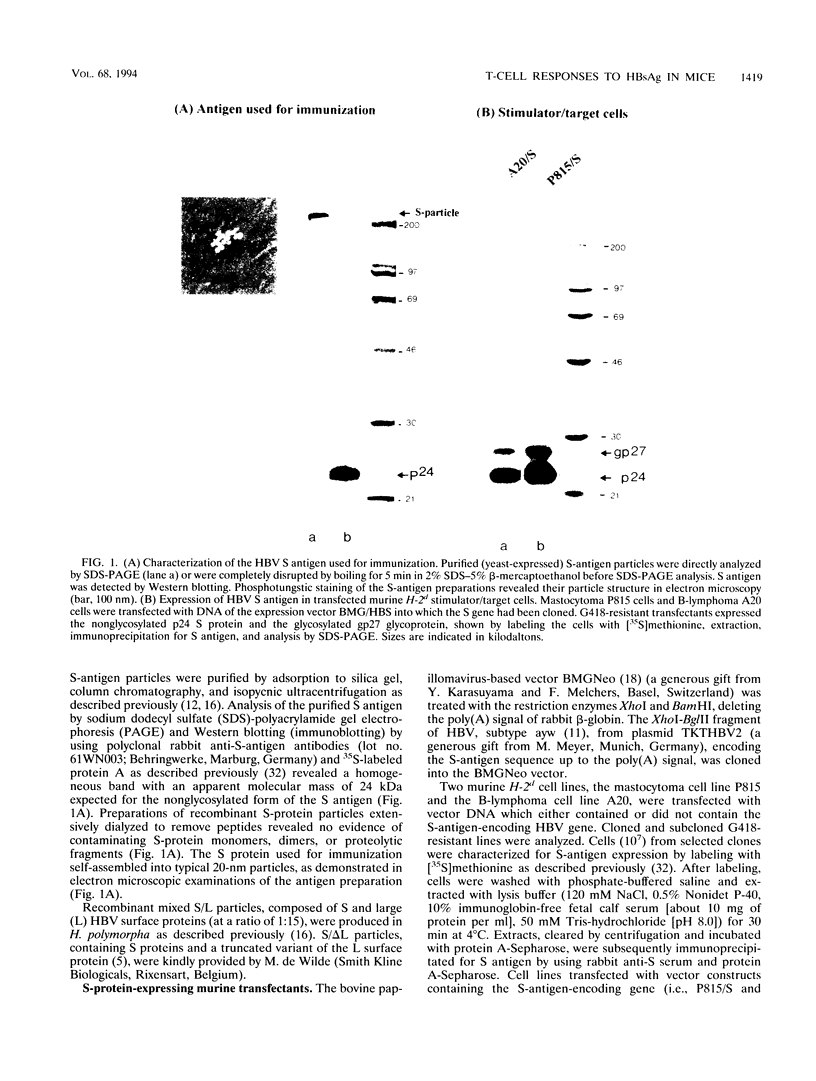
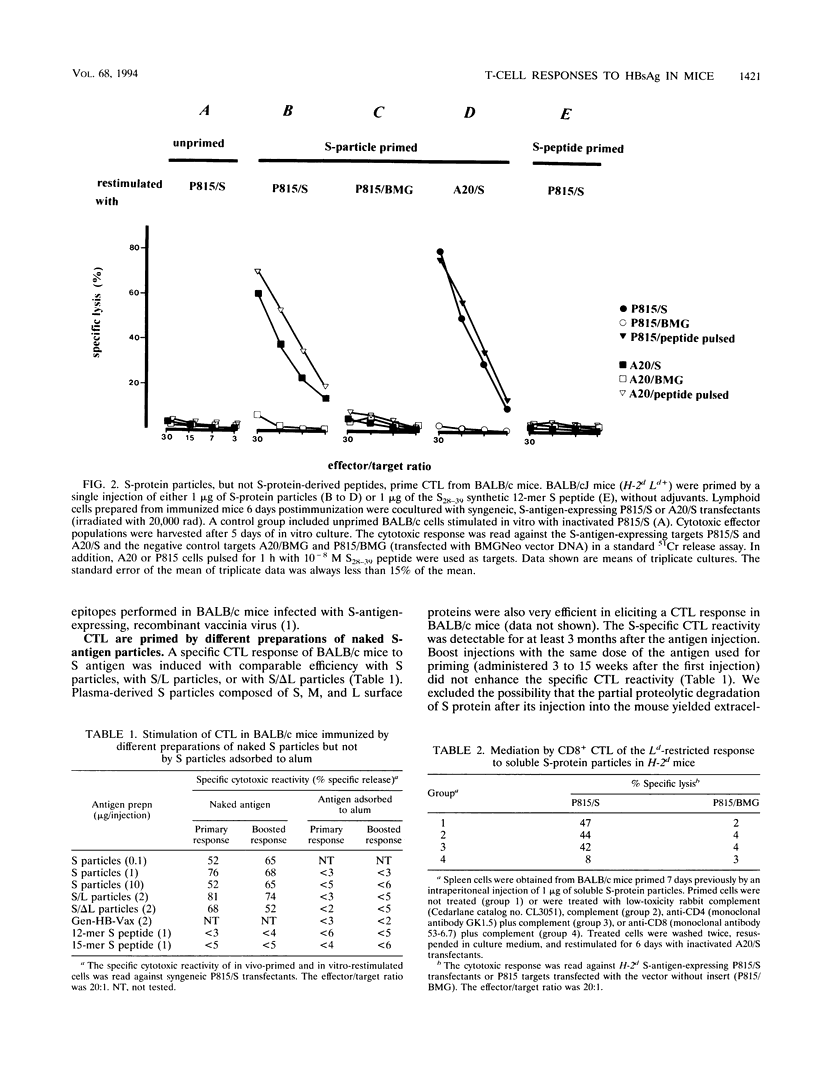
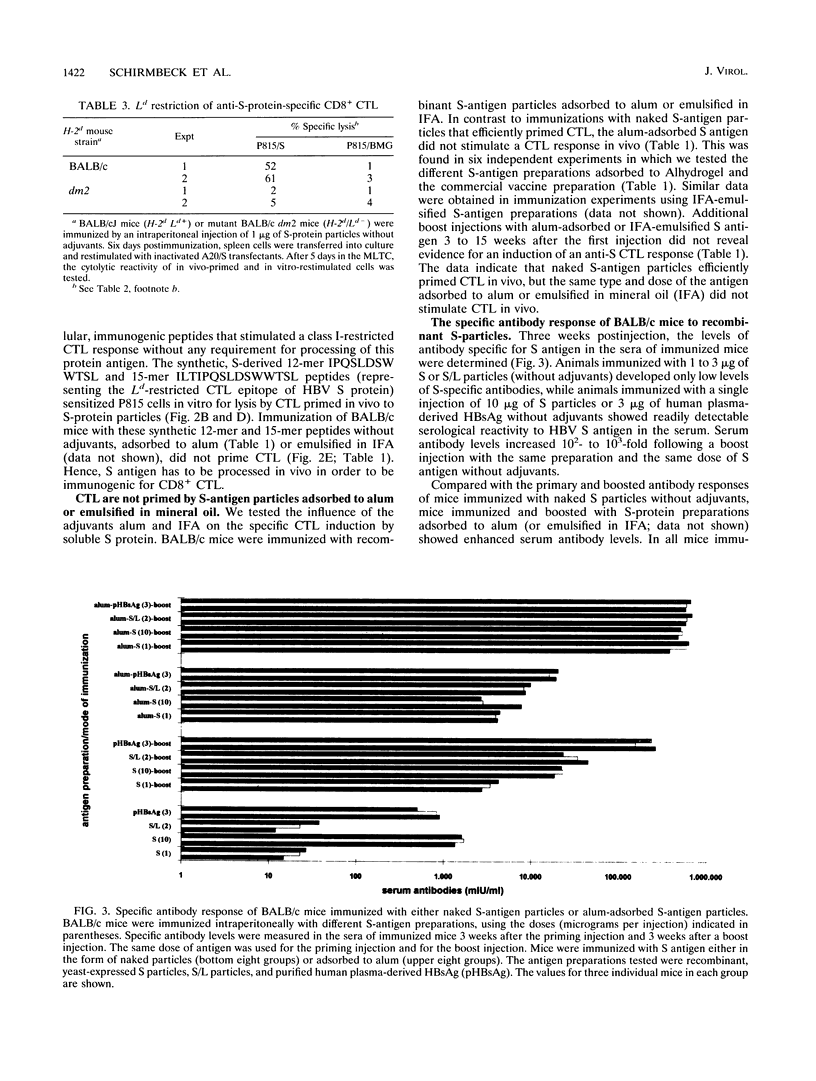
Abstract
Immune responses to components of hepatitis B virus (HBV) are assumed to play an essential role not only in the elimination of the virus but also in the pathogenesis of HBV-induced hepatitis. Protective humoral immunity to HBV is mediated by immune responses to HBV surface antigen (HBsAg). It is important to know which HBsAg preparations induce which type of cellular and humoral immune responses under which immunization conditions. We studied in BALB/c mice the humoral (antibody) response and the class I-restricted cytotoxic T-lymphocyte (CTL) response to different preparations of HBsAg particles: recombinant, small protein particles; plasma-derived, mixed particles formed by large, medium, and small surface proteins; and different preparations of recombinant, mixed particles formed by large and small surface proteins. Specific antibody levels appeared in the sera of immunized mice 2 to 3 weeks after immunization and were correlated with the antigen dose used for priming. HBsAg-specific antibody levels were enhanced by boost injections or by adsorbing the antigen to aluminum hydroxide. Injected in particulate form without adjuvants in the dose range of 0.1 to 10 micrograms per mouse, all HBsAg preparations tested efficiently primed specific CD8+ CTL of defined restriction and epitope specificity. Specific CTL reactivity was detectable from 5 days to more than 4 months postimmunization. In the dose range tested, it was independent of the antigen dose used for immunization and not enhanced by repeated boost injections. CTL were not elicited by HBsAg adsorbed to aluminum hydroxide. We have thus defined conditions under which HBsAg induced preferentially either a cellular immune response or a humoral immune response. These findings may be relevant for the interpretation of HBV-associated immunopathologic phenomena.
Full text
PDF

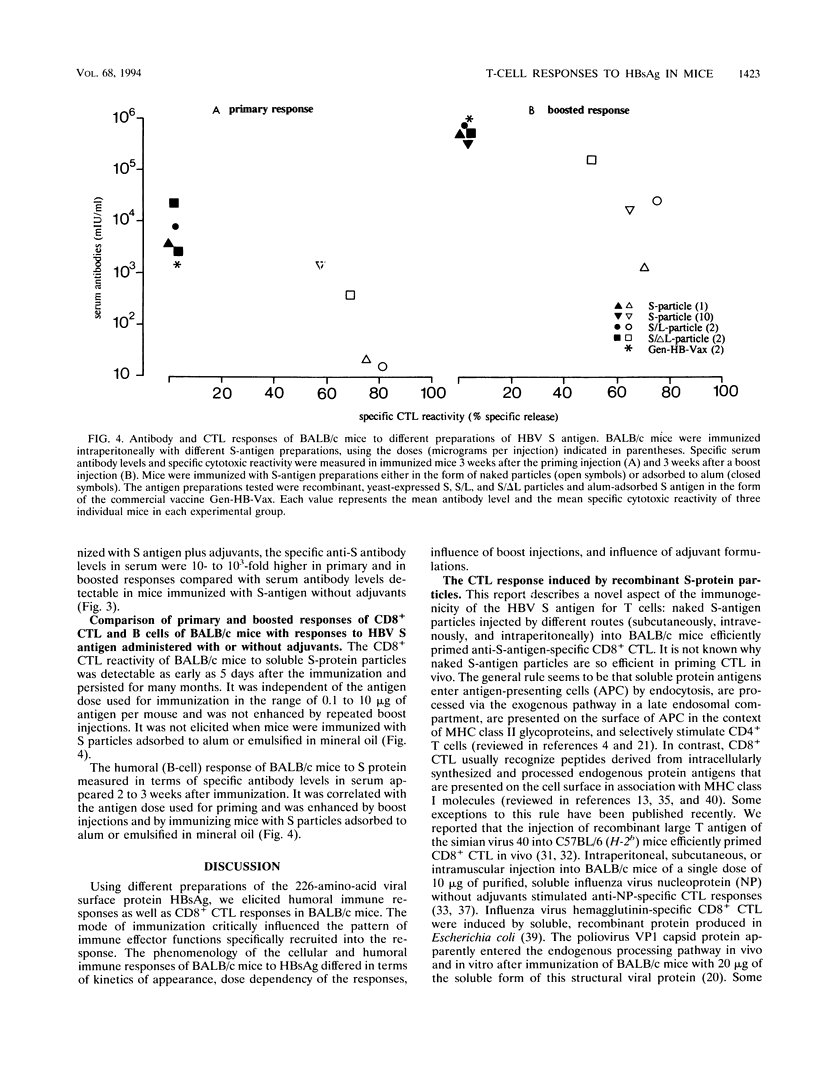


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando K., Moriyama T., Guidotti L. G., Wirth S., Schreiber R. D., Schlicht H. J., Huang S. N., Chisari F. V. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med. 1993 Nov 1;178(5):1541–1554. doi: 10.1084/jem.178.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnaba V., Franco A., Alberti A., Balsano C., Benvenuto R., Balsano F. Recognition of hepatitis B virus envelope proteins by liver-infiltrating T lymphocytes in chronic HBV infection. J Immunol. 1989 Oct 15;143(8):2650–2655. [PubMed] [Google Scholar]
- Barnaba V., Franco A., Alberti A., Benvenuto R., Balsano F. Selective killing of hepatitis B envelope antigen-specific B cells by class I-restricted, exogenous antigen-specific T lymphocytes. Nature. 1990 May 17;345(6272):258–260. doi: 10.1038/345258a0. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Guagliardi L. E. The cell biology of antigen processing and presentation. Annu Rev Immunol. 1991;9:707–744. doi: 10.1146/annurev.iy.09.040191.003423. [DOI] [PubMed] [Google Scholar]
- Collins D. S., Findlay K., Harding C. V. Processing of exogenous liposome-encapsulated antigens in vivo generates class I MHC-restricted T cell responses. J Immunol. 1992 Jun 1;148(11):3336–3341. [PubMed] [Google Scholar]
- Deres K., Schild H., Wiesmüller K. H., Jung G., Rammensee H. G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989 Nov 30;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Franco A., Paroli M., Testa U., Benvenuto R., Peschle C., Balsano F., Barnaba V. Transferrin receptor mediates uptake and presentation of hepatitis B envelope antigen by T lymphocytes. J Exp Med. 1992 May 1;175(5):1195–1205. doi: 10.1084/jem.175.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Gellissen G., Janowicz Z. A., Merckelbach A., Piontek M., Keup P., Weydemann U., Hollenberg C. P., Strasser A. W. Heterologous gene expression in Hansenula polymorpha: efficient secretion of glucoamylase. Biotechnology (N Y) 1991 Mar;9(3):291–295. doi: 10.1038/nbt0391-291. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Margulies D. H. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- Griffiths J. C., Harris S. J., Layton G. T., Berrie E. L., French T. J., Burns N. R., Adams S. E., Kingsman A. J. Hybrid human immunodeficiency virus Gag particles as an antigen carrier system: induction of cytotoxic T-cell and humoral responses by a Gag:V3 fusion. J Virol. 1993 Jun;67(6):3191–3198. doi: 10.1128/jvi.67.6.3191-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofnagle J. H., Shafritz D. A., Popper H. Chronic type B hepatitis and the "healthy" HBsAg carrier state. Hepatology. 1987 Jul-Aug;7(4):758–763. doi: 10.1002/hep.1840070424. [DOI] [PubMed] [Google Scholar]
- Janowicz Z. A., Melber K., Merckelbach A., Jacobs E., Harford N., Comberbach M., Hollenberg C. P. Simultaneous expression of the S and L surface antigens of hepatitis B, and formation of mixed particles in the methylotrophic yeast, Hansenula polymorpha. Yeast. 1991 Jul;7(5):431–443. doi: 10.1002/yea.320070502. [DOI] [PubMed] [Google Scholar]
- Jin Y., Shih W. K., Berkower I. Human T cell response to the surface antigen of hepatitis B virus (HBsAg). Endosomal and nonendosomal processing pathways are accessible to both endogenous and exogenous antigen. J Exp Med. 1988 Jul 1;168(1):293–306. doi: 10.1084/jem.168.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Brandt R. M., Melief C. J. Strict peptide length is not required for the induction of cytotoxic T lymphocyte-mediated antiviral protection by peptide vaccination. Eur J Immunol. 1993 May;23(5):1189–1192. doi: 10.1002/eji.1830230534. [DOI] [PubMed] [Google Scholar]
- Kutubuddin M., Simons J., Chow M. Poliovirus-specific major histocompatibility complex class I-restricted cytolytic T-cell epitopes in mice localize to neutralizing antigenic regions. J Virol. 1992 Oct;66(10):5967–5974. doi: 10.1128/jvi.66.10.5967-5974.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- Lopes L. M., Chain B. M. Liposome-mediated delivery stimulates a class I-restricted cytotoxic T cell response to soluble antigen. Eur J Immunol. 1992 Jan;22(1):287–290. doi: 10.1002/eji.1830220143. [DOI] [PubMed] [Google Scholar]
- Martinon F., Gras-Masse H., Boutillon C., Chirat F., Deprez B., Guillet J. G., Gomard E., Tartar A., Levy J. P. Immunization of mice with lipopeptides bypasses the prerequisite for adjuvant. Immune response of BALB/c mice to human immunodeficiency virus envelope glycoprotein. J Immunol. 1992 Nov 15;149(10):3416–3422. [PubMed] [Google Scholar]
- Moriyama T., Guilhot S., Klopchin K., Moss B., Pinkert C. A., Palmiter R. D., Brinster R. L., Kanagawa O., Chisari F. V. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science. 1990 Apr 20;248(4953):361–364. doi: 10.1126/science.1691527. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Donachie A. M., Reid G., Jarrett O. Immune-stimulating complexes containing Quil A and protein antigen prime class I MHC-restricted T lymphocytes in vivo and are immunogenic by the oral route. Immunology. 1991 Mar;72(3):317–322. [PMC free article] [PubMed] [Google Scholar]
- Nair S., Zhou X., Huang L., Rouse B. T. Class I restricted CTL recognition of a soluble protein delivered by liposomes containing lipophilic polylysines. J Immunol Methods. 1992 Aug 10;152(2):237–243. doi: 10.1016/0022-1759(92)90145-j. [DOI] [PubMed] [Google Scholar]
- Newman M. J., Wu J. Y., Gardner B. H., Munroe K. J., Leombruno D., Recchia J., Kensil C. R., Coughlin R. T. Saponin adjuvant induction of ovalbumin-specific CD8+ cytotoxic T lymphocyte responses. J Immunol. 1992 Apr 15;148(8):2357–2362. [PubMed] [Google Scholar]
- Reimann J., Bellan A., Conradt P. Development of autoreactive L3T4+ T cells from double-negative (L3T4-/Ly-2-) Thy-1+ spleen cells of normal mice. Eur J Immunol. 1988 Jul;18(7):989–999. doi: 10.1002/eji.1830180704. [DOI] [PubMed] [Google Scholar]
- Schild H., Norda M., Deres K., Falk K., Rötzschke O., Wiesmüller K. H., Jung G., Rammensee H. G. Fine specificity of cytotoxic T lymphocytes primed in vivo either with virus or synthetic lipopeptide vaccine or primed in vitro with peptide. J Exp Med. 1991 Dec 1;174(6):1665–1668. doi: 10.1084/jem.174.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmbeck R., Zerrahn J., Kuhröber A., Deppert W., Reimann J. Immunization of mice with the N-terminal (1-272) fragment of simian virus 40 large T antigen (without adjuvants) specifically primes cytotoxic T lymphocytes. Eur J Immunol. 1993 Jul;23(7):1528–1534. doi: 10.1002/eji.1830230720. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R., Zerrahn J., Kuhröber A., Kury E., Deppert W., Reimann J. Immunization with soluble simian virus 40 large T antigen induces a specific response of CD3+ CD4- CD8+ cytotoxic T lymphocytes in mice. Eur J Immunol. 1992 Mar;22(3):759–766. doi: 10.1002/eji.1830220320. [DOI] [PubMed] [Google Scholar]
- Stitz L., Schmitz C., Binder D., Zinkernagel R., Paoletti E., Becht H. Characterization and immunological properties of influenza A virus nucleoprotein (NP): cell-associated NP isolated from infected cells or viral NP expressed by vaccinia recombinant virus do not confer protection. J Gen Virol. 1990 May;71(Pt 5):1169–1179. doi: 10.1099/0022-1317-71-5-1169. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Takeshita T., Morein B., Putney S., Germain R. N., Berzofsky J. A. Induction of CD8+ cytotoxic T cells by immunization with purified HIV-1 envelope protein in ISCOMs. Nature. 1990 Apr 26;344(6269):873–875. doi: 10.1038/344873a0. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Wraith D. C., Vessey A. E., Askonas B. A. Purified influenza virus nucleoprotein protects mice from lethal infection. J Gen Virol. 1987 Feb;68(Pt 2):433–440. doi: 10.1099/0022-1317-68-2-433. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Gardner B. H., Murphy C. I., Seals J. R., Kensil C. R., Recchia J., Beltz G. A., Newman G. W., Newman M. J. Saponin adjuvant enhancement of antigen-specific immune responses to an experimental HIV-1 vaccine. J Immunol. 1992 Mar 1;148(5):1519–1525. [PubMed] [Google Scholar]
- Yamada A., Ziese M. R., Young J. F., Yamada Y. K., Ennis F. A. Influenza virus hemagglutinin-specific cytotoxic T cell response induced by polypeptide produced in Escherichia coli. J Exp Med. 1985 Aug 1;162(2):663–674. doi: 10.1084/jem.162.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. Cell biology of antigen processing and presentation to major histocompatibility complex class I molecule-restricted T lymphocytes. Adv Immunol. 1992;52:1–123. doi: 10.1016/s0065-2776(08)60875-5. [DOI] [PubMed] [Google Scholar]
- van Binnendijk R. S., van Baalen C. A., Poelen M. C., de Vries P., Boes J., Cerundolo V., Osterhaus A. D., UytdeHaag F. G. Measles virus transmembrane fusion protein synthesized de novo or presented in immunostimulating complexes is endogenously processed for HLA class I- and class II-restricted cytotoxic T cell recognition. J Exp Med. 1992 Jul 1;176(1):119–128. doi: 10.1084/jem.176.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]