Abstract
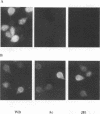
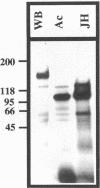
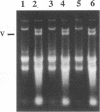
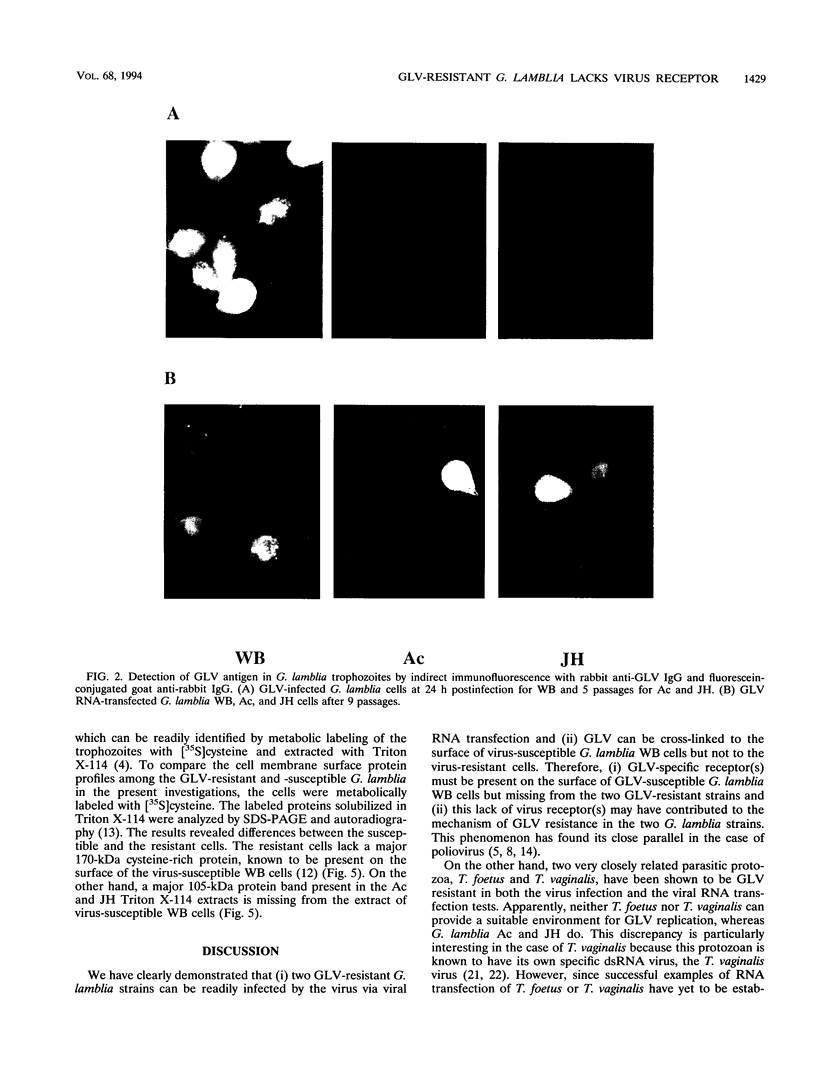
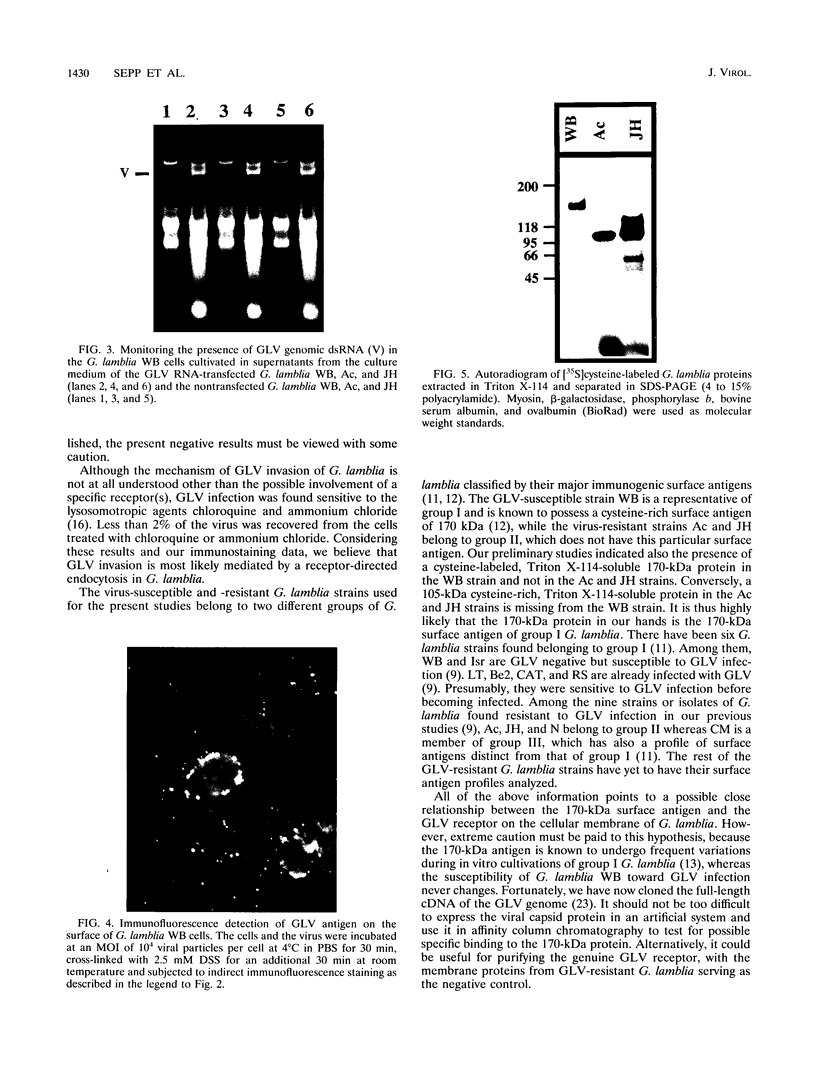
Giardia lamblia virus (GLV) is a small nonenveloped double-stranded RNA virus that infects specifically the parasitic protozoan G. lamblia. Among the many collected strains of G. lamblia, a few turn out to be highly resistant to the virus infection. Two of these strains, Ac and JH, were subjected to electroporation with the RNA from GLV-infected G. lamblia WB strain. Subsequent studies indicated the presence of GLV double-stranded RNA and GLV protein in the electroporated and propagated cells. Virus particles, released by the transfected cells into the culture medium, were capable of infecting the virus-sensitive G. lamblia WB strain. When the WB cells were incubated with GLV at 4 degrees C and treated with the bifunctional cross-linking reagent disuccinimidyl suberate, little GLV protein was detectable inside the cells by immunofluorescent staining. However, patches of fluorescent granules were found on the membrane surface of the cells, suggesting cross-linking of the viruses with a certain membrane component(s). Similar treatment of the resistant strains Ac and JH showed no fluorescence either inside or outside of the cells. Two other closely related parasitic protozoa, Tritrichomonas foetus and Trichomonas vaginalis, cannot be infected by GLV via either viral infection or RNA transfection. The [35S]cysteine-labeled protein profiles in Triton X-114 extracts of G. lamblia WB, Ac, and JH were compared. The profile of the WB strain differs clearly from that of Ac and JH. It remains to be seen, however, whether this difference is related at all to the different susceptibilities to GLV infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam R. D., Aggarwal A., Lal A. A., de La Cruz V. F., McCutchan T., Nash T. E. Antigenic variation of a cysteine-rich protein in Giardia lamblia. J Exp Med. 1988 Jan 1;167(1):109–118. doi: 10.1084/jem.167.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furfine E. S., Wang C. C. Transfection of the Giardia lamblia double-stranded RNA virus into giardia lamblia by electroporation of a single-stranded RNA copy of the viral genome. Mol Cell Biol. 1990 Jul;10(7):3659–3662. doi: 10.1128/mcb.10.7.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furfine E. S., White T. C., Wang A. L., Wang C. C. A single-stranded RNA copy of the Giardia lamblia virus double-stranded RNA genome is present in the infected Giardia lamblia. Nucleic Acids Res. 1989 Sep 25;17(18):7453–7467. doi: 10.1093/nar/17.18.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Hagblom P., Harwood J., Aley S. B., Reiner D. S., McCaffery M., So M., Guiney D. G. Isolation and expression of the gene for a major surface protein of Giardia lamblia. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4463–4467. doi: 10.1073/pnas.87.12.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C., SYVERTON J. T. The mammalian cell-virus relationship. IV. Infection of naturally insusceptible cells with enterovirus ribonucleic acid. J Exp Med. 1959 Jul 1;110(1):65–80. doi: 10.1084/jem.110.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz T. L. The recognition event between virus and host cell receptor: a target for antiviral agents. J Gen Virol. 1990 Apr;71(Pt 4):751–766. doi: 10.1099/0022-1317-71-4-751. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C., Johnson B., Lionetti K. A., Nobis P., Wimmer E., Racaniello V. R. Transformation of a human poliovirus receptor gene into mouse cells. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7845–7849. doi: 10.1073/pnas.83.20.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L., Wang A. L., Wang C. C. Identification of Giardia lamblia isolates susceptible and resistant to infection by the double-stranded RNA virus. Exp Parasitol. 1988 Jun;66(1):118–123. doi: 10.1016/0014-4894(88)90056-2. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Wang A. L., Wang C. C. Purification and characterization of the Giardia lamblia double-stranded RNA virus. Mol Biochem Parasitol. 1988 Apr;28(3):189–195. doi: 10.1016/0166-6851(88)90003-5. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Conrad J. T., Merritt J. W., Jr Variant specific epitopes of Giardia lamblia. Mol Biochem Parasitol. 1990 Aug;42(1):125–132. doi: 10.1016/0166-6851(90)90120-b. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Keister D. B. Differences in excretory-secretory products and surface antigens among 19 isolates of Giardia. J Infect Dis. 1985 Dec;152(6):1166–1171. doi: 10.1093/infdis/152.6.1166. [DOI] [PubMed] [Google Scholar]
- Nash T. Surface antigen variability and variation in Giardia lamblia. Parasitol Today. 1992 Jul;8(7):229–234. doi: 10.1016/0169-4758(92)90119-m. [DOI] [PubMed] [Google Scholar]
- Nobis P., Zibirre R., Meyer G., Kühne J., Warnecke G., Koch G. Production of a monoclonal antibody against an epitope on HeLa cells that is the functional poliovirus binding site. J Gen Virol. 1985 Dec;66(Pt 12):2563–2569. doi: 10.1099/0022-1317-66-12-2563. [DOI] [PubMed] [Google Scholar]
- Sawyer S. T., Krantz S. B., Luna J. Identification of the receptor for erythropoietin by cross-linking to Friend virus-infected erythroid cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3690–3694. doi: 10.1073/pnas.84.11.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai J. H., Ong S. J., Chang S. C., Su H. M. Giardiavirus enters Giardia lamblia WB trophozoite via endocytosis. Exp Parasitol. 1993 Mar;76(2):165–174. doi: 10.1006/expr.1993.1019. [DOI] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Contribution of a p75 interleukin 2 binding peptide to a high-affinity interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4215–4218. doi: 10.1073/pnas.84.12.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Miller R. L., Wang C. C. Antibodies to the Giardia lamblia double-stranded RNA virus major protein can block the viral infection. Mol Biochem Parasitol. 1988 Sep;30(3):225–232. doi: 10.1016/0166-6851(88)90091-6. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. Discovery of a specific double-stranded RNA virus in Giardia lamblia. Mol Biochem Parasitol. 1986 Dec;21(3):269–276. doi: 10.1016/0166-6851(86)90132-5. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7956–7960. doi: 10.1073/pnas.83.20.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. Viruses of the protozoa. Annu Rev Microbiol. 1991;45:251–263. doi: 10.1146/annurev.mi.45.100191.001343. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Yang H. M., Shen K. A., Wang C. C. Giardiavirus double-stranded RNA genome encodes a capsid polypeptide and a gag-pol-like fusion protein by a translation frameshift. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8595–8599. doi: 10.1073/pnas.90.18.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Double-stranded RNA replication in yeast: the killer system. Annu Rev Biochem. 1986;55:373–395. doi: 10.1146/annurev.bi.55.070186.002105. [DOI] [PubMed] [Google Scholar]
- Wright B. S., Tyler G. A., O'Brien R., Caporale L. H., Rosenblatt M. Immunoprecipitation of the parathyroid hormone receptor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):26–30. doi: 10.1073/pnas.84.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]