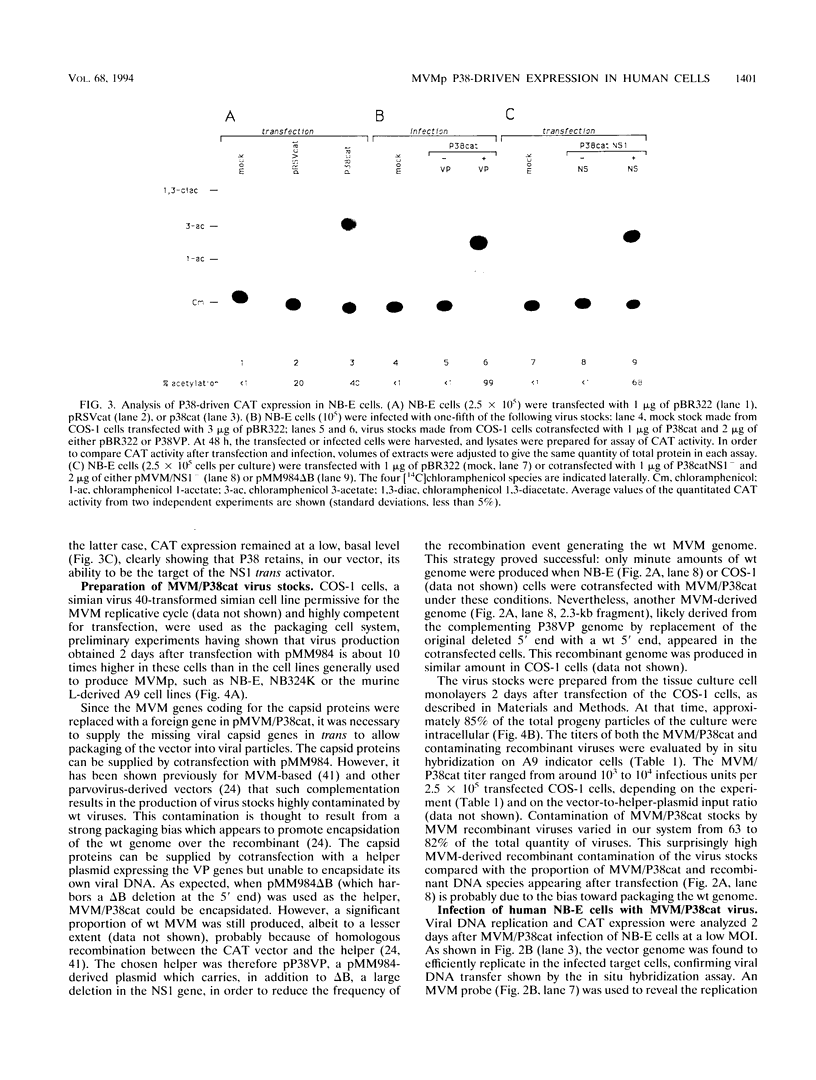
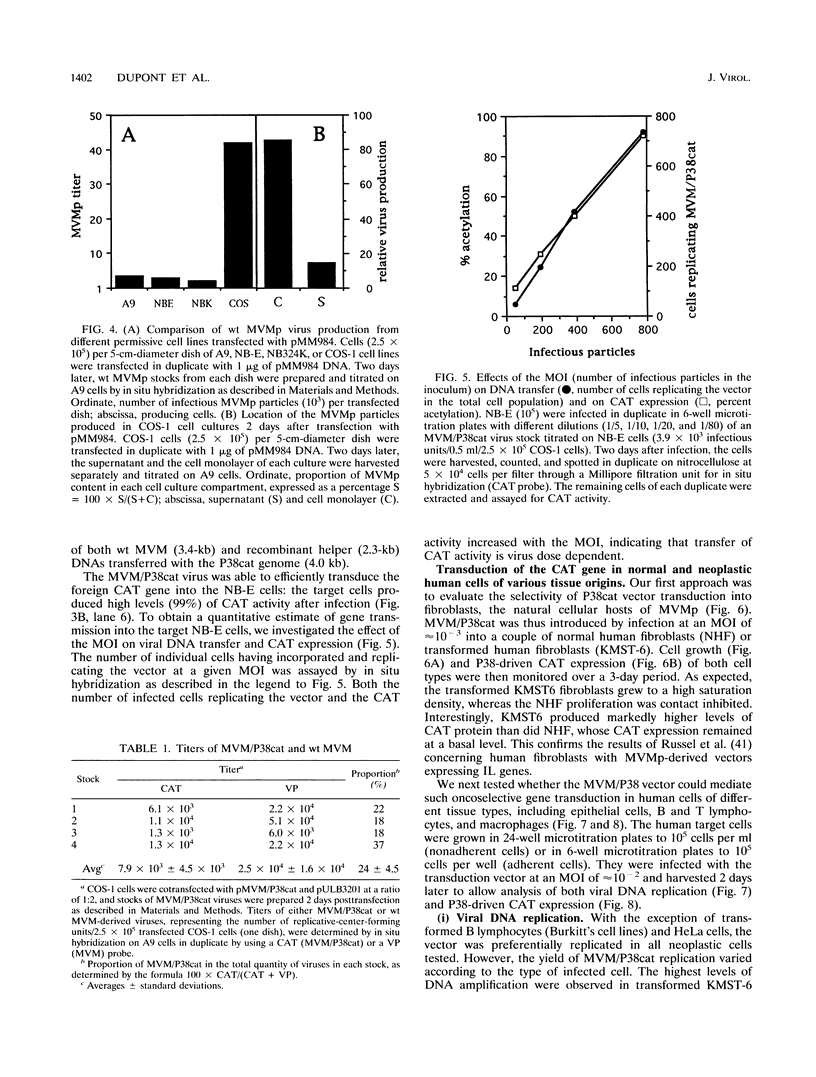
Abstract
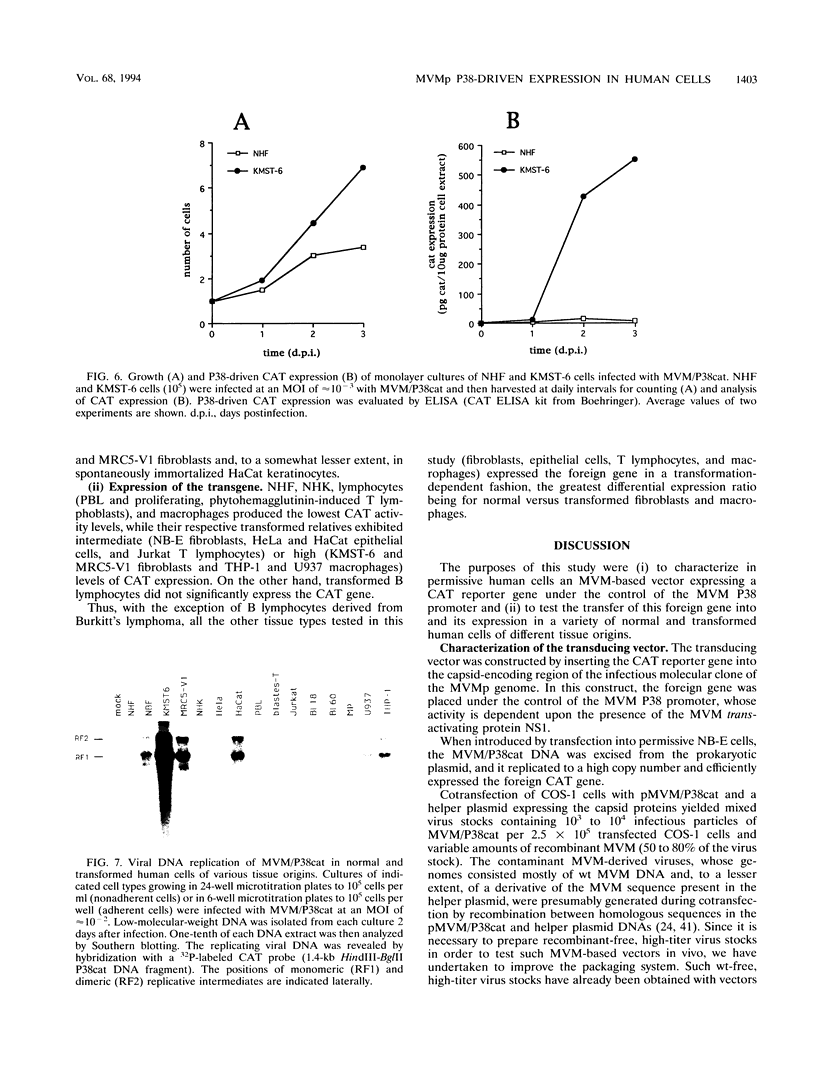
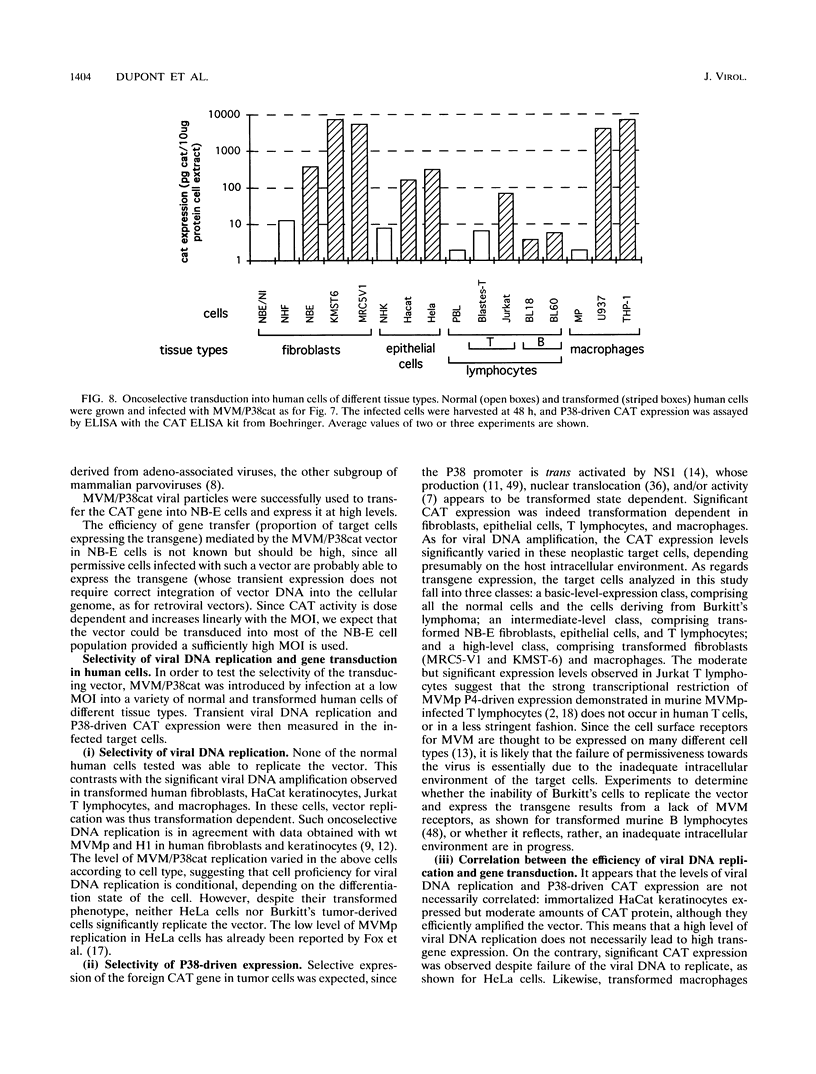
In this work, we report the transduction of a chloramphenicol acetyltransferase (CAT) reporter gene into a variety of normal and transformed human cells of various tissue origins. The vector used was MVM/P38cat, a recombinant of the prototype strain of the autonomous parvovirus minute virus of mice (MVMp). The CAT gene was inserted into the capsid-encoding region of the infectious molecular clone of MVMp genome, under the control of the MVM P38 promoter. When used to transfect permissive cells, the MVM/P38cat DNA was efficiently replicated and expressed the foreign CAT gene at high levels. By cotransfecting with a helper plasmid expressing the capsid proteins, it was possible to produce mixed virus stocks containing MVM/P38cat infectious particles and variable amounts of recombinant MVM. MVM/P38cat viral particles were successfully used to transfer the CAT gene and to express it in a variety of human cells. Both viral DNA replication and P38-driven CAT expression were achieved in fibroblasts, epithelial cells, T lymphocytes, and macrophages in a transformation-dependent way, but with an efficiency depending on the cell type. In transformed B lymphocytes, however, the vector was not replicated, nor did it express the CAT gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Antonietti J. P., Sahli R., Beard P., Hirt B. Characterization of the cell type-specific determinant in the genome of minute virus of mice. J Virol. 1988 Feb;62(2):552–557. doi: 10.1128/jvi.62.2.552-557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy R., Astell C. R. An Escherichia coli recBCsbcBrecF host permits the deletion-resistant propagation of plasmid clones containing the 5'-terminal palindrome of minute virus of mice. Gene. 1985;35(1-2):179–185. doi: 10.1016/0378-1119(85)90170-2. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburger A., Legendre D., Avalosse B., Rommelaere J. NS-1 and NS-2 proteins may act synergistically in the cytopathogenicity of parvovirus MVMp. Virology. 1990 Feb;174(2):576–584. doi: 10.1016/0042-6822(90)90110-d. [DOI] [PubMed] [Google Scholar]
- Brownstein D. G., Smith A. L., Johnson E. A., Pintel D. J., Naeger L. K., Tattersall P. The pathogenesis of infection with minute virus of mice depends on expression of the small nonstructural protein NS2 and on the genotype of the allotropic determinants VP1 and VP2. J Virol. 1992 May;66(5):3118–3124. doi: 10.1128/jvi.66.5.3118-3124.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Perros M., Brandenburger A., Spegelaere P., Rommelaere J. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding non-structural proteins. EMBO J. 1990 Sep;9(9):2989–2995. doi: 10.1002/j.1460-2075.1990.tb07491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Johnson P. R., Wong K. K., Jr Dual-target inhibition of HIV-1 in vitro by means of an adeno-associated virus antisense vector. Science. 1992 Nov 27;258(5087):1485–1488. doi: 10.1126/science.1359646. [DOI] [PubMed] [Google Scholar]
- Chen Y. Q., Tuynder M. C., Cornelis J. J., Boukamp P., Fusenig N. E., Rommelaere J. Sensitization of human keratinocytes to killing by parvovirus H-1 takes place during their malignant transformation but does not require them to be tumorigenic. Carcinogenesis. 1989 Jan;10(1):163–167. doi: 10.1093/carcin/10.1.163. [DOI] [PubMed] [Google Scholar]
- Clemens K. E., Pintel D. Minute virus of mice (MVM) mRNAs predominantly polyadenylate at a single site. Virology. 1987 Oct;160(2):511–514. doi: 10.1016/0042-6822(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Cornelis J. J., Becquart P., Duponchel N., Salomé N., Avalosse B. L., Namba M., Rommelaere J. Transformation of human fibroblasts by ionizing radiation, a chemical carcinogen, or simian virus 40 correlates with an increase in susceptibility to the autonomous parvoviruses H-1 virus and minute virus of mice. J Virol. 1988 May;62(5):1679–1686. doi: 10.1128/jvi.62.5.1679-1686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis J. J., Chen Y. Q., Spruyt N., Duponchel N., Cotmore S. F., Tattersall P., Rommelaere J. Susceptibility of human cells to killing by the parvoviruses H-1 and minute virus of mice correlates with viral transcription. J Virol. 1990 Jun;64(6):2537–2544. doi: 10.1128/jvi.64.6.2537-2544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Doerig C., Hirt B., Beard P., Antonietti J. P. Minute virus of mice non-structural protein NS-1 is necessary and sufficient for trans-activation of the viral P39 promoter. J Gen Virol. 1988 Oct;69(Pt 10):2563–2573. doi: 10.1099/0022-1317-69-10-2563. [DOI] [PubMed] [Google Scholar]
- Faust E. A., Ward D. C. Incomplete genomes of the parvovirus minute virus of mice: selective conservation of genome termini, including the origin for DNA replication. J Virol. 1979 Oct;32(1):276–292. doi: 10.1128/jvi.32.1.276-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fox E., Moen P. T., Jr, Bodnar J. W. Replication of minute virus of mice DNA in adenovirus-infected or adenovirus-transformed cells. Virology. 1990 Jun;176(2):403–412. doi: 10.1016/0042-6822(90)90010-o. [DOI] [PubMed] [Google Scholar]
- Gardiner E. M., Tattersall P. Evidence that developmentally regulated control of gene expression by a parvoviral allotropic determinant is particle mediated. J Virol. 1988 May;62(5):1713–1722. doi: 10.1128/jvi.62.5.1713-1722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Griffin G. E., Leung K., Folks T. M., Kunkel S., Nabel G. J. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature. 1989 May 4;339(6219):70–73. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L., Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huschtscha L. I., Holliday R. Limited and unlimited growth of SV40-transformed cells from human diploid MRC-5 fibroblasts. J Cell Sci. 1983 Sep;63:77–99. doi: 10.1242/jcs.63.1.77. [DOI] [PubMed] [Google Scholar]
- Jongeneel C. V., Sahli R., McMaster G. K., Hirt B. A precise map of splice junctions in the mRNAs of minute virus of mice, an autonomous parvovirus. J Virol. 1986 Sep;59(3):564–573. doi: 10.1128/jvi.59.3.564-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. THREE DEGREES OF GUANYLIC ACID--INOSINIC ACID PYROPHOSPHORYLASE DEFICIENCY IN MOUSE FIBROBLASTS. Nature. 1964 Sep 12;203:1142–1144. doi: 10.1038/2031142a0. [DOI] [PubMed] [Google Scholar]
- Lenoir G. M., Vuillaume M., Bonnardel C. The use of lymphomatous and lymphoblastoid cell lines in the study of Burkitt's lymphoma. IARC Sci Publ. 1985;(60):309–318. [PubMed] [Google Scholar]
- Merchlinsky M. J., Tattersall P. J., Leary J. J., Cotmore S. F., Gardiner E. M., Ward D. C. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice. J Virol. 1983 Jul;47(1):227–232. doi: 10.1128/jvi.47.1.227-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. R., Ward D. C. Three splicing patterns are used to excise the small intron common to all minute virus of mice RNAs. J Virol. 1986 Dec;60(3):1170–1174. doi: 10.1128/jvi.60.3.1170-1174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba M., Nishitani K., Hyodoh F., Fukushima F., Kimoto T. Neoplastic transformation of human diploid fibroblasts (KMST-6) by treatment with 60Co gamma rays. Int J Cancer. 1985 Feb 15;35(2):275–280. doi: 10.1002/ijc.2910350221. [DOI] [PubMed] [Google Scholar]
- Pintel D., Dadachanji D., Astell C. R., Ward D. C. The genome of minute virus of mice, an autonomous parvovirus, encodes two overlapping transcription units. Nucleic Acids Res. 1983 Feb 25;11(4):1019–1038. doi: 10.1093/nar/11.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz B. A., Ramabhadran T. V., Pintel D. The p39 promoter of minute virus of mice directs high levels of bovine growth hormone gene expression in the bovine papilloma virus shuttle vector. Gene. 1987;56(2-3):297–300. doi: 10.1016/0378-1119(87)90147-8. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus replication in normal and transformed human cells correlates with the nuclear translocation of the early protein NS1. J Virol. 1989 Jan;63(1):349–355. doi: 10.1128/jvi.63.1.349-355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R., Linser P., Armentrout R. W. Kinetics of assembly of a parvovirus, minute virus of mice, in synchronized rat brain cells. J Virol. 1977 Jun;22(3):778–793. doi: 10.1128/jvi.22.3.778-793.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Cornelis J. J. Antineoplastic activity of parvoviruses. J Virol Methods. 1991 Aug;33(3):233–251. doi: 10.1016/0166-0934(91)90024-t. [DOI] [PubMed] [Google Scholar]
- Russell S. J., Brandenburger A., Flemming C. L., Collins M. K., Rommelaere J. Transformation-dependent expression of interleukin genes delivered by a recombinant parvovirus. J Virol. 1992 May;66(5):2821–2828. doi: 10.1128/jvi.66.5.2821-2828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. J. Lymphokine gene therapy for cancer. Immunol Today. 1990 Jun;11(6):196–200. doi: 10.1016/0167-5699(90)90081-j. [DOI] [PubMed] [Google Scholar]
- SHEIN H. M., ENDERS J. F. Multiplication and cytopathogenicity of Simian vacuolating virus 40 in cultures of human tissues. Proc Soc Exp Biol Med. 1962 Mar;109:495–500. doi: 10.3181/00379727-109-27246. [DOI] [PubMed] [Google Scholar]
- Salvino R., Skiadopoulos M., Faust E. A., Tam P., Shade R. O., Astell C. R. Two spatially distinct genetic elements constitute a bipartite DNA replication origin in the minute virus of mice genome. J Virol. 1991 Mar;65(3):1352–1363. doi: 10.1128/jvi.65.3.1352-1363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider U., Schwenk H. U., Bornkamm G. Characterization of EBV-genome negative "null" and "T" cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1977 May 15;19(5):621–626. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spalholz B. A., Tattersall P. Interaction of minute virus of mice with differentiated cells: strain-dependent target cell specificity is mediated by intracellular factors. J Virol. 1983 Jun;46(3):937–943. doi: 10.1128/jvi.46.3.937-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spegelaere P., van Hille B., Spruyt N., Faisst S., Cornelis J. J., Rommelaere J. Initiation of transcription from the minute virus of mice P4 promoter is stimulated in rat cells expressing a c-Ha-ras oncogene. J Virol. 1991 Sep;65(9):4919–4928. doi: 10.1128/jvi.65.9.4919-4928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOOLAN H. W., SAUNDERS E. L., SOUTHAM C. M., MOORE A. E., LEVIN A. G. H-1 VIRUS VIREMIA IN THE HUMAN. Proc Soc Exp Biol Med. 1965 Jul;119:711–715. doi: 10.3181/00379727-119-30278. [DOI] [PubMed] [Google Scholar]
- Tenenbaum L., Dupont F., Spegelaere P., Zentilin L., Norio P., Giacca M., Riva S., Falaschi A., Rommelaere J. Inhibition of heterologous DNA replication by the MVMp nonstructural NS-1 protein: identification of a target sequence. Virology. 1993 Dec;197(2):630–641. doi: 10.1006/viro.1993.1638. [DOI] [PubMed] [Google Scholar]
- Tuynder M., Godfrine S., Cornelis J. J., Rommelaere J. Dose-dependent induction of resistance to terminal differentiation in x-irradiated cultures of normal human keratinocytes. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2638–2642. doi: 10.1073/pnas.88.7.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]