Abstract
Objective
Individuals with spinal cord injury (SCI) develop a severe form of osteoporosis below the level of injury that is poorly understood. We conducted a preliminary investigation to assess whether circulating markers of bone turnover and circulating RANKL/OPG levels are related to the severity of SCI, aging, or to differences in mobility (i.e., walking or using a wheelchair).
Methods
Sixty-four caucasian men ≥1.6 years since injury selected based on locomotive mode provided blood samples and completed a health questionnaire at the VA Boston Healthcare System from 10/2003 to 6/2005. Plasma sRANKL, osteoprotegerin (OPG), osteocalcin and carboxyterminal telopeptide of type I collagen (CTx) levels were determined.
Results
Increasing age was significantly associated with increased OPG and CTx. Injury severity was predictive of OPG levels, and adjusting for age, participants with cervical motor complete and ASIA C SCI (n=11) had significantly lower mean OPG (46.1 pg/ml) levels than others (63.4 pg/ml). Locomotive mode was not associated with differences in bone markers.
Conclusions
Severe cervical spinal cord injury is associated with decreased circulating OPG levels placing these patients at risk for accelerated bone loss that appears unrelated to locomotive mode.
Keywords: Osteoporosis, Osteoprotegerin, Aging, Spinal Cord Injury, Bone
Introduction
Spinal cord injury (SCI) is associated with a severe form of osteoporosis that is traditionally attributed to mechanical unloading of the sublesional limbs. Acutely, bone formation is decreased in the first two weeks directly following an injury and then returns to pre-injury levels. Bone resorption immediately and steadily increases causing an uncoupling of bone remodeling that can result in a loss of up to 4% per month of sublesional trabecular bone1-3. Increased resorption is most pronounced in the acute phase of spinal cord injury and slows after the first year with a cumulative sublesional trabecular bone loss as great as 41%4,5. The long bones and iliac crest are most affected with sparing of the vertebral bodies6,7. Hyper-resorption with trabecular wasting leads to structural modification of the affected bones, decreased mechanical strength, and increased fracture frequency8,9.
Receptor activator of nuclear factor kappa B (RANKL) and its decoy receptor, osteoprotegerin (OPG), are important regulators of bone homeostasis. RANKL is produced by osteoblasts, bone stromal cells, and activated T cells. The binding of RANKL to its receptor, RANK, is necessary and sufficient to induce osteoclast activation and resorption10,11. OPG is an osteoprotective molecule produced by osteoblasts and stromal cells that blocks RANKL-stimulated osteoclast activation by competitively binding to RANK12,13. While in vitro studies have clearly defined the role of RANKL/RANK/OPG in osteoclastogenesis, no clear consensus has emerged regarding the state of these cytokines in the pathogenesis of human disease.
Little is known about the clinical relevance of measuring circulating markers of bone turnover and the RANKL/OPG system in persons with chronic SCI. Our a priori hypothesis was that injury severity or mobility mode would be predictive of OPG/RANKL levels in this population. As part of an epidemiological study assessing chronic health outcomes in chronic SCI14, we conducted a preliminary investigation to assess whether these circulating markers are related to the severity of SCI, aging, body mass index (BMI) or to differences in mobility (i.e., walking or using a wheelchair).
Materials and methods
Subjects
Participants for the current study were selected from a larger epidemiological study assessing health in chronic spinal cord injury conducted at the VA Boston Healthcare System14. As part of the epidemiological study, each person completed a health questionnaire and answered the question, “How do you usually get around (usually means more than half the time)?“ Responses were recorded as motorized wheelchair, hand propelled wheelchair, walk with aid (crutch, cane, or similar aid), or walk without assistance. As part of this study, blood samples were collected from 82 white male participants between 10/2003 to 6/2005 who were 2 years or more years post SCI, were in their usual state of health, and who were not using cholesterol-lowering medications, including statins. For this preliminary investigation, we selected 64 of these 82 persons based on mobility mode. From this 82, all persons using a motorized wheelchair (n=12), an assistive device (n=17), and who walked independently were included (n=12). A random sample of the remaining persons who usually used a manual wheelchair (n=23) was selected. All subjects gave informed consent and the study was approved by the VA Boston Healthcare System, Harvard Medical School, and Brigham and Women's Hospital institutional review boards.
Motor score
Motor level and completeness of injury was assessed by physical exam. Level of injury was classified according to strength preservation in key muscle groups in the upper and lower extremity and reported regionally as cervical, thoracic, or lumbar. Injury completeness was reported according to guidelines suggested by the American Spinal Injury Association (ASIA)15. Participants were assigned as motor complete (equivalent to ASIA motor score of A or B, i.e., no motor function below the neurological level of injury), C (motor incomplete, motor function preserved below the neurological level and more than half the key muscles below the neurological level are not strong enough to overcome gravity), or D (motor incomplete, preservation of motor function below the neurological level and more than half the key muscles below the neurological level are strong enough to overcome gravity). SCI level and severity of injury was considered in 3 groups that included cervical motor complete and ASIA C; thoracic and lumbar motor complete or ASIA C, and ASIA D. By using participants in each of these 3 impairment groups, we can examine the effect of spinal cord injury in a “dose response“ manner ranging from profound neurological impairment to minimal-no neurological impairment.
Biochemical analyses
Plasma samples were drawn into an EDTA tube, immediately stored in an insulated container with a cooler pack, and shipped overnight to the VA Boston/Massachusetts Veterans Epidemiology Research and Information Center core blood laboratory. The samples were centrifuged for 15 minutes at 2600 rpm (1459 x g) at 4°C and stored at −80°C until batch analysis. The analyses were conducted by the Clinical & Epidemiologic Research Laboratory, Department of Laboratory Medicine at Children's Hospital in Boston. Circulating levels of osteocalcin and CTx were quantified by electrochemiluminescence immunoassay with a detection limit of 0.50 ng/mL for osteocalcin and 0.07 ng/mL for CTx. Circulating osteoprotegerin and sRANKL were quantified by ELISA assay (Alpco Diagnostics – Windham, NH) with a detection limit of 0.14 pmol/l (OPG) and 1.56 pg/ml (sRANKL).
Variable definition
Persons completed a health questionnaire based on the American Thoracic Society adult respiratory questionnaire16. Smokers were defined as smoking 20 or more packs of cigarettes or using 12 ounces of tobacco or more in a lifetime, or smoking one or more cigarettes per day for at least one year. Current smokers reported cigarette use within one month of testing. Hypertension and diabetes were defined if diagnosed by a doctor, heart disease was defined as receiving treatment for “heart trouble“ in the 10 years prior to blood draw. As part of the study protocol, subject length was measured in 59 persons, was available by self-report in 2, and from SCI clinic notes in 3. In 57 persons weight was measured, was available by self-report in 3, and was obtained from SCI clinic notes in 4 participants. Length and weight were used to calculate body mass index (BMI). For 3 persons whose health questionnaires were completed at a time other than the blood draw date, SCI clinic progress notes were reviewed to confirm previously reported questionnaire responses.
Statistical analysis
Generalized linear models (PROC GLM in SAS version 8.2) were used to assess cross-sectional determinants of OPG, CTx, sRANKL, and osteocalcin. Residual plots were examined for goodness of fit. Since the distributions of OPG, CTx, and sRANKL were skewed, natural log-transformation was used to normalize the distribution of the outcome and stabilize the variance.
Results
Participant characteristics
The average age at time of blood draw was 56 (±15) years with an average of 21(±13) years since injury. After selection, one subject was found to be 1.6 years post-SCI. Other participant characteristics are presented in Table 1 based on motor level and neurologic completeness of injury (cervical motor complete or ASIA C; thoracic and lumbar motor complete or ASIA C SCI, and all others with ASIA D SCI).
Table 1.
Participant characteristics.
| Participants by Motor Level and Severity of Injury | All Participants | |||
|---|---|---|---|---|
| Motor Complete and ASIA C | Motor CompleteThoracic and Lumbar and ASIA C | All ASIA D | ||
| N=11 | N=20 | N=33 | N=64 | |
| mean (sd) [range] | ||||
| Years since injury | 28.1 (8.9) [12.5−41.0] | 20.5 (10.4) [4.0−50.4] | 19.3 (14.2) [1.6−59.8] | 21.2 (12.6) [1.6−59.8] |
| Age (yr) | 52.8 (9.3) [35.9−63.1] | 52.2 (14.8) [26.7−84.8] | 59.1 (15.3) [24.8−87.8] | 55.9 (14.5) [24.8−87.8] |
| BMI (kg/m2) | 25.5 (3.6) [19.0−30.1] | 27.3 (4.3) [19.6−35.2] | 28.2 (5.7) [18.6−41.3] | 27.5 (5.0) [18.6−41.3] |
| Mobility | n (%) | |||
| Motorized Wheelchair | 4 (36.4%) | 4 (20%) | 4 (12.1%) | 12 (18.8%) |
| Manual Wheelchair | 6 (54.6%) | 15 (75%) | 2 (6.1%) | 23 (35.9%) |
| Assistive Device | 1 (9.1%) | 1 (5%) | 15 (45.5%) | 17 (26.6%) |
| Independent Walker | 0 (0%) | 0 (0%) | 12 (36.4%) | 12 (18.8%) |
| Smoking | ||||
| Current | 1 (9.1%) | 2 (10%) | 7 (21.2%) | 10 (15.6%) |
| Former | 7 (63.6%) | 6 (30%) | 17 (51.5%) | 30 (46.9%) |
| Never | 3 (27.3%) | 12 (60%) | 9 (27.3%) | 24 (37.5%) |
| Heart Disease | 3 (27.3%) | 2 (10%) | 6 (18.2%) | 11 (17.2%) |
| Hypertension | 1 (9.1%) | 6 (30%) | 11 (33.3%) | 18 (28.1%) |
| Diabetes | 0 (0.0%) | 3 (15%) | 6 (18.2%) | 9 (14.1%) |
| Markers of Bone Turnover | median [25% − 75%ile] | |||
| Osteocalcin (ng/ml) | 22.67 [17.87−33.90] | 21.11 [15.32−25.17] | 21.62 [18.79−27.85] | 21.54 [17.82−28.63] |
| C-telopeptide (ng/ml) | 0.24 [0.10−0.28] | 0.20 [0.14−0.37] | 0.19 [0.16−0.30] | 0.20 [0.15−0.31] |
| sRANKL (pg/ml) | 0.16 [0.08−0.27] | 0.09 [0.03−0.29] | 0.07 [0.04−0.16] | 0.08 [0.04−0.20] |
| OPG (pg/ml) | 49.00 [35.46−53.80] | 58.64 [48.20−70.30] | 69.50 [44.32−93.78] | 58.39 [43.93−79.60] |
Predictors of bone markers
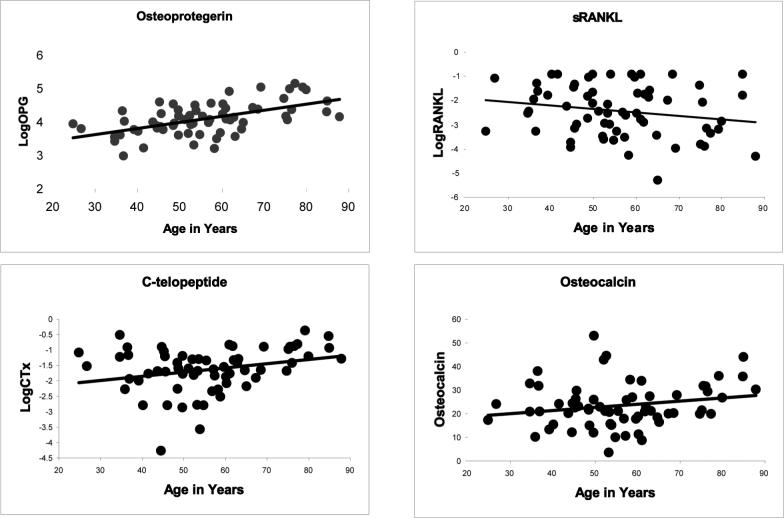
Mobility mode, age, injury duration, BMI, smoking history, SCI level and severity, and history of heart disease, hypertension, and diabetes were examined as predictors of circulating levels of osteocalcin and CTx (markers of bone turnover) sRANKL and OPG. Mobility was not a predictor of sRANKL, OPG, osteocalcin, and CTx (p=0.23 to 0.45, and shown in Table 2 for OPG). Likewise, BMI, years since injury, and smoking history (current, former, never) were not significant predictors (p=0.14 to 0.91). The concentrations of CTx (p=0.03) and OPG (p<.001) increased significantly with increasing age (Figure 1). Plots (Figure 1) suggested a similar relationship with osteocalcin and an inverse relationship between age and sRANKL, but these were not significant (p=0.10 and 0.11, respectively). OPG was significantly lower in persons with cervical complete motor or ASIA C SCI compared to all others (Table 2), but differences in other bone markers based on SCI level were not significant (p=0.12 to 0.66). In a model adjusting for age (β=0.017±0.003 (SE), p<0.001), OPG remained significantly lower in cervical complete motor or ASIA C SCI (Table 3).
Table 2.
Univariate predictors of ln OPG.
| Co-efficient (β) | SE | eθ | p | |
|---|---|---|---|---|
| Years post SCI (yrs) | 0.007 | 0.005 | 1.007 pg/yr | 0.14 |
| BMI (kg/m2) | 0.005 | 0.012 | 1.005 pg/kg/m2 | 0.68 |
| Mean ln OPG (+/− SE) | e(opg) pg/ml | p | ||
| Mobility | 0.29 | |||
| Motorized Wheelchair | 4.01 (0.14) | 55.06 | ||
| Manual Wheelchair | 4.00 (0.10) | 54.48 | ||
| Assistive Device | 4.27 (0.11) | 71.78 | ||
| Independent Walker | 4.11 (0.14) | 61.01 | ||
| Level/Completeness | 0.01 | |||
| Cervical motor complete and ASIA C | 3.78 (0.14) | 43.68 | ||
| All Others | 4.16 (0.06) | 64.20 | ||
| Smoking | 0.36 | |||
| Current | 4.21 (0.15) | 67.66 | ||
| Former | 4.14 (0.09) | 62.71 | ||
| Never | 3.99 (0.10) | 54.00 | ||
There are slight differences in exponentiated values due to rounding.
Figure 1.
Relationship between age and bone markers. Plots of the relationship between osteoprotegerin (p<0.001, r=0.56), c-telopeptide (p=0.03, r=0.28), sRANKL (p=0.11, r=−0.20), and osteocalcin (p=0.10, r=0.21) with age. Osteoprotegerin, c-telopeptide, and sRANKL are plotted using natural log-transformed data.
Table 3.
OPG levels adjusted for age.
| |
Mean ln OPG (+/− SE) |
e(opg) pg/ml |
p |
|---|---|---|---|
|
Level/Completeness |
0.02 |
||
| Cervical motor complete and / ASIA C |
3.83 (0.12) | 46.11 | |
| All Others | 4.15 (0.05) | 63.37 |
There are slight differences in exponentiated values due to rounding.
Hypertension, heart disease, and diabetes
OPG levels were higher in participants with a history of hypertension, diabetes, or heart disease (Table 4), and osteocalcin (p=0.03) and CTx (p=0.02) were higher in participants with a history of hypertension. Hypertension, heart disease, and diabetes were each included in separate regression models with age and SCI level and assessed as predictors of OPG. Although OPG levels were still greater in persons with hypertension, heart disease, or diabetes, the differences were not statistically significant (Table 4). In similar multivariate models, osteocalcin (p=0.05) and CTx (p=0.08) were also greater in persons with hypertension (data not shown). We also assessed whether OPG values increased more rapidly with increasing age in persons with cervical A/C SCI by examining the interaction between age and SCI level and found it to be insignificant (p=0.66). This suggests that OPG levels increase with advancing age in quadriplegic patients as it does in patients with all other levels of injury.
Table 4.
OPG levels for participants with and without hypertension, heart disease, and diabetes, adjusted and unadjusted for SCI level/completeness and age.
| Unadjusted | Adjusted for Age and Level/Completeness | |||||
|---|---|---|---|---|---|---|
| Mean ln OPG (+/− SE) | e(opg) pg/ml | p | Mean ln OPG (+/− SE) | e(opg) pg/ml | p | |
| Comorbidity | ||||||
| Hypertension | 4.35 (0.11) | 77.5 | <0.01 | 4.10 (0.11) | 60.58 | 0.20 |
| No Hypertension | 3.99 (0.07) | 54.3 | 3.96 (0.07) | 52.37 | ||
| Heart Disease | 4.37 (0.14) | 78.6 | 0.04 | 4.14 (0.12) | 62.70 | 0.16 |
| No Heart Disease | 4.04 (0.06) | 56.7 | 3.95 (0.07) | 51.81 | ||
| Diabetes | 4.50 (0.15) | 90.4 | <0.01 | 4.18 (0.15) | 65.30 | 0.16 |
| No Diabetes | 4.03 (0.06) | 56.1 | 3.97 (0.06) | 53.00 | ||
There are slight differences in exponentiated values due to rounding.
Discussion
Osteoporosis is a common sequela of spinal cord injury resulting in increased fractures, worsening functional impairment, and increased health care costs. The pathogenesis of SCI-related bone loss is currently poorly understood and treatment options remain limited in this population. As major modulators of bone homeostasis, alterations in the RANKL/OPG system may be involved in SCI-related bone loss and have not been previously described in chronic SCI. In this study, we examine the effects of mobility mode, age, BMI, and SCI level and severity of injury on the RANKL/OPG system and on circulating markers of bone turnover (CTx and osteocalcin) in the chronic phase of SCI. We found a significant relationship between increased age and greater levels of OPG and CTx. The finding that increasing age was significantly related to greater levels of circulating OPG and CTx are in agreement with reports in the able-bodied17-19 and increased OPG with age is considered to represent a physiologic response to counter bone loss that normally accompanies aging.
SCI-related bone loss is most pronounced in the acute phase and relatively little is known about bone metabolism in chronic SCI (i.e., beyond the first 12−24 months after injury). While the pathophysiology is poorly understood, many consider disuse to be the primary factor leading to SCI-related bone loss. An association between immobility and bone turnover has been described in the able-bodied elderly. Chen et al. assessed the effect of mobility mode on circulating markers of bone turnover in a population of frail elderly nursing home residents. Serum levels of another marker of bone formation, aminoterminal propeptide of type I collagen (PINP), increased with increasing hypomobility and were greatest in wheelchair users20. Accordingly, we hypothesized that usual ambulatory mode, a surrogate for the degree of weight-bearing, would also influence these modulators of bone homeostasis and turnover in patients with chronic SCI. Participants who walk without an assistive device weight-bear normally through the affected limbs while those who use a motorized wheelchair bear minimal weight. However, we found little effect of mobility mode on markers of bone turnover or RANKL/OPG levels.
We also examined the effect of the severity of neurological injury, determined by spinal level and ASIA classification, on bone mediators. Alteration of the RANKL/OPG system has been described in other clinical conditions where pathological bone loss is common irrespective of mobility level, including inflammatory bowel disease and chronic liver disease21,22. After adjusting for age, participants with motor complete cervical or ASIA C had significantly lower levels of circulating OPG than others with less severe injuries. Although our data were limited by sample size, there was no evidence that the effect of age on OPG level in quadriplegia was greater than in others with SCI. Lower OPG levels may promote bone resorption leading to lower bone mass, and suggests that patients with quadriplegia may be prone to more severe bone loss than others with SCI. This suggests that on a chronic basis, injury level/severity in SCI contributes more to bone loss than degree of immobility. This is consistent with findings reported by Dauty et al. who found that extent of neurological impairment, not lack of mechanical loading, was the primary factor contributing to loss of sublesional BMC in 31 men with chronic (more than 1 year post-injury) SCI7. Similarly, Wilmet et al. reported that spinal cord injured patients who regained near normal mobility after injury still had substantial sublesional reduction in BMC at one year post-injury5. There is mounting evidence that the nervous system is a major regulator of bone homeostasis23-30. Spinal cord injury, therefore, may result in abnormal neuromodulation of bone metabolism leading to SCI-related bone loss. Taken together, these observations suggest that disrupted neuroosteogenic signaling likely contributes to the severity of SCI-related bone loss and this disruption persists into the chronic phase of injury. Suppression of OPG may result from abnormal neuronal-bone interactions. There is also a growing body of data showing that the perturbation of fluid-flow dynamics associated with prolonged recumbence, in the absence of SCI, can have dramatic effects on bone mineral density in different regions of the skeleton31,32. How OPG levels in SCI might relate to this observation, and how these dramatic changes in fluid-flow dynamics might interact with disrupted neuro-osteogenic signaling to produce changes in regional bone mineral density, are avenues for further research.
The only other study assessing the RANKL/OPG system in SCI was conducted by Maimoun et al. They assessed circulating RANKL and OPG levels of 7 male patients with acute spinal cord injury over the first year compared to 12 neurologically intact men. They reported higher OPG and lower RANKL levels in the injured participants than in the able-bodied, but no relationship with time since injury33. In our study in chronic SCI, we also found no relationship between circulating markers and time since injury.
The metabolic role of OPG has been incompletely elucidated, but appears to extend beyond that directly related to bone homeostasis. Although its role in neurologic diseases is unknown, OPG is expressed in the spinal cord and cerebrospinal fluid in persons with various inflammatory and non-inflammatory neurologic diseases and increases in the cerebrospinal fluid with age34. Greater levels of OPG have been associated with atherosclerosis in epidemiological studies35, and high bone turnover in the elderly has been associated with all causes of mortality, and in particular, cardiovascular mortality36,37. In OPG-deficient mice, arterial calcification occurs38. Several recent epidemiologic studies have also suggested a relationship between the development of coronary artery disease and osteoporosis. An inverse relationship between metacarpal cortical thickness and incidence of coronary artery disease was found in female participants of the Framingham Study39. Similarly, greater metacarpal cortical bone loss was associated with greater progression of abdominal aortic calcification in females, but not in males40. Taken together, these studies suggest a relationship between bone homeostasis and systemic diseases. In a study of mortality in persons in our accompanying epidemiological study in chronic SCI, the most common cause of death was due to cardiovascular diseases, and a history of heart disease was significantly related to overall mortality14. Given the prevalence of osteoporosis in the population, we explored the relationship between history of heart disease, hypertension, and diabetes and markers of bone metabolism. Heart disease history, hypertension, or diabetes was associated with greater OPG levels, and hypertension history was associated with osteocalcin and CTx, although these differences were no longer significant after adjusting for age and SCI level. This observation warrants further exploration with larger numbers of participants.
One potential explanation for the results reported in this study might be an underlying vitamin D deficiency. We did not assay vitamin D levels in this study and this represents a limitation and an area for further investigation. However, we do believe it is unlikely that individuals with motor complete spinal cord injury are more prone to vitamin D deficiency than those with other injury levels in our community-based cohort.
These findings suggest that bone resorption increases with age in patients with spinal cord injury and that SCI level and severity of injury is predictive of bone turnover not degree of immobility. Circulating OPG levels increase with age (as in the able-bodied) and decrease with severe cervical spinal cord injury. Chronic suppression of systemic OPG may contribute to the severity of osteoporosis in patients with cervical spinal cord injury, although the mechanism whereby this may occur is speculative.
Conclusions
While bone loss is most rapid in the first 2 years following spinal cord injury, the mean time to first fracture is nine years post-injury1,4. Therefore, an improved understanding of the ongoing bone loss in the chronic phase of spinal cord injury is of great clinical relevance. Seventy-six percent of patients will sustain a traumatic fracture at some point following their injury3; however, there is currently no standard of care for prevention or treatment of osteoporosis. Our work suggests that OPG is a potential biomarker of osteoporosis in these patients. These findings also raise the possibility that bone loss following neurological injury may be prevented by replacement with recombinant OPG.
Acknowledgements
Support: National Institute of Child Health and Human Development, National Institutes of Health (grant no. RO1 HD42141), The Rehabilitation Medicine Scientists Training program (grant no. K12 HD001097-08), the Massachusetts Veterans Epidemiology Research and Information Center, Department of Veterans Affairs, Co-operative Studies Program, and Health Services Research and Development, The Foundation for PMR.
References
- 1.Eser P, Frotzler A, Zehnder Y, Schiessl H, Denoth J. Assessment of anthropometric, systemic, and lifestyle factors influencing bone status in the legs of spinal cord injured individuals. Osteoporos Int. 2005;16:26–34. doi: 10.1007/s00198-004-1638-x. [DOI] [PubMed] [Google Scholar]
- 2.Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, McWhinney B, Hickman PE. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab. 1998;83:415–22. doi: 10.1210/jcem.83.2.4581. [DOI] [PubMed] [Google Scholar]
- 3.Szollar SM, Martin EM, Sartoris DJ, Parthemore JG, Deftos LJ. Bone mineral density and indexes of bone metabolism in spinal cord injury. Am J Phys Med Rehabil. 1998;77:28–35. doi: 10.1097/00002060-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Frey-Rindova P, de Bruin ED, Stussi E, Dambacher MA, Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38:26–32. doi: 10.1038/sj.sc.3100905. [DOI] [PubMed] [Google Scholar]
- 5.Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–7. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]
- 6.Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–5. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 7.Dauty M, Perrouin VB, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27:305–9. doi: 10.1016/s8756-3282(00)00326-4. [DOI] [PubMed] [Google Scholar]
- 8.Demirel G, Yilmaz H, Paker N, Onel S. Osteoporosis after spinal cord injury. Spinal Cord. 1998;36:822–5. doi: 10.1038/sj.sc.3100704. [DOI] [PubMed] [Google Scholar]
- 9.Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39:208–14. doi: 10.1038/sj.sc.3101139. [DOI] [PubMed] [Google Scholar]
- 10.Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001;79:243–53. doi: 10.1007/s001090100226. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi N, Udagawa N, Suda T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun. 1999;256:449–55. doi: 10.1006/bbrc.1999.0252. [DOI] [PubMed] [Google Scholar]
- 12.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 13.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 14.Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, Brown R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43:408–16. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marino RJ, Barros T, Biering-Sorenson F, Burns SP, Donovan WH, Graves DE, Haak M, Hudson LM, Priebe MM, ASIA Neurological Standards Committee 2002 International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26(Suppl1):S50–S56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- 16.Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 17.Kudlacek S, Schneider B, Woloszczuk W, Pietschmann P, Willvonseder R. Serum levels of osteoprotegerin increase with age in a healthy adult population. Bone. 2003;32:681–6. doi: 10.1016/s8756-3282(03)00090-5. [DOI] [PubMed] [Google Scholar]
- 18.Szulc P, Hofbauer LC, Heufelder AE, Roth S, Delmas PD. Osteoprotegerin serum levels in men: correlation with age, estrogen, and testosterone status. J Clin Endocrinol Metab. 2001;86:3162–5. doi: 10.1210/jcem.86.7.7657. [DOI] [PubMed] [Google Scholar]
- 19.Yano K, Tsuda E, Washida N, Kobayashi F, Goto M, Harada A, Ikeda K, Higashio K, Yamada Y. Immunological characterization of circulating osteoprotegerin/osteoclastogenesis inhibitory factor: increased serum concentrations in postmenopausal women with osteoporosis. J Bone Miner Res. 1999;14:518–27. doi: 10.1359/jbmr.1999.14.4.518. [DOI] [PubMed] [Google Scholar]
- 20.Chen JS, Cameron ID, Cumming RG, Lord SR, March LM, Sambrook PN, Simpson JM, Seibel MJ. Effect of age-related chronic immobility on markers of bone turnover. J Bone Miner Res. 2006;21:324–31. doi: 10.1359/JBMR.051014. [DOI] [PubMed] [Google Scholar]
- 21.Moschen AR, Kaser A, Stadlmann S, Millonig G, Kaser S, Muhllechner P, Habior A, Graziadei I, Vogel W, Tilg H. The RANKL/OPG system and bone mineral density in patients with chronic liver disease. J Hepatol. 2005;43:973–83. doi: 10.1016/j.jhep.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 22.Moschen AR, Kaser A, Enrich B, Ludwiczek O, Gabriel M, Obrist P, Wolf AM, Tilg H. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut. 2005;54:479–87. doi: 10.1136/gut.2004.044370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battaglino R, Fu J, Spate U, Ersoy U, Joe M, Sedaghat L, Stashenko P. Serotonin regulates osteoclast differentiation through its transporter. J Bone Miner Res. 2004;19:1420–31. doi: 10.1359/JBMR.040606. [DOI] [PubMed] [Google Scholar]
- 24.Battaglino R, Vokes M, Schulze-Spate U, Sharma A, Graves D, Kohler T, Muller R, Yoganathan S, Stashenko P. Fluoxetine treatment increases trabecular bone formation in mice. J Cell Biochem. 2007;100:1387–94. doi: 10.1002/jcb.21131. [DOI] [PubMed] [Google Scholar]
- 25.Cherruau M, Facchinetti P, Baroukh B, Saffar JL. Chemical sympathectomy impairs bone resorption in rats: a role for the sympathetic system on bone metabolism. Bone. 1999;25:545–51. doi: 10.1016/s8756-3282(99)00211-2. [DOI] [PubMed] [Google Scholar]
- 26.Cherruau M, Morvan FO, Schirar A, Saffar JL. Chemical sympathectomy-induced changes in TH-, VIP-, and CGRP-immunoreactive fibers in the rat mandible periosteum: influence on bone resorption. J Cell Physiol. 2003;194:341–8. doi: 10.1002/jcp.10209. [DOI] [PubMed] [Google Scholar]
- 27.Hill EL, Elde R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991;264:469–80. doi: 10.1007/BF00319037. [DOI] [PubMed] [Google Scholar]
- 28.Hill EL, Turner R, Elde R. Effects of neonatal sympathectomy and capsaicin treatment on bone remodeling in rats. Neuroscience. 1991;44:747–55. doi: 10.1016/0306-4522(91)90094-5. [DOI] [PubMed] [Google Scholar]
- 29.Serre CM, Farlay D, Delmas PD, Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone. 1999;25:623–9. doi: 10.1016/s8756-3282(99)00215-x. [DOI] [PubMed] [Google Scholar]
- 30.Sherman BE, Chole RA. Sympathectomy, which induces membranous bone remodeling, has no effect on endochondral long bone remodeling in vivo. J Bone Miner Res. 2000;15:1354–60. doi: 10.1359/jbmr.2000.15.7.1354. [DOI] [PubMed] [Google Scholar]
- 31.Turner CH. Effects of tissue viscoelasticity on mechanical loading models using rats. Bone. 1999;25:742. doi: 10.1016/s8756-3282(99)00233-1. [DOI] [PubMed] [Google Scholar]
- 32.Winet H. A bone fluid flow hypothesis for muscle pump-driven capillary filtration: II. Proposed role for exercise in erodible scaffold implant incorporation. Eur Cell Mater. 2003;6:1–10. doi: 10.22203/ecm.v006a01. [DOI] [PubMed] [Google Scholar]
- 33.Maimoun L, Fattal C, Micallef JP, Peruchon E, Rabischong P. Bone loss in spinal cord-injured patients: from physiopathology to therapy. Spinal Cord. 2006;44:203–10. doi: 10.1038/sj.sc.3101832. [DOI] [PubMed] [Google Scholar]
- 34.Hofbauer LC, Cepok S, Hemmer B. Osteoprotegerin is highly expressed in the spinal cord and cerebrospinal fluid. Acta Neuropathol (Berl) 2004;107:575–7. doi: 10.1007/s00401-004-0854-y. [DOI] [PubMed] [Google Scholar]
- 35.Barengolts EI, Berman M, Kukreja SC, Kouznetsova T, Lin C, Chomka EV. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int. 1998;62:209–13. doi: 10.1007/s002239900419. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook PN, Chen CJ, March L, Cameron ID, Cumming RG, Lord SR, Simpson JM, Seibel MJ. High bone turnover is an independent predictor of mortality in the frail elderly. J Bone Miner Res. 2006;21:549–55. doi: 10.1359/jbmr.060104. [DOI] [PubMed] [Google Scholar]
- 37.Tanko LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912–20. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 38.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–8. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samelson EJ, Kiel DP, Broe KE, Zhang Y, Cupples LA, Hannan MT, Wilson PW, Levy D, Williams SA, Vaccarino V. Metacarpal cortical area and risk of coronary heart disease: the Framingham Study. Am J Epidemiol. 2004;159:589–95. doi: 10.1093/aje/kwh080. [DOI] [PubMed] [Google Scholar]
- 40.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68:271–6. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 41.Ragnarsson KT, Sell GH. Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil. 1981;62:418–23. [PubMed] [Google Scholar]