Abstract
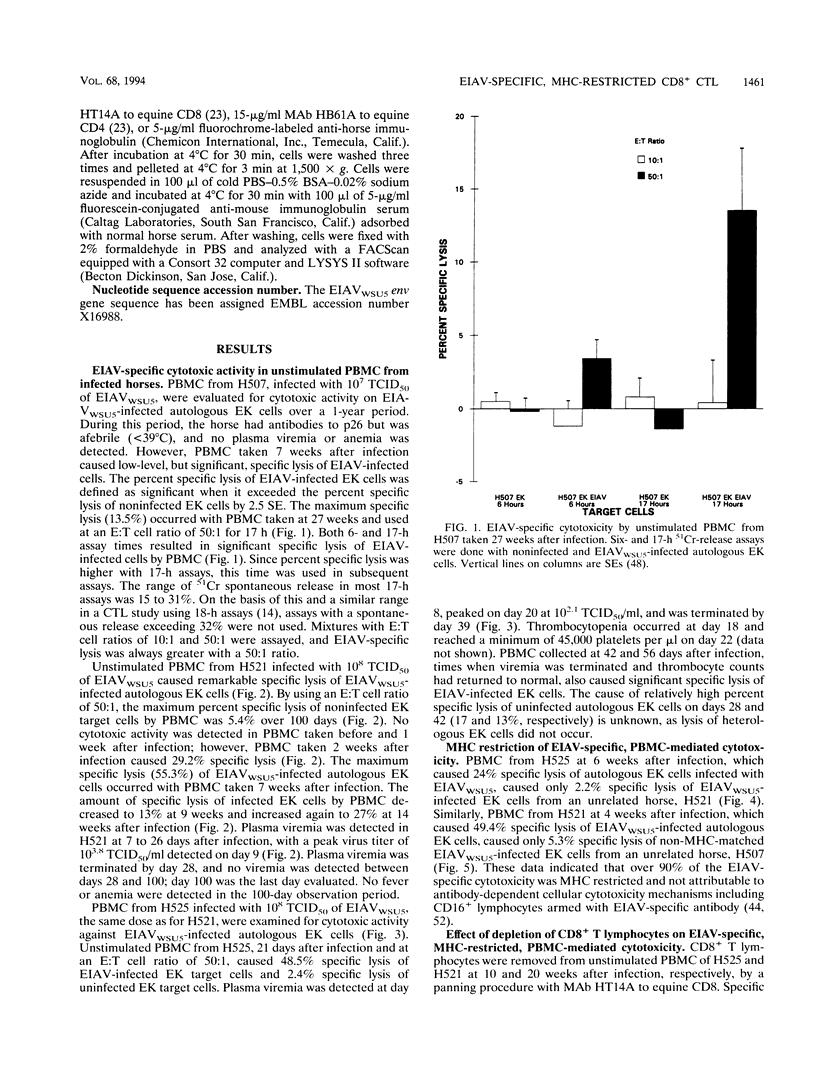
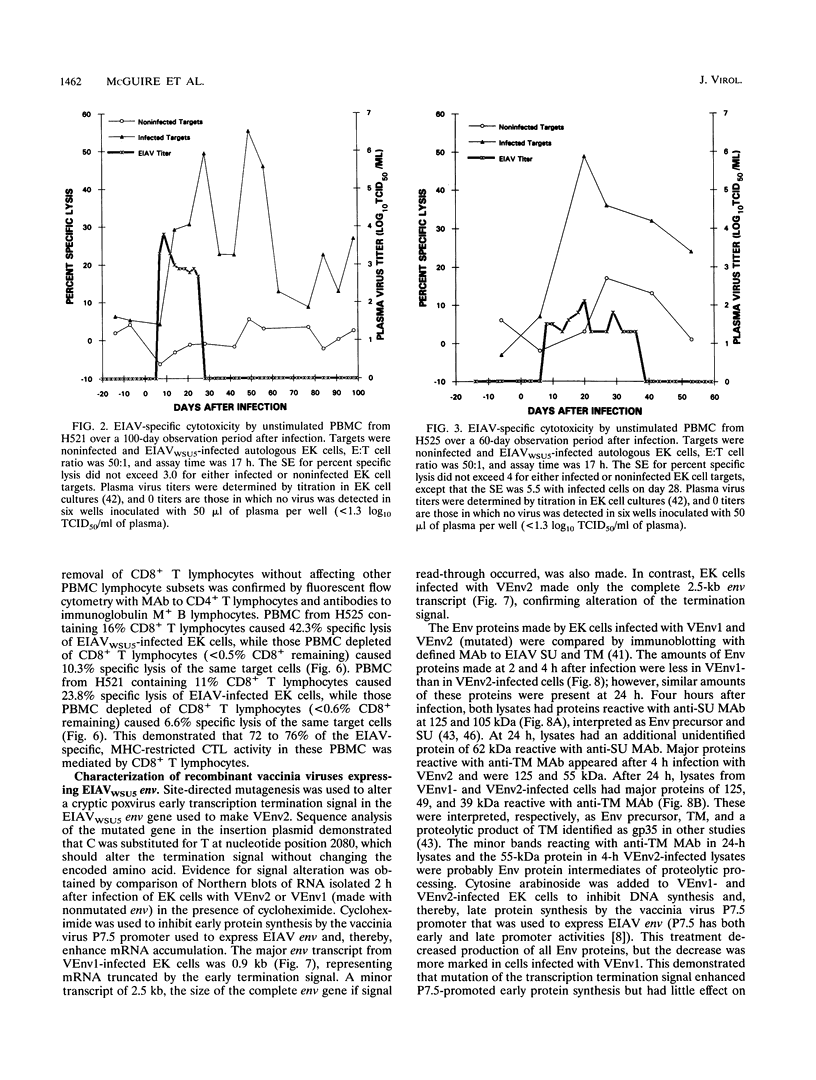
Cytotoxic T lymphocytes (CTL) can control some viral infections and may be important in the control of lentiviruses, including human immunodeficiency virus type 1. Since there is limited evidence for an in vivo role of CTL in control of lentiviruses, dissection of immune mechanisms in animal lentiviral infections may provide needed information. Horses infected with equine infectious anemia virus (EIAV) a lentivirus, have acute plasma viremia which is terminated in immunocompetent horses. Viremic episodes may recur, but most horses ultimately control infection and become asymptomatic carriers. To begin dissection of the immune mechanisms involved in EIAV control, peripheral blood mononuclear cells (PBMC) from infected horses were evaluated for CTL to EIAV-infected cells. By using noninfected and EIAV-infected autologous equine kidney (EK) cells in 51Cr-release assays, EIAV-specific cytotoxic activity was detected in unstimulated PBMC from three infected horses. The EIAV-specific cytotoxic activity was major histocompatibility complex (MHC) restricted, as determined by assaying EIAV-infected heterologous EK targets, and was mediated by CD8+ T lymphocytes, as determined by depleting these cells by a panning procedure with an anti-CD8 monoclonal antibody. MHC-restricted CD8+ CTL in unstimulated PBMC from infected horses caused significant specific lysis of autologous EK cells infected with recombinant vaccinia viruses expressing EIAV genes, either env or gag plus 5' pol. The EIAV-specific MHC-restricted CD8+ CTL were detected in two EIAV-infected horses within a few days after plasma viremia occurred and were present after viremia was terminated. The detection of these immune effector cells in EIAV-infected horses permits further studies to determine their in vivo role.
Full text
PDF

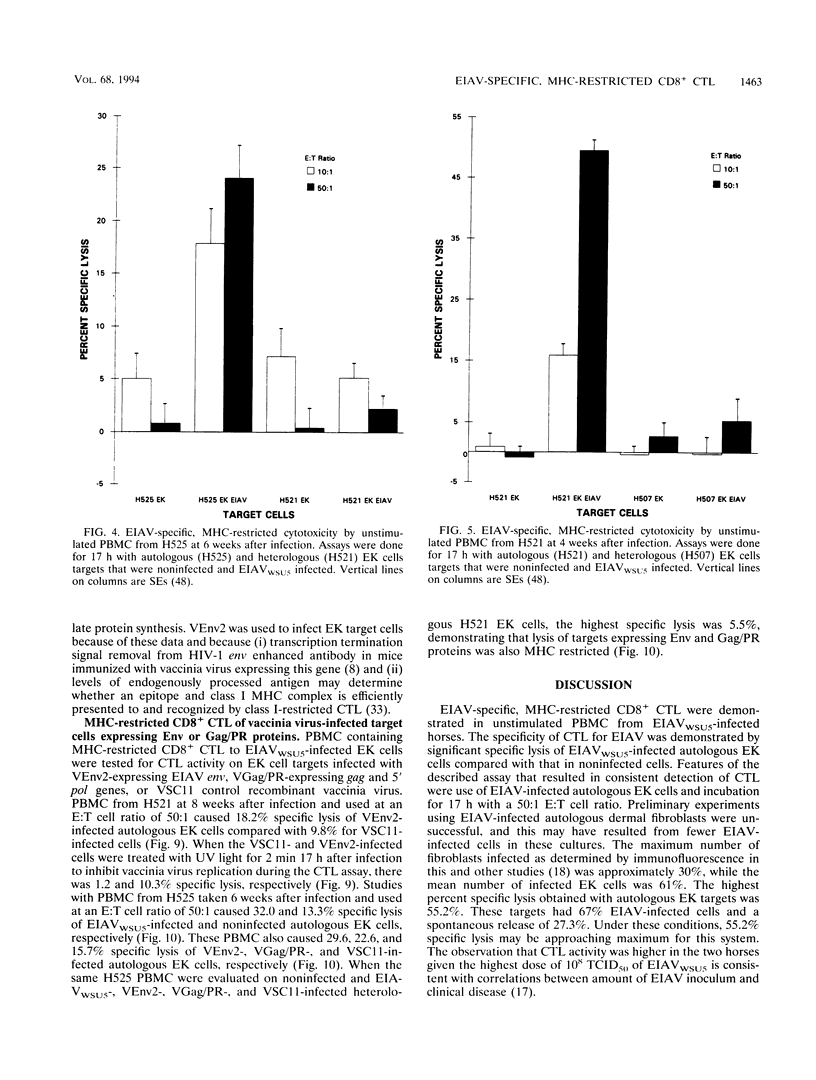
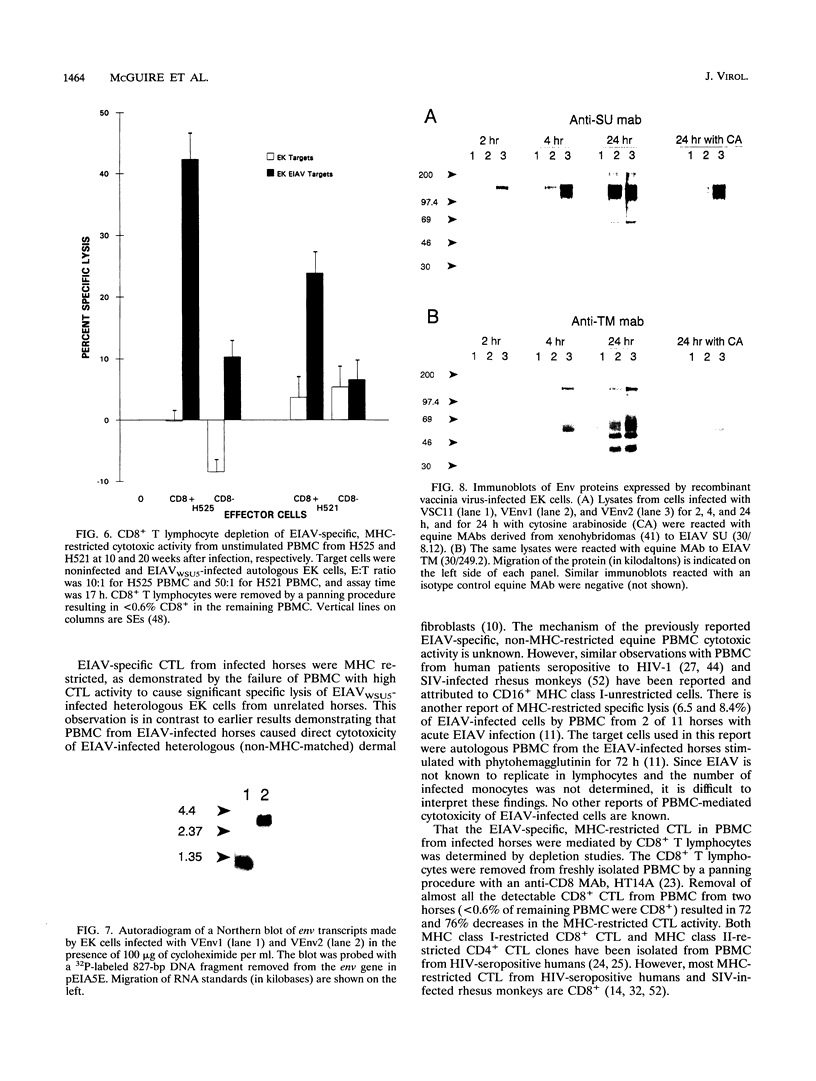
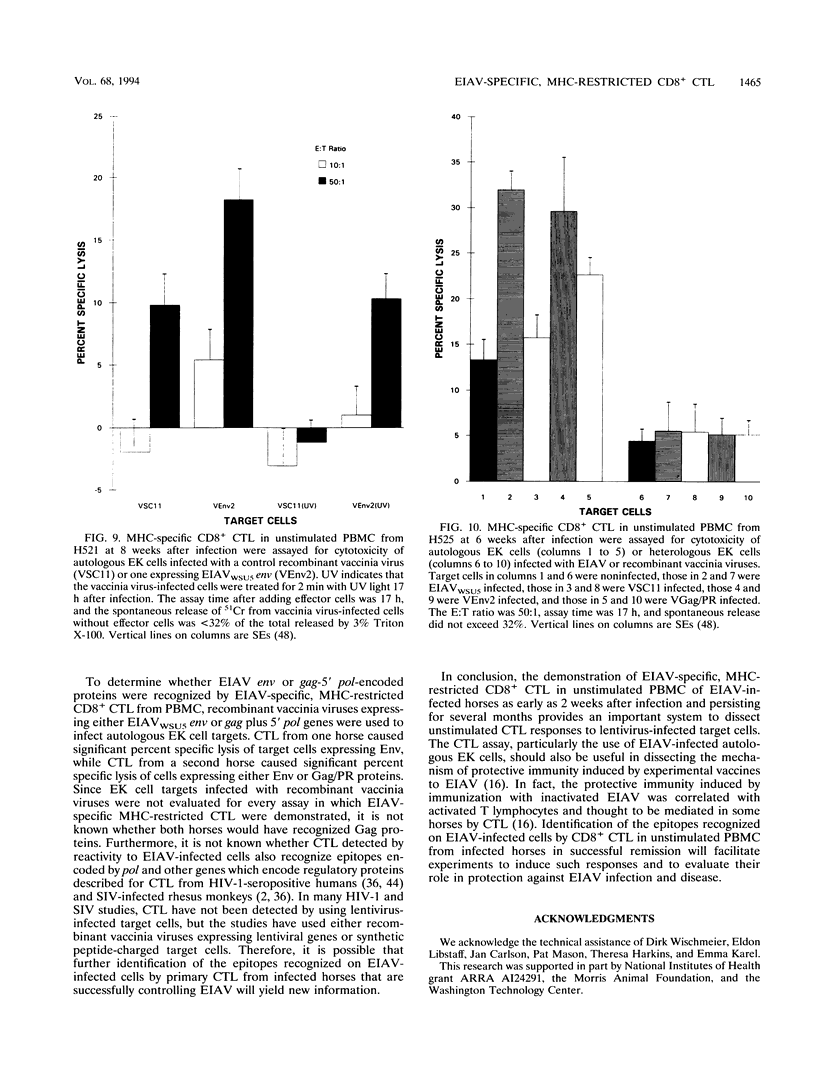


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgault I., Venet A., Levy J. P. Three epitopic peptides of the simian immunodeficiency virus Nef protein recognized by macaque cytolytic T lymphocytes. J Virol. 1992 Feb;66(2):750–756. doi: 10.1128/jvi.66.2.750-756.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson P., McCoy R., Sadler R., Gilligan K., Tursz T., Raab-Traub N. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J Virol. 1992 May;66(5):3257–3262. doi: 10.1128/jvi.66.5.3257-3262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clabough D. L., Gebhard D., Flaherty M. T., Whetter L. E., Perry S. T., Coggins L., Fuller F. J. Immune-mediated thrombocytopenia in horses infected with equine infectious anemia virus. J Virol. 1991 Nov;65(11):6242–6251. doi: 10.1128/jvi.65.11.6242-6251.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins L. Carriers of equine infectious anemia virus. J Am Vet Med Assoc. 1984 Feb 1;184(3):279–281. [PubMed] [Google Scholar]
- Coggins L., Norcross N. L., Nusbaum S. R. Diagnosis of equine infectious anemia by immunodiffusion test. Am J Vet Res. 1972 Jan;33(1):11–18. [PubMed] [Google Scholar]
- Crawford T. B., McGuire T. C., Henson J. B. Detection of equine infectious anemia virus in vitro by immunofluorescence. Arch Gesamte Virusforsch. 1971;34(4):332–339. doi: 10.1007/BF01242979. [DOI] [PubMed] [Google Scholar]
- Earl P. L., Hügin A. W., Moss B. Removal of cryptic poxvirus transcription termination signals from the human immunodeficiency virus type 1 envelope gene enhances expression and immunogenicity of a recombinant vaccinia virus. J Virol. 1990 May;64(5):2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Schleif W. A., Nunberg J. H., Conley A. J., Eda Y., Tokiyoshi S., Putney S. D., Matsushita S., Cobb K. E., Jett C. M. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992 Feb 20;355(6362):728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- Fujimiya Y., Perryman L. E., Crawford T. B. Leukocyte cytotoxicity in a persistent virus infection: presence of direct cytotoxicity but absence of antibody-dependent cellular cytotoxicity in horses infected with equine infectious anemia virus. Infect Immun. 1979 Jun;24(3):628–636. doi: 10.1128/iai.24.3.628-636.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerencer M., Valpotić I., Jukić B., Tomasković M., Basić I. Qualitative analyses of cellular immune functions in equine infectious anemia show homology with AIDS. Arch Virol. 1989;104(3-4):249–257. doi: 10.1007/BF01315547. [DOI] [PubMed] [Google Scholar]
- Gregersen J. P., Mehdi S., Baur A., Hilfenhaus J. Antibody- and complement-mediated lysis of HIV-infected cells and inhibition of viral replication. J Med Virol. 1990 Apr;30(4):287–293. doi: 10.1002/jmv.1890300411. [DOI] [PubMed] [Google Scholar]
- Haynes B. F. Scientific and social issues of human immunodeficiency virus vaccine development. Science. 1993 May 28;260(5112):1279–1286. doi: 10.1126/science.8493572. [DOI] [PubMed] [Google Scholar]
- Hoffenbach A., Langlade-Demoyen P., Dadaglio G., Vilmer E., Michel F., Mayaud C., Autran B., Plata F. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J Immunol. 1989 Jan 15;142(2):452–462. [PubMed] [Google Scholar]
- Hussain K. A., Issel C. J., Schnorr K. L., Rwambo P. M., West M., Montelaro R. C. Antigenic mapping of the envelope proteins of equine infectious anemia virus: identification of a neutralization domain and a conserved region on glycoprotein 90. Arch Virol. 1988;98(3-4):213–224. doi: 10.1007/BF01322170. [DOI] [PubMed] [Google Scholar]
- Issel C. J., Horohov D. W., Lea D. F., Adams W. V., Jr, Hagius S. D., McManus J. M., Allison A. C., Montelaro R. C. Efficacy of inactivated whole-virus and subunit vaccines in preventing infection and disease caused by equine infectious anemia virus. J Virol. 1992 Jun;66(6):3398–3408. doi: 10.1128/jvi.66.6.3398-3408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny L. J., Mott L. O., Pearson J. E. Titration of equine infectious anemia virus. Effect of dosage on incubation time and clinical signs. Cornell Vet. 1971 Oct;61(4):687–695. [PubMed] [Google Scholar]
- Klevjer-Anderson P., Cheevers W. P., Crawford T. B. Characterization of the infection of equine fibroblasts by equine infectious anemia virus. Arch Virol. 1979;60(3-4):279–289. doi: 10.1007/BF01317499. [DOI] [PubMed] [Google Scholar]
- Kono Y., Hirasawa K., Fukunaga Y., Taniguchi T. Recrudescence of equine infectious anemia by treatment with immunosuppressive drugs. Natl Inst Anim Health Q (Tokyo) 1976 Spring;16(1):8–15. [PubMed] [Google Scholar]
- Kono Y., Kobayashi K., Fukunaga Y. Antigenic drift of equine infectious anemia virus in chronically infected horses. Arch Gesamte Virusforsch. 1973;41(1):1–10. doi: 10.1007/BF01249923. [DOI] [PubMed] [Google Scholar]
- Kono Y. Viremia and immunological responses in horses infected with equine infectious anemia virus. Natl Inst Anim Health Q (Tokyo) 1969 Spring;9(1):1–9. [PubMed] [Google Scholar]
- Koup R. A., Sullivan J. L., Levine P. H., Brewster F., Mahr A., Mazzara G., McKenzie S., Panicali D. Antigenic specificity of antibody-dependent cell-mediated cytotoxicity directed against human immunodeficiency virus in antibody-positive sera. J Virol. 1989 Feb;63(2):584–590. doi: 10.1128/jvi.63.2.584-590.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littaua R. A., Oldstone M. B., Takeda A., Debouck C., Wong J. T., Tuazon C. U., Moss B., Kievits F., Ennis F. A. An HLA-C-restricted CD8+ cytotoxic T-lymphocyte clone recognizes a highly conserved epitope on human immunodeficiency virus type 1 gag. J Virol. 1991 Aug;65(8):4051–4056. doi: 10.1128/jvi.65.8.4051-4056.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littaua R. A., Oldstone M. B., Takeda A., Ennis F. A. A CD4+ cytotoxic T-lymphocyte clone to a conserved epitope on human immunodeficiency virus type 1 p24: cytotoxic activity and secretion of interleukin-2 and interleukin-6. J Virol. 1992 Jan;66(1):608–611. doi: 10.1128/jvi.66.1.608-611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McChesney M., Tanneau F., Regnault A., Sansonetti P., Montagnier L., Kieny M. P., Rivière Y. Detection of primary cytotoxic T lymphocytes specific for the envelope glycoprotein of HIV-1 by deletion of the env amino-terminal signal sequence. Eur J Immunol. 1990 Jan;20(1):215–220. doi: 10.1002/eji.1830200131. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Lacy P. A., O'Rourke K. I. cDNA sequence of the env gene of a pathogenic equine infectious anemia lentivirus variant. Nucleic Acids Res. 1990 Jan 11;18(1):196–196. doi: 10.1093/nar/18.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. D., Lord C. I., Stallard V., Mazzara G. P., Letvin N. L. The gag-specific cytotoxic T lymphocytes in rhesus monkeys infected with the simian immunodeficiency virus of macaques. J Immunol. 1990 Jan 1;144(1):122–128. [PubMed] [Google Scholar]
- Miller M. D., Yamamoto H., Hughes A. L., Watkins D. I., Letvin N. L. Definition of an epitope and MHC class I molecule recognized by gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J Immunol. 1991 Jul 1;147(1):320–329. [PubMed] [Google Scholar]
- Milligan G. N., Morrison L. A., Gorka J., Braciale V. L., Braciale T. J. The recognition of a viral antigenic moiety by class I MHC-restricted cytolytic T lymphocytes is limited by the availability of the endogenously processed antigen. J Immunol. 1990 Nov 15;145(10):3188–3193. [PubMed] [Google Scholar]
- Montelaro R. C., Lohrey N., Parekh B., Blakeney E. W., Issel C. J. Isolation and comparative biochemical properties of the major internal polypeptides of equine infectious anemia virus. J Virol. 1982 Jun;42(3):1029–1038. doi: 10.1128/jvi.42.3.1029-1038.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Parekh B., Orrego A., Issel C. J. Antigenic variation during persistent infection by equine infectious anemia virus, a retrovirus. J Biol Chem. 1984 Aug 25;259(16):10539–10544. [PubMed] [Google Scholar]
- Nixon D. F., McMichael A. J. Cytotoxic T-cell recognition of HIV proteins and peptides. AIDS. 1991 Sep;5(9):1049–1059. [PubMed] [Google Scholar]
- O'Rourke K., Perryman L. E., McGuire T. C. Antiviral, anti-glycoprotein and neutralizing antibodies in foals with equine infectious anaemia virus. J Gen Virol. 1988 Mar;69(Pt 3):667–674. doi: 10.1099/0022-1317-69-3-667. [DOI] [PubMed] [Google Scholar]
- Orrego A., Issel C. J., Montelaro R. C., Adams W. V., Jr Virulence and in vitro growth of a cell-adapted strain of equine infectious anemia virus after serial passage in ponies. Am J Vet Res. 1982 Sep;43(9):1556–1560. [PubMed] [Google Scholar]
- Pantaleo G., De Maria A., Koenig S., Butini L., Moss B., Baseler M., Lane H. C., Fauci A. S. CD8+ T lymphocytes of patients with AIDS maintain normal broad cytolytic function despite the loss of human immunodeficiency virus-specific cytotoxicity. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4818–4822. doi: 10.1073/pnas.87.12.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. L., Fang F. D., Liu C. P., Dhruva B. R., Rwambo P., Issel C. J., Montelaro R. C. Antigenic variation and lentivirus persistence: variations in envelope gene sequences during EIAV infection resemble changes reported for sequential isolates of HIV. Virology. 1987 Dec;161(2):321–331. doi: 10.1016/0042-6822(87)90124-3. [DOI] [PubMed] [Google Scholar]
- Perryman L. E., O'Rourke K. I., Mason P. H., McGuire T. C. Equine monoclonal antibodies recognize common epitopes on variants of equine infectious anaemia virus. Immunology. 1990 Dec;71(4):592–594. [PMC free article] [PubMed] [Google Scholar]
- Perryman L. E., O'Rourke K. I., McGuire T. C. Immune responses are required to terminate viremia in equine infectious anemia lentivirus infection. J Virol. 1988 Aug;62(8):3073–3076. doi: 10.1128/jvi.62.8.3073-3076.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., Henderson L. E., Sowder R. C., Copeland T. D., Oroszlan S., Edwards J. F. Synthesis and processing of the transmembrane envelope protein of equine infectious anemia virus. J Virol. 1990 Aug;64(8):3770–3778. doi: 10.1128/jvi.64.8.3770-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere Y., Tanneau-Salvadori F., Regnault A., Lopez O., Sansonetti P., Guy B., Kieny M. P., Fournel J. J., Montagnier L. Human immunodeficiency virus-specific cytotoxic responses of seropositive individuals: distinct types of effector cells mediate killing of targets expressing gag and env proteins. J Virol. 1989 May;63(5):2270–2277. doi: 10.1128/jvi.63.5.2270-2277.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow K., Peng X. X., Montelaro R. C., Shih D. S. Expression of the protease gene of equine infectious anemia virus in Escherichia coli: formation of the mature processed enzyme and specific cleavage of the gag precursor. Virology. 1992 May;188(1):396–401. doi: 10.1016/0042-6822(92)90773-i. [DOI] [PubMed] [Google Scholar]
- Salinovich O., Payne S. L., Montelaro R. C., Hussain K. A., Issel C. J., Schnorr K. L. Rapid emergence of novel antigenic and genetic variants of equine infectious anemia virus during persistent infection. J Virol. 1986 Jan;57(1):71–80. doi: 10.1128/jvi.57.1.71-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano R. F., Keegan A. D., Dintzis R. Z., Dintzis H. M., Shin H. S. The interaction of nominal antigen with T cell antigen receptors. I. Specific binding of multivalent nominal antigen to cytolytic T cell clones. J Immunol. 1985 Aug;135(2):906–914. [PubMed] [Google Scholar]
- Tsurushita N., Maki H., Korn L. J. Site-directed mutagenesis with Escherichia coli DNA polymerase III holoenzyme. Gene. 1988;62(1):135–139. doi: 10.1016/0378-1119(88)90587-2. [DOI] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Wyatt C. R., Davis W. C., McGuire T. C., Perryman L. E. T lymphocyte development in horses. I. Characterization of monoclonal antibodies identifying three stages of T lymphocyte differentiation. Vet Immunol Immunopathol. 1988 Feb;18(1):3–18. doi: 10.1016/0165-2427(88)90032-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Miller M. D., Watkins D. I., Snyder G. B., Chase N. E., Mazzara G. P., Gritz L., Panicali D. L., Letvin N. L. Two distinct lymphocyte populations mediate simian immunodeficiency virus envelope-specific target cell lysis. J Immunol. 1990 Dec 1;145(11):3740–3746. [PubMed] [Google Scholar]
- Yamamoto H., Ringler D. J., Miller M. D., Yasutomi Y., Hasunuma T., Letvin N. L. Simian immunodeficiency virus-specific cytotoxic T lymphocytes are present in the AIDS-associated skin rash in rhesus monkeys. J Immunol. 1992 Jul 15;149(2):728–734. [PubMed] [Google Scholar]