Abstract
Adenoviruses of subgroup C can establish persistent infections in human beings. The exact site of persistence has not been established, but lymphoid tissues are certainly one reservoir. Experimental evidence suggests that early transcription unit 3 (E3) of the virus is involved in this phenomenon. In particular, the most abundant protein of this region, the E3/19K protein, seems to fulfill an important role in viral escape from the immune response. We previously demonstrated that in nonlymphoid cells E3/19K interferes with the antigen presentation function of class I major histocompatibility complex (MHC) antigens by inhibiting their transport to the cell surface. However, the function of the E3 products in lymphoid cells was not investigated. To examine this, the T-lymphoma cell line Jurkat was transfected with a DNA fragment comprising the entire E3 region of adenovirus type 2. We show here that E3/19K is expressed in the absence of the viral transactivator E1A with a rate of biosynthesis similar to that in nonlymphoid 293 cells. Furthermore, inhibition of transport and down-regulation of MHC antigens was comparable in both cell lines. In contrast, various T-cell molecules containing immunoglobulin-like domains showed a normal expression pattern in the transfected cells. A detailed analysis of the interaction between E3/19K and the MHC class I antigens of Jurkat (HLA-A3 and HLA-B35) revealed a differential sensitivity for down-regulation by E3/19K. The data demonstrate that E3/19K exerts its function also in lymphoid cells without affecting other lymphoid cell surface molecules. The implications for persistence of adenovirus in lymphoid cells in vivo are discussed.
Full text
PDF

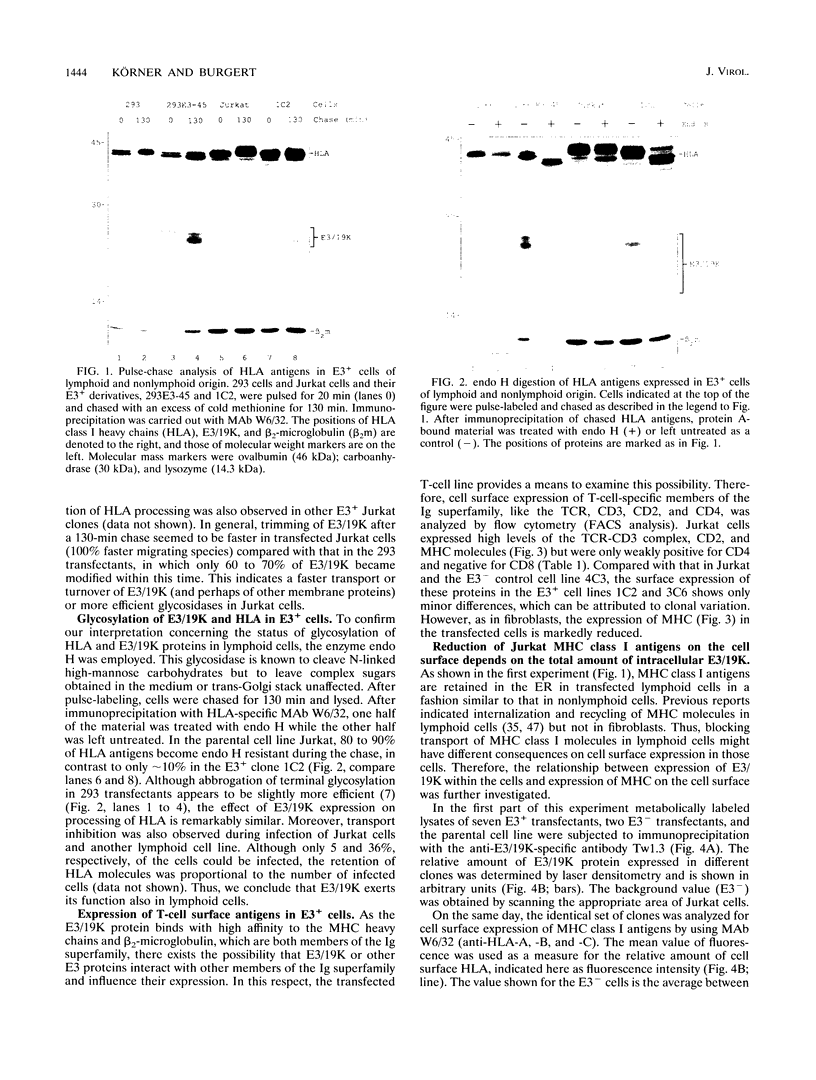
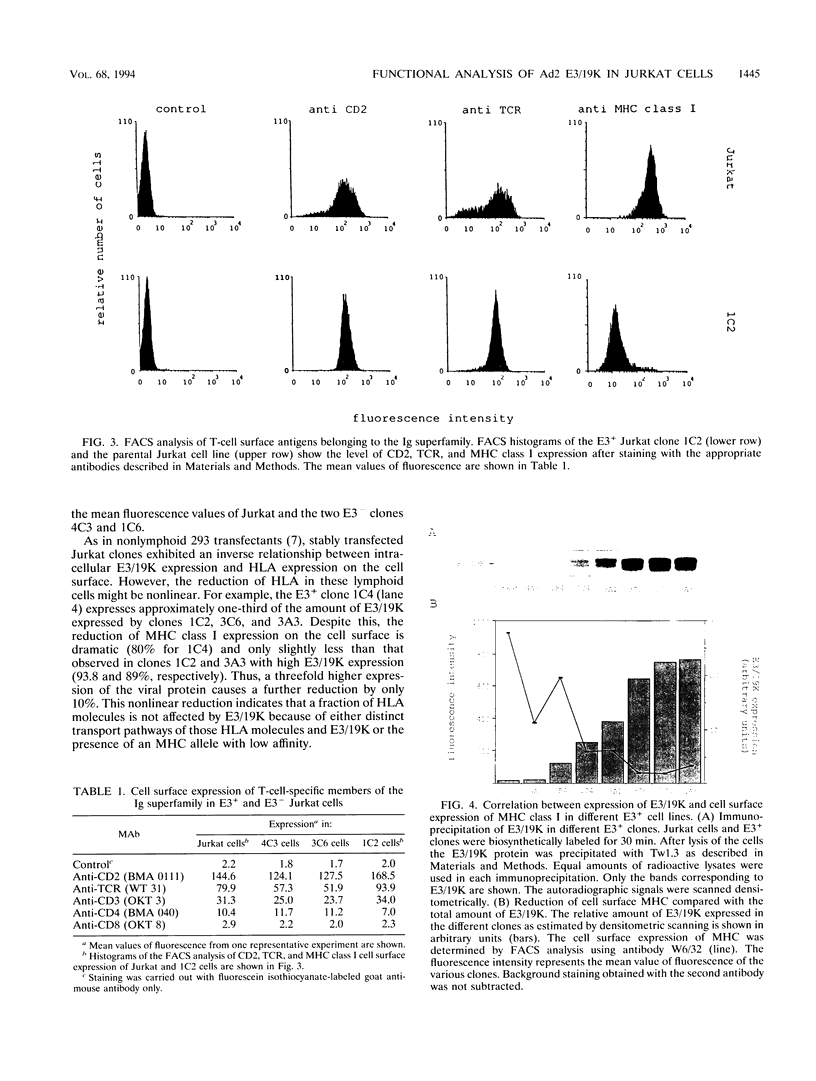
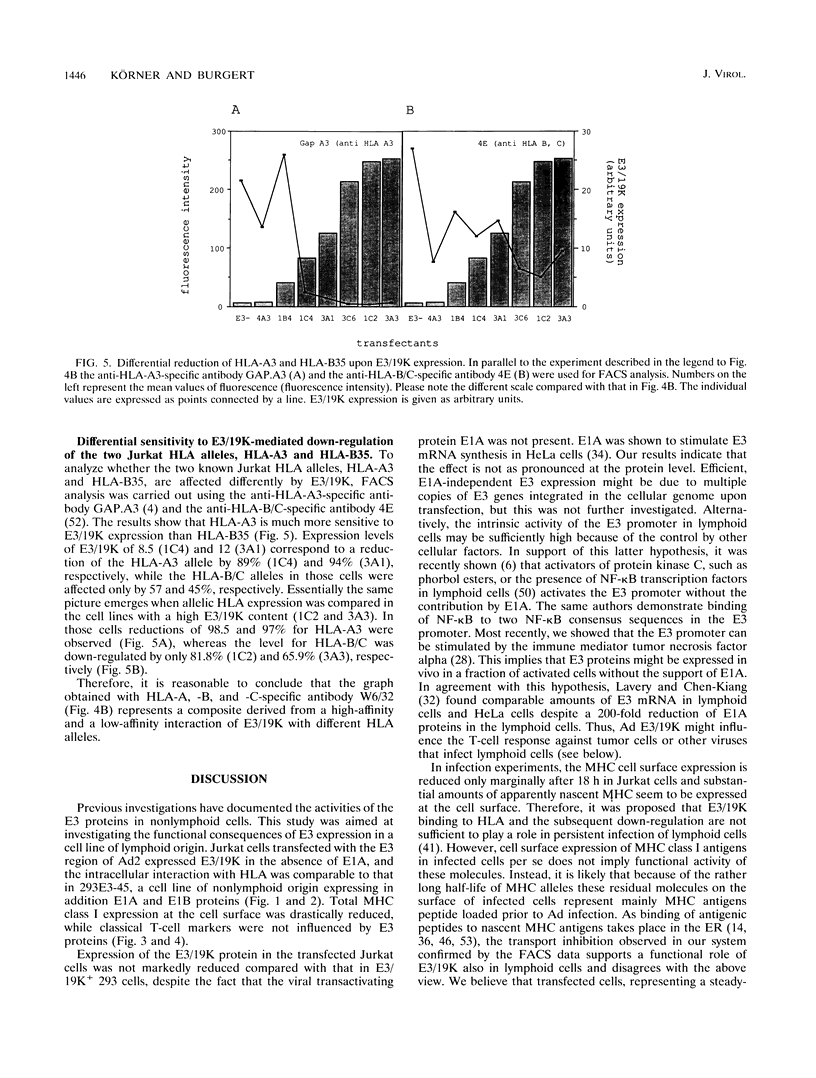


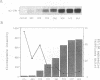
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M., McMichael A., Peterson P. A. Reduced allorecognition of adenovirus-2 infected cells. J Immunol. 1987 Jun 1;138(11):3960–3966. [PubMed] [Google Scholar]
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Berger A. E., Davis J. E., Cresswell P. Monoclonal antibody to HLA-A3. Hybridoma. 1982;1(2):87–90. doi: 10.1089/hyb.1.1982.1.87. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Buckbinder L., Miralles V. J., Reinberg D. TPA can overcome the requirement for EIa and together act synergistically in stimulating expression of the adenovirus EIII promoter. EMBO J. 1989 Dec 20;8(13):4239–4250. doi: 10.1002/j.1460-2075.1989.tb08609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. The E3/19K protein of adenovirus type 2 binds to the domains of histocompatibility antigens required for CTL recognition. EMBO J. 1987 Jul;6(7):2019–2026. doi: 10.1002/j.1460-2075.1987.tb02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H. G., Maryanski J. L., Kvist S. "E3/19K" protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H. G., White J., Weltzien H. U., Marrack P., Kappler J. W. Reactivity of V beta 17a+ CD8+ T cell hybrids. Analysis using a new CD8+ T cell fusion partner. J Exp Med. 1989 Dec 1;170(6):1887–1904. doi: 10.1084/jem.170.6.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin C. R., Tollefson A. E., Brady H. A., Hoffman B. L., Wold W. S. Epidermal growth factor receptor is down-regulated by a 10,400 MW protein encoded by the E3 region of adenovirus. Cell. 1989 Apr 7;57(1):135–144. doi: 10.1016/0092-8674(89)90179-7. [DOI] [PubMed] [Google Scholar]
- Chen I. S., Cann A. J., Shah N. P., Gaynor R. B. Functional relation between HTLV-II x and adenovirus E1A proteins in transcriptional activation. Science. 1985 Nov 1;230(4725):570–573. doi: 10.1126/science.2996140. [DOI] [PubMed] [Google Scholar]
- Cox J. H., Bennink J. R., Yewdell J. W. Retention of adenovirus E19 glycoprotein in the endoplasmic reticulum is essential to its ability to block antigen presentation. J Exp Med. 1991 Dec 1;174(6):1629–1637. doi: 10.1084/jem.174.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. H., Yewdell J. W., Eisenlohr L. C., Johnson P. R., Bennink J. R. Antigen presentation requires transport of MHC class I molecules from the endoplasmic reticulum. Science. 1990 Feb 9;247(4943):715–718. doi: 10.1126/science.2137259. [DOI] [PubMed] [Google Scholar]
- Fox J. P., Hall C. E., Cooney M. K. The Seattle Virus Watch. VII. Observations of adenovirus infections. Am J Epidemiol. 1977 Apr;105(4):362–386. doi: 10.1093/oxfordjournals.aje.a112394. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Aquino L., Duerksen-Hughes P. J., Day D., Horton T. M., Yei S. P., Wold W. S. The E1B 19,000-molecular-weight protein of group C adenoviruses prevents tumor necrosis factor cytolysis of human cells but not of mouse cells. J Virol. 1991 Jun;65(6):3083–3094. doi: 10.1128/jvi.65.6.3083-3094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Elmore L. W., Tollefson A. E., Brady H. A., Wold W. S. A 14,700 MW protein from the E3 region of adenovirus inhibits cytolysis by tumor necrosis factor. Cell. 1988 May 6;53(3):341–346. doi: 10.1016/0092-8674(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Gooding L. R., Ranheim T. S., Tollefson A. E., Aquino L., Duerksen-Hughes P., Horton T. M., Wold W. S. The 10,400- and 14,500-dalton proteins encoded by region E3 of adenovirus function together to protect many but not all mouse cell lines against lysis by tumor necrosis factor. J Virol. 1991 Aug;65(8):4114–4123. doi: 10.1128/jvi.65.8.4114-4123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S., Mackey J. K., Rigden P. Analysis of human tonsil and cancer DNAs and RNAs for DNA sequences of group C (serotypes 1, 2, 5, and 6) human adenoviruses. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6606–6610. doi: 10.1073/pnas.76.12.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Ishii A., Yonehara S. The E1b oncogene of adenovirus confers cellular resistance to cytotoxicity of tumor necrosis factor and monoclonal anti-Fas antibody. Int Immunol. 1991 Apr;3(4):343–351. doi: 10.1093/intimm/3.4.343. [DOI] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Chambon P. Repression of the immunoglobulin heavy chain enhancer by the adenovirus-2 E1A products. Science. 1985 Dec 20;230(4732):1391–1394. doi: 10.1126/science.2999984. [DOI] [PubMed] [Google Scholar]
- Horvath J., Palkonyay L., Weber J. Group C adenovirus DNA sequences in human lymphoid cells. J Virol. 1986 Jul;59(1):189–192. doi: 10.1128/jvi.59.1.189-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath J., Weber J. M. Nonpermissivity of human peripheral blood lymphocytes to adenovirus type 2 infection. J Virol. 1988 Jan;62(1):341–345. doi: 10.1128/jvi.62.1.341-345.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Ostberg L., Persson H., Philipson L., Peterson P. A. Molecular association between transplantation antigens and cell surface antigen in adenovirus-transformed cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5674–5678. doi: 10.1073/pnas.75.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner H., Fritzsche U., Burgert H. G. Tumor necrosis factor alpha stimulates expression of adenovirus early region 3 proteins: implications for viral persistence. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11857–11861. doi: 10.1073/pnas.89.24.11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambriex M., Van der Veen J. Comparison of replication of adenovirus type 2 and type 4 in human lymphocyte cultures. Infect Immun. 1976 Sep;14(3):618–622. doi: 10.1128/iai.14.3.618-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky R. D., Troy F. A. Possible DNA-RNA tumor virus interaction in human lymphomas: expression of retroviral proteins in Ramos lymphoma lines is enhanced after conversion with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1984 Jan;81(1):33–37. doi: 10.1073/pnas.81.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery D. J., Chen-Kiang S. Adenovirus E1A and E1B genes are regulated posttranscriptionally in human lymphoid cells. J Virol. 1990 Nov;64(11):5349–5359. doi: 10.1128/jvi.64.11.5349-5359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery D., Fu S. M., Lufkin T., Chen-Kiang S. Productive infection of cultured human lymphoid cells by adenovirus. J Virol. 1987 May;61(5):1466–1472. doi: 10.1128/jvi.61.5.1466-1472.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff T., Elkaim R., Goding C. R., Jalinot P., Sassone-Corsi P., Perricaudet M., Kédinger C., Chambon P. Individual products of the adenovirus 12S and 13S EIa mRNAs stimulate viral EIIa and EIII expression at the transcriptional level. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4381–4385. doi: 10.1073/pnas.81.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monos D. S., Cooper H. L. Rapid turnover of HLA proteins in quiescent lymphocytes: proposed connection with immunologic surveillance. J Immunol. 1983 Jul;131(1):341–346. [PubMed] [Google Scholar]
- Neefjes J. J., Schumacher T. N., Ploegh H. L. Assembly and intracellular transport of major histocompatibility complex molecules. Curr Opin Cell Biol. 1991 Aug;3(4):601–609. doi: 10.1016/0955-0674(91)90029-x. [DOI] [PubMed] [Google Scholar]
- Oettgen H. C., Kappler J., Tax W. J., Terhorst C. Characterization of the two heavy chains of the T3 complex on the surface of human T lymphocytes. J Biol Chem. 1984 Oct 10;259(19):12039–12048. [PubMed] [Google Scholar]
- Päbo S., Nilsson T., Peterson P. A. Adenoviruses of subgenera B, C, D, and E modulate cell-surface expression of major histocompatibility complex class I antigens. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9665–9669. doi: 10.1073/pnas.83.24.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawle F. C., Tollefson A. E., Wold W. S., Gooding L. R. Mouse anti-adenovirus cytotoxic T lymphocytes. Inhibition of lysis by E3 gp19K but not E3 14.7K. J Immunol. 1989 Sep 15;143(6):2031–2037. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routes J. M., Cook J. L. Resistance of human cells to the adenovirus E3 effect on class I MHC antigen expression. Implications for antiviral immunity. J Immunol. 1990 Apr 1;144(7):2763–2770. [PubMed] [Google Scholar]
- Severinsson L., Martens I., Peterson P. A. Differential association between two human MHC class I antigens and an adenoviral glycoprotein. J Immunol. 1986 Aug 1;137(3):1003–1009. [PubMed] [Google Scholar]
- Silver L., Anderson C. W. Interaction of human adenovirus serotype 2 with human lymphoid cells. Virology. 1988 Aug;165(2):377–387. doi: 10.1016/0042-6822(88)90582-x. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tollefson A. E., Stewart A. R., Yei S. P., Saha S. K., Wold W. S. The 10,400- and 14,500-dalton proteins encoded by region E3 of adenovirus form a complex and function together to down-regulate the epidermal growth factor receptor. J Virol. 1991 Jun;65(6):3095–3105. doi: 10.1128/jvi.65.6.3095-3105.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Tse D. B., Pernis B. Spontaneous internalization of Class I major histocompatibility complex molecules in T lymphoid cells. J Exp Med. 1984 Jan 1;159(1):193–207. doi: 10.1084/jem.159.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Sabbatini P., Debbas M., Wold W. S., Kusher D. I., Gooding L. R. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol Cell Biol. 1992 Jun;12(6):2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. L., Garcia J., Harrich D., Pearson L., Wu F., Gaynor R. Lymphoid specific gene expression of the adenovirus early region 3 promoter is mediated by NF-kappa B binding motifs. EMBO J. 1990 Dec;9(13):4435–4442. doi: 10.1002/j.1460-2075.1990.tb07894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Gooding L. R. Region E3 of adenovirus: a cassette of genes involved in host immunosurveillance and virus-cell interactions. Virology. 1991 Sep;184(1):1–8. doi: 10.1016/0042-6822(91)90815-s. [DOI] [PubMed] [Google Scholar]
- Yang S. Y., Morishima Y., Collins N. H., Alton T., Pollack M. S., Yunis E. J., Dupont B. Comparison of one-dimensional IEF patterns for serologically detectable HLA-A and B allotypes. Immunogenetics. 1984;19(3):217–231. doi: 10.1007/BF00364765. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. Cell biology of antigen processing and presentation to major histocompatibility complex class I molecule-restricted T lymphocytes. Adv Immunol. 1992;52:1–123. doi: 10.1016/s0065-2776(08)60875-5. [DOI] [PubMed] [Google Scholar]
- de Jong P. J., Valderrama G., Spigland I., Horwitz M. S. Adenovirus isolates from urine of patients with acquired immunodeficiency syndrome. Lancet. 1983 Jun 11;1(8337):1293–1296. doi: 10.1016/s0140-6736(83)92411-x. [DOI] [PubMed] [Google Scholar]
- van der Veen J., Lambriex M. Relationship of adenovirus to lymphocytes in naturally infected human tonsils and adenoids. Infect Immun. 1973 Apr;7(4):604–609. doi: 10.1128/iai.7.4.604-609.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]