Abstract
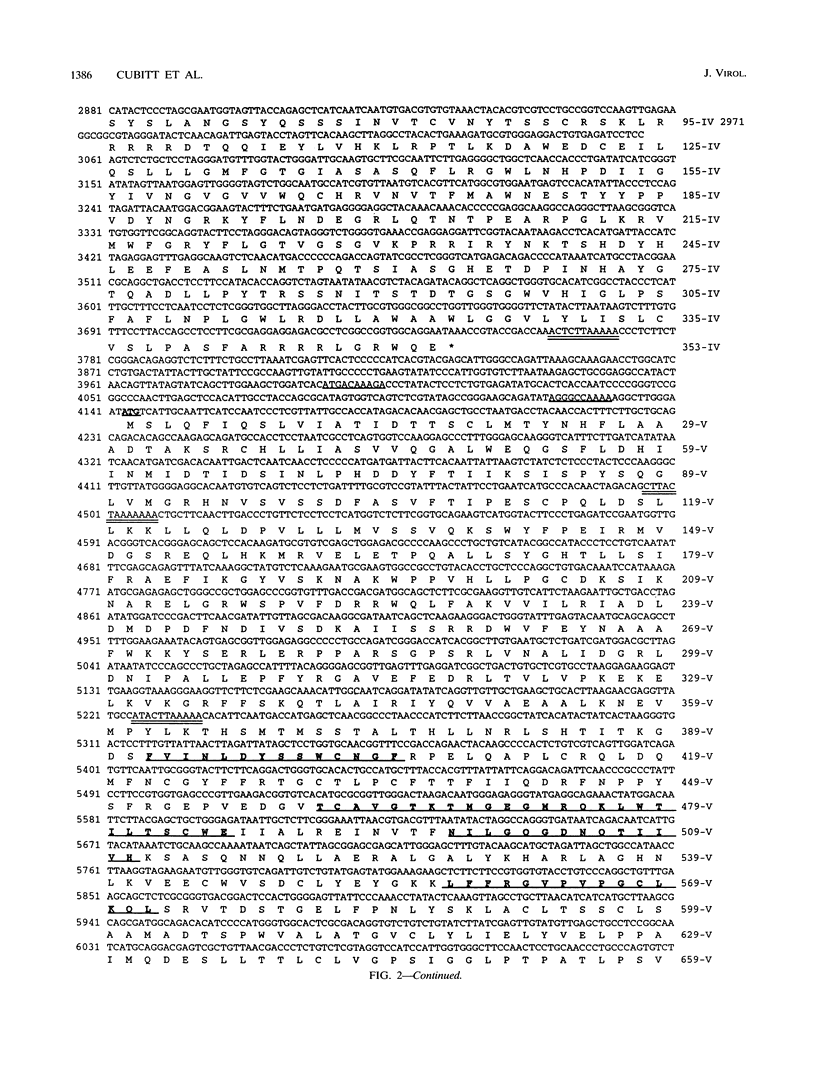
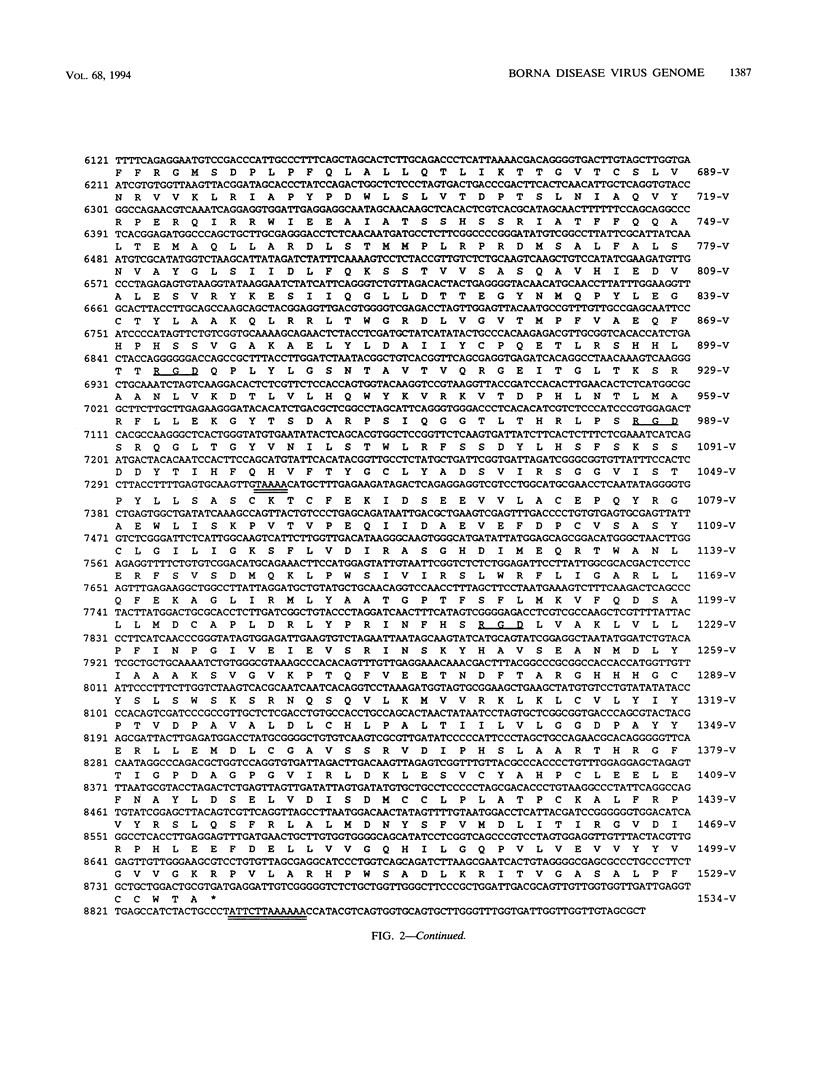
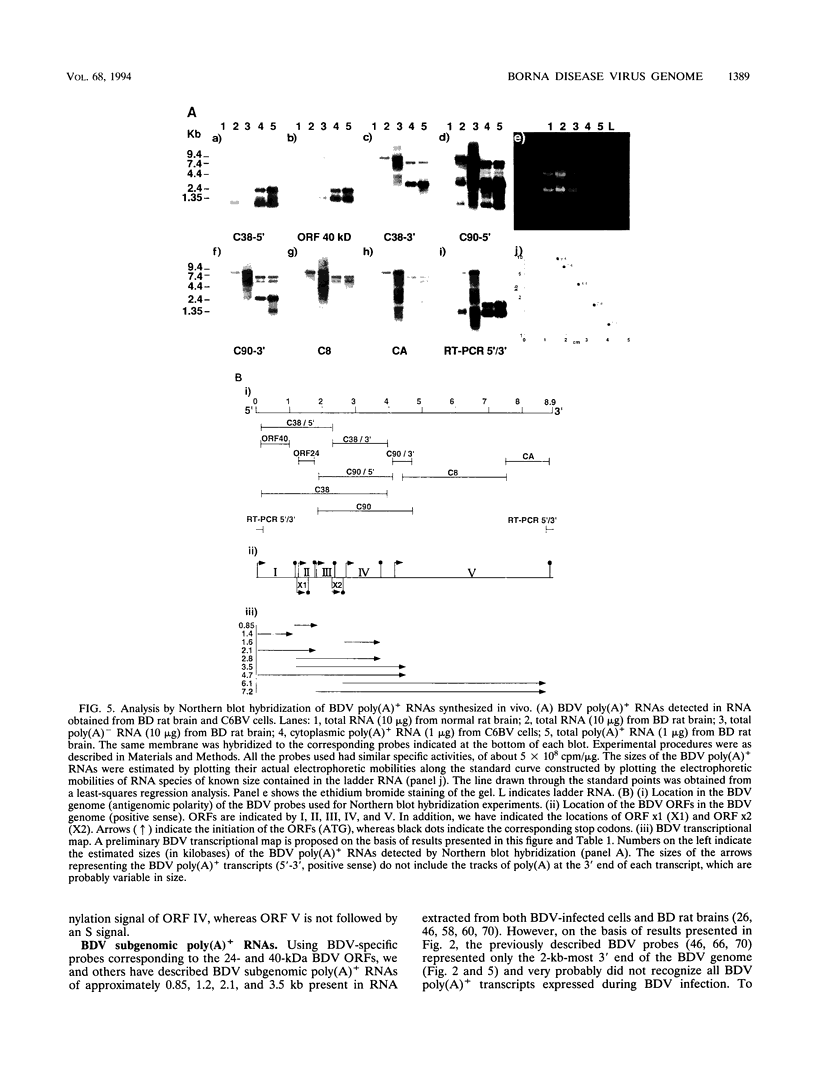
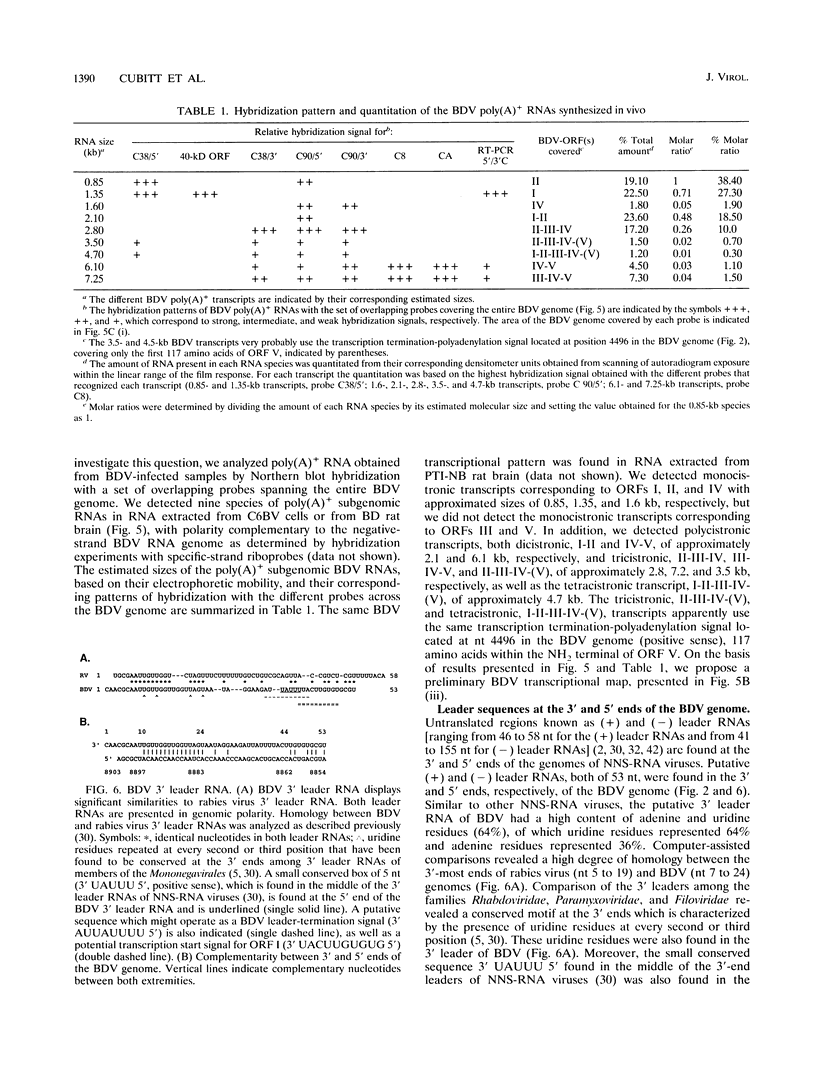
We have previously demonstrated that Borna disease virus (BDV) has a negative nonsegmented single-stranded (NNS) RNA genome that replicates in the nucleus of infected cells. Here we report for the first time the cloning and complete sequence of the BDV genome. Our results revealed that BDV has a genomic organization similar to that of other members of the Mononegavirales order. We have identified five main open reading frames (ORFs). The largest ORF, V, is located closest to the 5' end in the BDV genome and, on the basis of strong homology with other NNS-RNA virus polymerases, is a member of the L-protein family. The intercistronic regions vary in length and nucleotide composition and contain putative transcriptional start and stop signals. BDV untranslated 3' and 5' RNA sequences resemble those of other NNS-RNA viruses. Using a set of overlapping probes across the BDV genome, we identified nine in vivo synthesized species of polyadenylated subgenomic RNAs complementary to the negative-strand RNA genome, including monocistronic transcripts corresponding to ORFs I, II, and IV, as well as six polycistronic polyadenylated BDV RNAs. Interestingly, although ORFs III and V were detected within polycistronic transcripts, their corresponding monocistronic transcripts were not detected. Our data indicate that BDV is a member of the Mononegavirales, specially related to the family Rhabdoviridae. However, in contrast to the rest of the NNS-RNA animal viruses, BDV replication and transcription occur in the nucleus of infected cells. These findings suggest a possible relationship between BDV and the plant rhabdoviruses, which also replicate and transcribe in the nucleus.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam J. D., Winokur A., Dyson W., Herzog S., Gonzalez F., Rott R., Koprowski H. Borna disease virus. A possible etiologic factor in human affective disorders? Arch Gen Psychiatry. 1985 Nov;42(11):1093–1096. doi: 10.1001/archpsyc.1985.01790340077011. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Barik S., Rud E. W., Luk D., Banerjee A. K., Kang C. Y. Nucleotide sequence analysis of the L gene of vesicular stomatitis virus (New Jersey serotype): identification of conserved domains in L proteins of nonsegmented negative-strand RNA viruses. Virology. 1990 Mar;175(1):332–337. doi: 10.1016/0042-6822(90)90218-g. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Bode L., Riegel S., Lange W., Ludwig H. Human infections with Borna disease virus: seroprevalence in patients with chronic diseases and healthy individuals. J Med Virol. 1992 Apr;36(4):309–315. doi: 10.1002/jmv.1890360414. [DOI] [PubMed] [Google Scholar]
- Bode L., Riegel S., Ludwig H., Amsterdam J. D., Lange W., Koprowski H. Borna disease virus-specific antibodies in patients with HIV infection and with mental disorders. Lancet. 1988 Sep 17;2(8612):689–689. doi: 10.1016/s0140-6736(88)90505-3. [DOI] [PubMed] [Google Scholar]
- Briese T., de la Torre J. C., Lewis A., Ludwig H., Lipkin W. I. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutlag D. L., Dautricourt J. P., Maulik S., Relph J. Improved sensitivity of biological sequence database searches. Comput Appl Biosci. 1990 Jul;6(3):237–245. doi: 10.1093/bioinformatics/6.3.237. [DOI] [PubMed] [Google Scholar]
- Carbone K. M., Park S. W., Rubin S. A., Waltrip R. W., 2nd, Vogelsang G. B. Borna disease: association with a maturation defect in the cellular immune response. J Virol. 1991 Nov;65(11):6154–6164. doi: 10.1128/jvi.65.11.6154-6164.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone K. M., Rubin S. A., Sierra-Honigmann A. M., Lederman H. M. Characterization of a glial cell line persistently infected with borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J Virol. 1993 Mar;67(3):1453–1460. doi: 10.1128/jvi.67.3.1453-1460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda S. J., Wong T. C. Measles virus synthesizes both leaderless and leader-containing polyadenylated RNAs in vivo. J Virol. 1989 Jul;63(7):2977–2986. doi: 10.1128/jvi.63.7.2977-2986.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi T. J., Kuwata S., Koonin E. V., Heaton L. A., Jackson A. O. Structure of the L (polymerase) protein gene of sonchus yellow net virus. Virology. 1992 Jul;189(1):31–39. doi: 10.1016/0042-6822(92)90678-i. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Olmsted R. A., Spriggs M. K., Johnson P. R., Buckler-White A. J. Gene overlap and site-specific attenuation of transcription of the viral polymerase L gene of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5134–5138. doi: 10.1073/pnas.84.15.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988 Nov 25;16(22):10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley J. C., Dowling P. C., Menonna J., Silverman J. I., Schuback D., Cook S. D., Blumberg B. M. Sequence variability and function of measles virus 3' and 5' ends and intercistronic regions. Virology. 1988 Jun;164(2):498–506. doi: 10.1016/0042-6822(88)90564-8. [DOI] [PubMed] [Google Scholar]
- Cubitt B., de la Torre J. C. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J Virol. 1994 Mar;68(3):1371–1381. doi: 10.1128/jvi.68.3.1371-1381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J. A., Kolakofsky D. Rescue of a Sendai virus DI genome by other parainfluenza viruses: implications for genome replication. Virology. 1991 May;182(1):168–176. doi: 10.1016/0042-6822(91)90660-4. [DOI] [PubMed] [Google Scholar]
- Danner K., Mayr A. In vitro studies on Borna virus. II. Properties of the virus. Arch Virol. 1979;61(4):261–271. doi: 10.1007/BF01315012. [DOI] [PubMed] [Google Scholar]
- Delarue M., Poch O., Tordo N., Moras D., Argos P. An attempt to unify the structure of polymerases. Protein Eng. 1990 May;3(6):461–467. doi: 10.1093/protein/3.6.461. [DOI] [PubMed] [Google Scholar]
- Duchala C. S., Carbone K. M., Narayan O. Preliminary studies on the biology of Borna disease virus. J Gen Virol. 1989 Dec;70(Pt 12):3507–3511. doi: 10.1099/0022-1317-70-12-3507. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feldmann H., Mühlberger E., Randolf A., Will C., Kiley M. P., Sanchez A., Klenk H. D. Marburg virus, a filovirus: messenger RNAs, gene order, and regulatory elements of the replication cycle. Virus Res. 1992 Jun;24(1):1–19. doi: 10.1016/0168-1702(92)90027-7. [DOI] [PubMed] [Google Scholar]
- Fu Z. F., Amsterdam J. D., Kao M., Shankar V., Koprowski H., Dietzschold B. Detection of Borna disease virus-reactive antibodies from patients with affective disorders by western immunoblot technique. J Affect Disord. 1993 Jan;27(1):61–68. doi: 10.1016/0165-0327(93)90098-5. [DOI] [PubMed] [Google Scholar]
- Galinski M. S. Paramyxoviridae: transcription and replication. Adv Virus Res. 1991;39:129–162. doi: 10.1016/s0065-3527(08)60794-0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Bye J., Skehel J., Waterfield M. Cloning and DNA sequence of double-stranded copies of haemagglutinin genes from H2 and H3 strains elucidates antigenic shift and drift in human influenza virus. Nature. 1980 Sep 25;287(5780):301–306. doi: 10.1038/287301a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Polytranscripts of Sendai virus do not contain intervening polyadenylate sequences. Virology. 1985 Feb;141(1):102–109. doi: 10.1016/0042-6822(85)90186-2. [DOI] [PubMed] [Google Scholar]
- Herman R. C., Schubert M., Keene J. D., Lazzarini R. A. Polycistronic vesicular stomatitis virus RNA transcripts. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4662–4665. doi: 10.1073/pnas.77.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog S., Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168(3):153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Jackson A. O. Replication of sonchus yellow net virus in infected protoplasts. Virology. 1990 Dec;179(2):815–820. doi: 10.1016/0042-6822(90)90149-l. [DOI] [PubMed] [Google Scholar]
- Kao M., Hamir A. N., Rupprecht C. E., Fu Z. F., Shankar V., Koprowski H., Dietzschold B. Detection of antibodies against Borna disease virus in sera and cerebrospinal fluid of horses in the USA. Vet Rec. 1993 Mar 6;132(10):241–244. doi: 10.1136/vr.132.10.241. [DOI] [PubMed] [Google Scholar]
- Kurilla M. G., Stone H. O., Keene J. D. RNA sequence and transcriptional properties of the 3' end of the Newcastle disease virus genome. Virology. 1985 Sep;145(2):203–212. doi: 10.1016/0042-6822(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Lipkin W. I., Briese T., de la Torre J. C. Borna disease virus: molecular analysis of a neurotropic infectious agent. Microb Pathog. 1992 Sep;13(3):167–170. doi: 10.1016/0882-4010(92)90017-i. [DOI] [PubMed] [Google Scholar]
- Lipkin W. I., Travis G. H., Carbone K. M., Wilson M. C. Isolation and characterization of Borna disease agent cDNA clones. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H., Bode L., Gosztonyi G. Borna disease: a persistent virus infection of the central nervous system. Prog Med Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- Masters P. S., Samuel C. E. Detection of in vivo synthesis of polycistronic mRNAs of vesicular stomatitis virus. Virology. 1984 Apr 30;134(2):277–286. doi: 10.1016/0042-6822(84)90297-6. [DOI] [PubMed] [Google Scholar]
- McClure M. A., Thibault K. J., Hatalski C. G., Lipkin W. I. Sequence similarity between Borna disease virus p40 and a duplicated domain within the paramyxovirus and rhabdovirus polymerase proteins. J Virol. 1992 Nov;66(11):6572–6577. doi: 10.1128/jvi.66.11.6572-6577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A., Galinski M. S. Characterization of human parainfluenza virus type 3 persistent infection in cell culture. J Virol. 1990 Jul;64(7):3212–3218. doi: 10.1128/jvi.64.7.3212-3218.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Smallwood-Kentro S., Haddad A., Prevec L. Assembly and transcription of synthetic vesicular stomatitis virus nucleocapsids. J Virol. 1991 May;65(5):2170–2178. doi: 10.1128/jvi.65.5.2170-2178.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollis D. L., Brick P., Hamlin R., Xuong N. G., Steitz T. A. Structure of large fragment of Escherichia coli DNA polymerase I complexed with dTMP. 1985 Feb 28-Mar 6Nature. 313(6005):762–766. doi: 10.1038/313762a0. [DOI] [PubMed] [Google Scholar]
- Parvin J. D., Palese P., Honda A., Ishihama A., Krystal M. Promoter analysis of influenza virus RNA polymerase. J Virol. 1989 Dec;63(12):5142–5152. doi: 10.1128/jvi.63.12.5142-5152.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch O., Blumberg B. M., Bougueleret L., Tordo N. Sequence comparison of five polymerases (L proteins) of unsegmented negative-strand RNA viruses: theoretical assignment of functional domains. J Gen Virol. 1990 May;71(Pt 5):1153–1162. doi: 10.1099/0022-1317-71-5-1153. [DOI] [PubMed] [Google Scholar]
- Poch O., Sauvaget I., Delarue M., Tordo N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989 Dec 1;8(12):3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyper J. M., Richt J. A., Brown L., Rott R., Narayan O., Clements J. E. Genomic organization of the structural proteins of borna disease virus revealed by a cDNA clone encoding the 38-kDa protein. Virology. 1993 Jul;195(1):229–238. doi: 10.1006/viro.1993.1364. [DOI] [PubMed] [Google Scholar]
- Richt J. A., Vande Woude S., Zink M. C., Narayan O., Clements J. E. Analysis of Borna disease virus-specific RNAs in infected cells and tissues. J Gen Virol. 1991 Sep;72(Pt 9):2251–2255. doi: 10.1099/0022-1317-72-9-2251. [DOI] [PubMed] [Google Scholar]
- Richt J. A., VandeWoude S., Zink M. C., Clements J. E., Herzog S., Stitz L., Rott R., Narayan O. Infection with Borna disease virus: molecular and immunobiological characterization of the agent. Clin Infect Dis. 1992 Jun;14(6):1240–1250. doi: 10.1093/clinids/14.6.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. Nuclear location signal-mediated protein transport. Biochim Biophys Acta. 1989 Aug 14;1008(3):263–280. doi: 10.1016/0167-4781(89)90016-x. [DOI] [PubMed] [Google Scholar]
- Rott R., Herzog S., Bechter K., Frese K. Borna disease, a possible hazard for man? Arch Virol. 1991;118(3-4):143–149. doi: 10.1007/BF01314025. [DOI] [PubMed] [Google Scholar]
- Schädler R., Diringer H., Ludwig H. Isolation and characterization of a 14500 molecular weight protein from brains and tissue cultures persistently infected with borna disease virus. J Gen Virol. 1985 Nov;66(Pt 11):2479–2484. doi: 10.1099/0022-1317-66-11-2479. [DOI] [PubMed] [Google Scholar]
- Sippel A. E. Purification and characterization of adenosine triphosphate: ribonucleic acid adenyltransferase from Escherichia coli. Eur J Biochem. 1973 Aug 1;37(1):31–40. doi: 10.1111/j.1432-1033.1973.tb02953.x. [DOI] [PubMed] [Google Scholar]
- Thierer J., Riehle H., Grebenstein O., Binz T., Herzog S., Thiedemann N., Stitz L., Rott R., Lottspeich F., Niemann H. The 24K protein of Borna disease virus. J Gen Virol. 1992 Feb;73(Pt 2):413–416. doi: 10.1099/0022-1317-73-2-413. [DOI] [PubMed] [Google Scholar]
- Tsurudome M., Bando H., Kawano M., Matsumura H., Komada H., Nishio M., Ito Y. Transcripts of simian virus 41 (SV41) matrix gene are exclusively dicistronic with the fusion gene which is also transcribed as a monocistron. Virology. 1991 Sep;184(1):93–100. doi: 10.1016/0042-6822(91)90825-v. [DOI] [PubMed] [Google Scholar]
- VandeWoude S., Richt J. A., Zink M. C., Rott R., Narayan O., Clements J. E. A borna virus cDNA encoding a protein recognized by antibodies in humans with behavioral diseases. Science. 1990 Nov 30;250(4985):1278–1281. doi: 10.1126/science.2244211. [DOI] [PubMed] [Google Scholar]
- Wilde A., Morrison T. Structural and functional characterization of Newcastle disease virus polycistronic RNA species. J Virol. 1984 Jul;51(1):71–76. doi: 10.1128/jvi.51.1.71-76.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. C., Hirano A. Structure and function of bicistronic RNA encoding the phosphoprotein and matrix protein of measles virus. J Virol. 1987 Feb;61(2):584–589. doi: 10.1128/jvi.61.2.584-589.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C., Carbone K. M., Lipkin W. I. Molecular characterization of the Borna disease agent. Virology. 1990 Dec;179(2):853–856. doi: 10.1016/0042-6822(90)90154-j. [DOI] [PubMed] [Google Scholar]




