethylsulfoxide
- DMTP
delayed matching to position
- DOE
differential outcomes effect
- DOP
differential outcomes procedure
- ERK/MAPK
extracellular signal-regulated kinase/mitogen-activated protein kinases
- FR
fixed ratio
- GABA
γ-aminobutyric acid
- i.c.v.
intracerebroventricular
- ITI
intertrial interval
- LTM
long-term memory
- MTP
matching to position
- NOP
non-differential outcomes procedure
- OFC
orbital frontal cortex
- R–O
response–outcome
- S–O
stimulus–outcome
- S–R
stimulus–response
- STM
short-term memory
References
- Alvarez-Jaimes L, Feliciano-Rivera M, Centeno-Gonzalez M, Maldonado-Vlaar C. Contributions of the mitogen-activated protein kinase and protein kinase C cascades in spatial learning and memory mediated by the nucleus accumbens. J. Pharmacol. Exp. Ther. 2005;314:1144–1157. doi: 10.1124/jpet.105.086140. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. Working memory. In: Bower GH, editor. The Psychology of Learning and Motivation: Advances in Research & Theory. Academic Press; New York: 1974. pp. 47–89. [Google Scholar]
- Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J. Neurosci. 2003;23:666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Basolateral lesions do not prevent memory of context-footshock training. Learn. Mem. 2003;10:495–502. doi: 10.1101/lm.64003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. J. Neurosci. 2001;21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Preserved sensitivity to outcome value after lesions of the basolateral amygdala. J. Neurosci. 2003;23:7702–7709. doi: 10.1523/JNEUROSCI.23-20-07702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett SB, Martel FL. Proactive interference effects on short-term memory in rats: basic parameters and drug effects. Behav. Neurosci. 1990;104:655–665. doi: 10.1037//0735-7044.104.5.655. [DOI] [PubMed] [Google Scholar]
- van Duuren E, Escámez FA, Joosten RN, Visser R, Mulder AB, Pennartz CM. Neural coding of reward magnitude in the orbitofrontal cortex of the rat during a five-odor olfactory discrimination task. Learn. Mem. 2007;14:446–456. doi: 10.1101/lm.546207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Nade RK, LeDoux JE. Activation of extracellular signal-regulated kinase-mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur. J. Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Jan JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of the basolateral, but not central, amygdala interfere with Pavlovian second order conditioning and reinforcer devaluation effects. J. Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J. Neurosci. 1999;19:9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC. Statistical Methods Psychology. 5th Edn. Duxbury-Wadsworth; Pacific Grove, CA: 2002. [Google Scholar]
- Igaz LM, Winograd M, Cammarota M, Izquierdo LA, Alonso M, Izquierdo I, Medina JH. Early activation of extracellular signal-regulated kinase signaling pathway in the hippocampus is required for short-term memory formation of a fear-motivated learning. Cell. Mol. Neurobiol. 2006;26:989–1002. doi: 10.1007/s10571-006-9116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- McDannald MA, Saddoris MP, Gallagher M, Holland PC. Lesions of orbitofrontal cortex impair rats' differential outcome expectancy but not conditioned stimulus-potentiated feeding. J. Neurosci. 2005;25:4626–4632. doi: 10.1523/JNEUROSCI.5301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmier BJ, Linwick D. Conditional choice – unique outcomes establish expectancies that mediate choice behavior. Integrative Physiol. Behav. Sci. 2001;36:173–181. doi: 10.1007/BF02734091. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Edn Academic Press; San Diego: 1998. [Google Scholar]
- Pickens CP, Saddoris MP, Setlow B, Gallagher M, Holland PC. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J. Neurosci. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DR, Buzzetti RA, Savage LM. The role of the GABAA agonist muscimol on memory performance: reward contingencies determine the nature of the deficit. Neurobiol. Learn. Mem. 2005;84:184–191. doi: 10.1016/j.nlm.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Ramirez DR, Savage LM. Differential involvement of the basolateral amygdala, orbital frontal cortex and nucleus accumbens core in the acquisition and use of reward expectancies. Behav. Neurosci. 2007;121:896–906. doi: 10.1037/0735-7044.121.5.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LM. In search of the neurobiological underpinnings of the differential outcomes effect. Integrative Physiol. Behav. Sci. 2001;36:182–195. doi: 10.1007/BF02734092. [DOI] [PubMed] [Google Scholar]
- Savage LM, Buzzetti RA, Ramirez DR. The effect of hippocampal lesions on learning, memory and reward expectancies. Neurobiol. Learn. Mem. 2004;82:109–119. doi: 10.1016/j.nlm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Savage LM, Langlais PJ. Differential outcomes attenuate spatial memory impairments on matching-to-position following pyrithiamine-induced thiamine deficiency in rats. Psychobiology. 1995;23:153–160. [Google Scholar]
- Savage LM, Pitkin SR, Careri JM. Memory enhancement in aged rats: the differential outcomes effect. Dev. Psychobiol. 1999a;35:318–327. [PubMed] [Google Scholar]
- Savage LM, Pitkin SR, Knitowski KM. Rats exposed to acute pyrithiamine-induced thiamine deficiency are more sensitive to the amnestic effects of scopolamine and MK-801: examination of working memory, response selection and reinforcement contingencies. Behav. Brain Res. 1999b;104:13–26. doi: 10.1016/s0166-4328(99)00049-2. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J. Neurosci. 2000;10:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nadar K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitalfrontal cortex during cue sampling depends upon input from the basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. Official Pub. Fed. Am. Soc. Exp. Biol. 1995;9:726–735. [PubMed] [Google Scholar]
- Spanis CW, Bianchin MM, Izquierdo I, McGaugh JL. Excitotoxic basolateral amygdala lesions potentiate the memory impairment effect of muscimol injected into the medial septal area. Brain Res. 1999;816:329–336. doi: 10.1016/s0006-8993(98)01104-4. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signaling and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Touzani K, Puthanveettil SV, Kandel ER. Consolidation of learning strategies during spatial working memory task requires protein synthesis in the prefrontal cortex. Proc. Natl Acad. Sci. USA. 2007;104:5632–5637. doi: 10.1073/pnas.0611554104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapold MA. Are expectancies based upon different positive reinforcing events discriminably different. J. Learn. Mot. 1970;1:129–140. [Google Scholar]
- Trapold MA, Overmier JB. The second learning process in instrumental conditioning. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Theory and Research. Appleton Century Crafts; New York: 1972. pp. 237–452. [Google Scholar]
- Urcuioli PJ. Behavioral and associative effects of differential outcomes in discrimination learning. Learn. Behav. 2005;33:1–21. doi: 10.3758/bf03196047. [DOI] [PubMed] [Google Scholar]
- Wallis J. Orbitofrontal cortex and it's contribution to decision-making. Annu. Rev. Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Wellman LL, Gale K, Malkova L. GABAA-mediated inhibition of basolateralamygdala blocks reward devaluation in macaques. J. Neurosci. 2005;25:4577–4586. doi: 10.1523/JNEUROSCI.2257-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox RR. New designs in the analysis of variance. Rev. Psychol. 1987;61:165–170. [Google Scholar]
- Wilkinson L, Task Force on Statistical Inference, APA Board of Scientific Affairs Statistical methods in psychology journals: guidelines and explanations. Am. Psychol. 1999;54:594–604. [Google Scholar]
- Zhang H, Zhao Y, Huang Y, Dorairaj NR, Chandler LJ, O'Donnell JM. Inhibition of the phosphodiesterase 4 (PDE4) enzyme reverses memory deficits produced by infusion of the MEK inhibitor U0126 into the CA1 subregion of the rat hippocampus. Neuropsychopharmacology. 2004;29:1432–1439. doi: 10.1038/sj.npp.1300440. [DOI] [PubMed] [Google Scholar]