Abstract
Under usual conditions, the role of IGF-I in vascular cell types is to maintain cellular protein synthesis and cell size, and even excess IGF-I does not stimulate proliferation. In pathophysiologic states, such as hyperglycemia, smooth muscle cells (SMC) de-differentiate and change their responsiveness to IGF-I. During hyperglycemia IGF-I stimulates both SMC migration and proliferation. Our laboratory has investigated the molecular mechanism by which this change is mediated. Following hyperglycemia SMC secrete increased concentrations of thrombospondin, vitronectin and osteopontin, ligands for the integrin αVβ3. Activation of αVβ3 stimulates recruitment of a tyrosine phosphatase, SHP-2. Exposure of SMC to IGF-I results in phosphorylation of the transmembrane protein, SHPS-1, which provides a docking site for αVβ3-associated SHP-2. After IGF-I stimulation SHP-2 associates with Src kinase, which associates with the signaling protein Shc. Src phosphorylates Shc, resulting in activation of MAP kinases, which are necessary both for stimulation of cell proliferation and migration. Blocking activation of αVβ3 results in an inability of IGF-I to stimulate Shc phosphorylation. Under conditions of normoglycemia, there are insufficient αVβ3 ligands to recruit SHP-2, and no increase in Shc phosphorylation can be demonstrated in SMC. In contrast, if αVβ3 ligands are added to cells in normal glucose, the signaling events that are necessary for Shc phosphorylation can be reconstituted. Therefore when SMC are exposed to normal glucose they are protected from excessive stimulation of mitogenesis by IGF-I. With hyperglycemia there is a marked increased in αVβ3 ligands and Shc phosphorylation in response to IGF-I is sustained. These findings indicate that in SMC hyperglycemic stress may leads to altered IGF-I signaling, which allows the cells to undergo a mitogenic response, and which may contribute to the development of atherosclerosis.
Keywords: Insulin resistance, diabetes, atherosclerosis, cell migration
Introduction
Insulin-like growth factor-I (IGF-I) is a potent mitogen for anchorage dependent cells; however, when cells are placed in suspension, IGF-I has much less capacity to stimulate cell division. Generally when anchorage dependent cells are placed in suspension, they require exposure to multiple mitogens in order to increase DNA synthesis. One major mediator of the ability of stably attached cells to respond to mitogens is a class of receptors termed integrins. Integrins are alpha/beta heterodimers which mediate communication between the extracellular matrix and cytoskeletal proteins (1). Integrins are critical for cells to assume a normal architecture following attachment. For several years investigators have been interested in how integrins modulate responses of cells to mitogens. Specifically it has been shown that integrins can interact directly with growth factor receptors (demonstrated primarily for VEGF and PDGF receptors (2, 3)). Other cell surface proteins, such as heparan sulfate containing proteoglycans and proteins with multiple membrane spanning domains such as tetraspanin, also can modulate integrin function (4–5).
Various elements of the insulin-like growth factor (IGF) signaling system have been shown to interact with integrins. Initially it was found that if cells over-expressed the adaptor protein IRS-1 and the β3 integrin subunit that these two proteins could co-associate (6). One study was able to demonstrate association of the αVβ3 integrin and the IGF-I receptor following their overexpression (7). Other studies have reported that IGF binding proteins such as IGFBP-1 could bind to integrins such as α5β1; following binding they could modulate cellular functions (8). Similarly it was shown that following secretion of IGFBP-5 it was localized in the extracellular matrix (ECM ), where it altered cellular responsiveness to IGF-I, but whether this was integrin mediated was not proven (9).
Some integrins such as α5β1 are expressed on the surface of multiple cell types, while others such as αVβ3 have a restricted pattern of cell surface expression. When normal cell types are analyzed, αVβ3 is primarily expressed in vascular endothelial and smooth muscle cells (SMC), as well as osteoclasts (10). Ligand occupancy of αVβ3 has been shown in many cases to stimulate cell motility, and aberrant expression of this integrin has been implicated in metastatic behavior of tumor cells, a process which is dependent upon cell migration (11).
α Vβ3 Integrin Activation
Following ligand occupancy of αVβ3 by extracellular matrix proteins (ECM) such as osteopontin, thrombospondin and vitronectin, it undergoes tyrosine phosphorylation. Although αVβ3 contains no intrinsic tyrosine kinase activity in its intracellular domain, the β3 subunit contains two tyrosines which are phosphorylated by an unknown intracellular kinase. Several protein kinases have been implicated in phosphorylating β3 including Src kinase and integrin-linked kinase; however, definitive identification of this kinase (12). Following tyrosine phosphorylation, a variety of molecules have been shown to bind to the phosphorylated tyrosine residues on β3 and several of these are believed to be involved in activating intracellular signaling pathways (13).
Most studies that have analyzed intracellular signaling in response to integrin activation have been conducted using one specific paradigm (e.g. cellular attachment). This paradigm consists of plating cells on an ECM that is enriched in a specific protein that is a ligand for a specific integrin (14). While this experimental paradigm results in marked activation of a particular integrin, it provides very little insight into how integrins function when cells are stably attached. In contrast, when cells are exposed to increasing concentrations of soluble ECM proteins, the degree of integrin activation is considerably less compared to what is attained following cell attachment. Although addition of soluble ligands is much more relevant to most physiologic and pathophysiologic conditions, there are very few experimental models that have utilized this paradigm. Consequently it is difficult to extrapolate from attachment assay data to the behavior of stably attached cells. Nevertheless, it is clear that following attachment intracellular kinases such as Fak and Src are activated, which then activate intracellular signaling proteins such as paxcillin and Gab-1 (15–18). In some cases it has been shown that these activated signaling proteins interact with components of growth factor signaling pathways. For example, cooperation between αVβ3 and signaling molecules downstream of the VEGF and FGF receptors has been demonstrated (2,3). Therefore there is a reasonable body of experimental evidence to suggest that there might be interactions between IGF-I receptor linked signaling mechanisms and the changes in signaling molecules that occur following increases in integrin ligand occupancy.
Interaction between changes in αVβ3 ligand occupancy and IGF-I receptor-linked signaling
To test the hypothesis that interactions occurred between the IGF-I receptor and αVβ3, we initially determined whether following ligand occupancy the two proteins could coprecipitate. Using either SMC or endothelial cells, we were consistently unsuccessful in being able to coprecipitate αVβ3 and the IGF-I receptor. Likewise we were not able to immunoprecipitate IRS-1 and αVβ3. This is particularly striking since IRS-1 contains a phosphotyrosine binding (PTB) domain, and the β3 subunit of αVβ3 has a known PTB domain binding site. Since coassociation of the receptors did not occur, we next wanted to determine if blocking ligand occupancy of αVβ3 would result in attenuation of IGF-I actions. The addition of a monoclonal antibody that inhibited ligand occupancy was able to reduce the ability of IGF-I to stimulate SMC migration or proliferation (19). Furthermore the addition of excess ligand, either vitronectin or osteopontin, was able to enhance the effects of IGF-I in stimulating these two processes (20, 21).
To determine the molecular mechanism by which this occurred, we began to dissect the specific intracellular events that followed ligand occupancy of both receptors. We were able to show that following ligand occupancy of αVβ3 there was stimulation of tyrosine phosphorylation of β3, and that this tyrosine phosphorylation led to the recruitment of DOK1, a PTB domain containing protein (22). Additionally, following IGF-I receptor stimulation, DOK1 was phosphorylated by an unknown kinase on sites that contained YXXL/I motifs, and phosphorylation of these sites resulted in recruitment of a tyrosine phosphatase SHP-2 to DOK1. Our studies then confirmed that tyrosine phosphorylation of β3 led to the recruitment of the DOK1/SHP-2 complex. Since SHP-2 is constitutively localized in the cytoskeleton, this recruitment of SHP-2 to β3 in the plasma membrane was significant, and we proposed that it might be involved in IGF-I signaling. To directly test this question we used a mutant form of SHP-2 that destroyed its phosphatase activity. When over-expressed in SMC, the SHP2 C/S mutant was not recruited to the plasma membrane, and these cells did not migrate in response to IGF-I (23). These results indicated that recruitment of SHP-2 to the plasma membrane was necessary for IGF-I-mediated biological effects, but did not identify the downstream pathways.
To assess the mechanisms of action of SHP-2, we examined effects on known SHP-2 binding proteins following IGF-I stimulation. SHPS-1 contains a large extracellular domain, a trans-membrane segment and a cytoplasmic region that contains 4 tyrosine residues (24). These tyrosine residues are contained in YXXL/I motifs, which when phosphorylated become docking sites for SH2 domains contained within SHP-2. Other investigators had shown that growth factors such as PDGF and insulin could stimulate SHPS-1 phosphorylation, resulting in SHP-2 recruitment. Initially we determined that following IGF-I exposure there was enhanced phosphorylation of SHPS1, and that this was required for recruitment of SHP-2. We further showed that blocking SHP-2 recruitment to SHPS-1 inhibited stimulation of SMC migration by IGF-I. To determine whether recruitment of SHP-2 to αVβ3 was required, we used mutagenesis and cell permeable blocking peptides (22). Either mutating the cytoplasmic domain tyrosines on β3 or using a cell permeable peptide that inhibited DOK1 binding to β3 eliminated transfer of SHP-2 to SHPS-1, and prevented IGF-I stimulated mitogenesis. We further demonstrated with cell permeable peptides and by mutating the tyrosines on SHPS-1, that inhibiting transfer of SHP-2 to SHPS-1 also blocked IGF-I stimulated mitogenesis and cell migration (25). Therefore, recruitment of SHP-2 to β3 following αVβ3 ligand occupancy was critical for its subsequent transfer to SHPS-1, and recruitment of SHP-2 to SHPS-1 was required for mitogenic stimulation.
To further evaluate the role of SHP-2 binding to SHPS-1, we sought to assess the effect of this process on downstream signaling events. Initially we showed that blocking MAP kinase activation inhibited stimulation of mitogenesis completely, and resulted in a 70% decrease in cell migration in response to IGF-I (26). To determine what was required for activation of MAP kinases we examined both IRS-1 and Shc phosphorylation in response to IGF-I in SMC. IRS-1 was minimally detectible in these cells and its tyrosine phosphorylation did not increase in response to IGF-I. In contrast, Shc was abundant and its phosphorylation increased dramatically (27). To definitively prove the role of Shc, we prepared a mutant in which the three tyrosines that were phoshorylated in response to IGF-I stimulation were changed to phenylalanines. Cells expressing this mutant form of Shc had markedly reduced activation of MAP kinase and decreased mitogenic and cell migration responses to IGF-I. Therefore tyrosine phosphorylation of Shc and subsequent MAP kinase activation appeared to be critical for both mitogenesis and migration. We next determined if SHP-2 had a role in recruiting Shc to SHPS-1. We found that blocking the association of SHP-2 and Shc inhibited the binding of Shc to SHPS-1, and resulted in attenuation of the ability of IGF-I to activate MAP kinase or to stimulate cell migration and proliferation (27). Therefore it appeared that the association of Shc with SHP-2 was required for full IGF-I activation. As recruitment of Shc could be inhibited either by blocking αVβ3 ligand occupancy, or IGF-I receptor ligand occupancy, activation of both pathways was required for Shc recruitment to the membrane and its phosphorylation.
To further characterize the components of this interaction and to identify the kinase necessary for Shc phosphorylation, we evaluated the role of c-Src in mediating signaling interactions between αVβ3 and the IGF-I receptor in SMC. Initially we were able to show that c-Src is phosphorylated in response to IGF-I and that its enzymatic activity is activated (28). Subsequently we found that c-Src bound to SHP-2 through its SH3 domain and that disassociation of c-Src from SHP-2 resulted in an inability to recruit Src to SHPS-1 and an inability to recruit Shc. This suggested that Shc was binding directly to Src. To test this idea, we prepared a Src mutant in which two YXXL motifs were altered. This resulted in complete disassociation of Src and Shc and failure to phosphorylate Shc in response to IGF-I. We were further able to show that the recruitment of Shc to SHPS-1 was disrupted by inhibiting binding of Src to Shc. Using a Src mutant that had no tyrosine kinase we found that there was no increase in Shc phosphorylation following IGF-I stimulation, no downstream signaling to activate MAP kinase, and no stimulation of either cell migration or proliferation (28). Therefore, in vascular SMC phosphorylation of SHPS-1 results in the recruitment of a complex consisting of SHP-2, Src, and Shc. Src is auto-activated in response to its binding to SHP-2, thus leading to Shc phosphorylation within the complex, which is required for MAP kinase activation. Therefore, stimulation of Src by IGF-I is critical for subsequent signaling in SMC.
Role of Hyperglycemia
To extend these findings to a pathophysiologic condition, we examined the effects of hyperglycemia. SMC are the cell type that plays a major role in the development of atherosclerosis, and hyperglycemia is known to be an important risk factor for atherosclerotic lesion development (29). Other studies have shown that IGF-I plays a role in the development of atherosclerosis, and have implicated local IGF-I production in stimulation of vascular SMC proliferation and an increasing the size of atherosclerotic lesions (30–32). Conversely, if IGF-I action is inhibited locally then atherosclerotic lesion development is inhibited (33, 34). Therefore, a possible role of hyperglycemia is to alter SMC responsiveness to IGF-I. To test this idea, we first compared cells grown in normal glucose (final concentration of 5 mM) with cells grown in high glucose (25 mM) in terms of the ability of IGF-I to stimulate actions which might be related to atherogenesis. We found initially that IGF-I could only stimulate SMC migration or proliferation in the presence of 25 mM glucose, but not in 5 mM glucose, even though under the latter conditions IGF-I could induce phosphorylation of IRS-1 and could stimulate protein synthesis.
To determine the mechanisms by which exposure to high glucose augmented IGF-I actions, we examined IGF-I receptor phosphorylation, since exposure of cells to high glucose had been shown to enhance ligand-stimulated PDGF receptor phosphorylation (35). Unlike the PDGF receptor, there was no detectible alteration in phosphorylation of the IGF-I receptor in 25 mM glucose. High glucose did result in suppression of IRS-1 expression, and there was no detectible rise in IRS-1 tyrosine phosphorylation following IGF-I treatment. Since we had previously shown that Shc phosphorylation was an important signaling element in mediating the effect of IGF-I when these cells had been cultured in high glucose, we reasoned that high glucose must be inducing a process which would allow maximum Shc phosphorylation. Since we had shown that ligand occupancy of αVβ3 was critical to induce maximum Shc phosphorylation, we reviewed the literature for whether high glucose was known to increase either β3 activation or ligand occupancy of αVβ3. Several reports showed that exposure to high glucose resulted in marked increases in expression of osteopontin and thrombospondin, and that in diabetic animals the kidney had been shown to contain increased vitronectin (36–38). Therefore it was logical to assume that high glucose was inducing the production of αVβ3 ligands, which resulted in enhanced αVβ3 ligand occupancy and β3 activation. We then wished to test the hypothesis of whether enhanced β3 activation stimulate IGF-I receptor signaling, and whether this mediated the effect of high glucose on the ability of IGF-I to increase cell migration and proliferation. We found that cells maintained in 25 mM glucose produced substantially more vitronectin, as well as osteopontin and thrombospondin. In addition, when SMC were cultured in low glucose, SHPS-1 phosphorylation in response to IGF-I, Shc recruitment to SHPS-1, Shc phosphorylation, and MAP kinase activation were all attenuated. Therefore the signaling responses that are augmented by increased αVβ3 ligand occupancy are diminished when cells are incubated in low glucose.
To confirm that this effect was specific for αVβ3 we identified the binding site on the β3 subunit for its ligands. We found that a 6 amino acid loop (positions 203–209) contained this site (39). An antibody to this region inhibited vitronectin binding, and prevented phosphorylation of β3 stimulated by vitronectin. More importantly, the antibody was found to inhibit IGF-I-stimulated activation of Shc and MAP kinase, and blocked cell migration and proliferation. To confirm that hyperglycemia induced β3 ligands, vitronectin was added to SMC in low glucose, and the ability of IGF-I to stimulate signaling leading to enhanced cell migration and proliferation was assessed (40). Under these conditions vitronectin stimulated Shc and MAP kinase phosphorylation, and enhanced the ability of IGF-I to promote cell growth. In additon, these responses were completely inhibited by exposure to the β3 antibody.
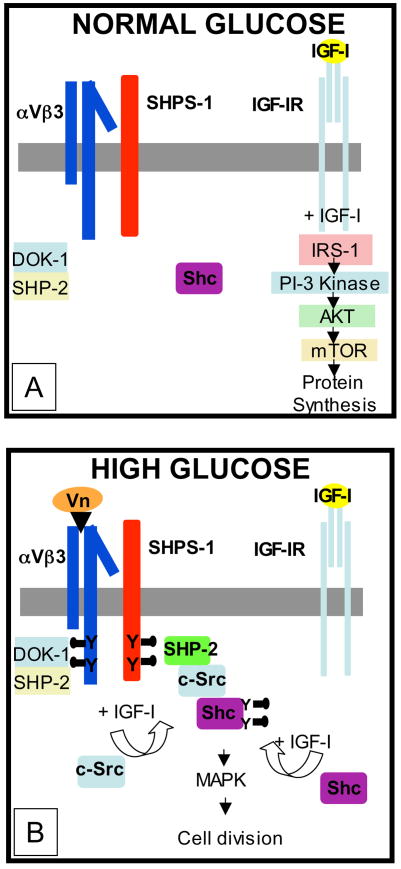
In summary, our studies have demonstrated that exposure of cells to high glucose results in activation of the αVβ3 mediated signaling cascade which then functions cooperatively with IGF-I receptor activation to activate Shc phosphorylation by c-Src (Figure 1). Phosphorylated Shc then stimulates downstream signaling leading to enhanced mitogenesis. In contrast, in cells cultured in normal glucose IGF-I does not induce Shc phosphorylation to any significant extent. Therefore overexpression of IGF-I in normoglycemic animals results in increased vascular smooth muscle cell size and hypertrophy but not in hyperplastic or increased cell migration responses. Therefore unlike other serum mitogens such as PDGF, the effects of IGF-I on vascular cells are relatively restricted to cells that have undergone injury and have partially dedifferentiated, and are thus able to respond to IGF-I with increased Shc induction. Since with atherosclerotic lesions, there are mixed cell populations, some of which are increasing their rates of migration and proliferation and others that remain quiescent, it is likely that only a subpopulation of cells have undergone dedifferentiation in response to hyperglycemic stress. Our observations provide a framework for the rational testing of the hypothesis that that inhibition of this pathway may lead to attenuation of atherosclerotic lesion formation that occurs in response to hyperglycemia.
Figure 1.
Smooth muscle cells that are exposed to hyperglycemia respond by increasing their synthesis of αVβ3 ligands such as vitronectin (Vn). This increase in ligand occupancy leads to recruitment of the SHP-2/c-Src/Shc complex to SHPS-1. Following IGF-I exposure and Shc phosphorylation, Shc subsequently activates MAP kinase which is essential for cell proliferation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senger Dr, Claffey KP, Benes JE, et al. Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci USA. 1997;94:13612–13617. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yayon A, Kglasburn M, Esko JD, et al. Cell surface, heparin-like molecules are required for binding or basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 5.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 6.Vuori K, Ruoslahti E. Association of insulin receptor substrate-1 with integrins. Science. 1994;266:1576–1578. doi: 10.1126/science.7527156. [DOI] [PubMed] [Google Scholar]
- 7.Schneller M, Vuori K, Ruoslahte E. α Vβ3 Integrin associates with activated insulin and PDGF βreceptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones JI, Gockerman A, Busby WH, Jr, et al. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proc Natl Acad Sci USA. 1993;90:10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones JI, Gockerman A, Busby WH, Jr, et al. Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-I. J Cell Biol. 1993;121:679–687. doi: 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horton MA. The alpha V beta 3 integrin “vitronectin receptor”. In J Biochem Cell Biol. 1997;29:721–725. doi: 10.1016/s1357-2725(96)00155-0. [DOI] [PubMed] [Google Scholar]
- 11.Samanna V, Wei H, Ego-Osuala D, Chellajah MA. Alpha-V-dependent outside-in signaling is required for the regulation of CD44 surface expression, MMP-2 secretion and cell migration by osteopontin in human melanoma cells. Exp Cell Res. 2006;312:2214–2230. doi: 10.1016/j.yexcr.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Phillips DR, Prasad KS, Manganello J, et al. Integrin tyrosine phosphorylation in platelet signaling. Curr Opin Cell Biol. 2001;13:546–554. doi: 10.1016/s0955-0674(00)00250-7. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins AL, Nannizzi-Alaimo L, Silver D, et al. Tyrosine phosphorylation of the beta3 cytoplasmic domain mediates integrin-cytoskeletal interactions. J Biol Chem. 1998;273:13878–13885. doi: 10.1074/jbc.273.22.13878. [DOI] [PubMed] [Google Scholar]
- 14.Gaus K, Le Lay S, Balasubramanian N, Schwartz MA, et al. Integrin-mediated adhesion regulates membrane order. J Cell Biol. 2006;174:725–734. doi: 10.1083/jcb.200603034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen LA, Guan JL. Mechanisms of focal adhesion kinase regulation. Curr Cancer Drug Targets. 2005;5:629–643. doi: 10.2174/156800905774932798. [DOI] [PubMed] [Google Scholar]
- 16.Frame MC. Src in cancer: deregulation and consequences for cell behavior. Biochim Biophys Acta. 2002;1602:114–130. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 17.Turner CE. Paxillin interactions. J Cell Sci. 2000;23:4139–4140. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- 18.Duong LT, Rodan GA. Integrin-mediated signaling in the regulation of osteoclast adhesion and activation. Front Biosci. 1998;3:757–768. doi: 10.2741/A319. [DOI] [PubMed] [Google Scholar]
- 19.Gockerman A, Prevette T, Jones JI, Clemmons DR. Insulin-like growth factor (IGF) binding proteins inhibit the smooth muscle cell migration responses to IGF-I and IGF-II. Endocrinol. 1995;135:4168–4173. doi: 10.1210/endo.136.10.7545099. [DOI] [PubMed] [Google Scholar]
- 20.Jones JI, Doerr ME, Clemmons DR. Cell migration: interactions among integrins, IGFs and IGFBPs. 1995;6:319–327. doi: 10.1016/0955-2235(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 21.Zheng B, Clemmons DR. Blocking ligand occupancy of the alphaVbeta3 integrin inhibits insulin-like growth factor-I signaling in vascular smooth muscle cells. Proc Natl Acad Sci USA. 1998;95:11217–11222. doi: 10.1073/pnas.95.19.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling Y, Maile LA, Badley-Clarke J, Clemmons DR. DOK-1 mediates SHP-2 binding to the alphaVbeta3 integrin and thereby regulates insulin-like growth factor I signaling in cultured vascular smooth muscle cells. J Biol Chem. 2005;280:3151–3158. doi: 10.1074/jbc.M411035200. [DOI] [PubMed] [Google Scholar]
- 23.Maile LA, Clemmons DR. Regulation of insulin-like growth factor I receptor dephosphorylation by SHPS-1 and the tyrosine phosphatase SHP-2. J Biol Chem. 2002;277:8955–8960. doi: 10.1074/jbc.M109258200. [DOI] [PubMed] [Google Scholar]
- 24.Oshima K, Ruhul Amin AR, Suzuki A, et al. SHPS-1, a multifunctional transmembrane glycoprotein. FEBS Lett. 2002;519:1–7. doi: 10.1016/s0014-5793(02)02703-5. [DOI] [PubMed] [Google Scholar]
- 25.Ling Y, Maile LA, Clemmons DR. Tyrosine phosphorylation of the beta3-subunit of the alphaVbeta3 integrin is required for membrane association of the tyrosine phosphatase SHP-2 and its further recruitment to the insulin-like growth factor I receptor. Mol Endocrinol. 2003;17:1824–1833. doi: 10.1210/me.2003-0143. [DOI] [PubMed] [Google Scholar]
- 26.Imai Y, Clemmons DR. Roles of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways in stimulation of vascular smooth muscle cell migration and deoxyriboncleic acid synthesis by insulin-like growth factor-I. Endocrinol. 1999;140:4228–4235. doi: 10.1210/endo.140.9.6980. [DOI] [PubMed] [Google Scholar]
- 27.Ling Y, Maile LA, Lieskovska J, et al. Role of SHPS-1 in the regulation of insulin-like growth factor I-stimulated Shc and mitogen-activated protein kinase activation in vascular smooth muscle cells. Mol Biol Chem. 2005;16:3353–3364. doi: 10.1091/mbc.E04-10-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieskovska J, Ling Y, Badley-Clarke J, Clemmons DR. The role of Src kinase in insulin-like growth factor-dependent mitogenic signaling in vascular smooth muscle cells. J Biol Chem. 2006;281:25041–25053. doi: 10.1074/jbc.M602866200. [DOI] [PubMed] [Google Scholar]
- 29.Robertson LA, Kim AJ, Werstuck GH. Mechanisms linking diabetes mellitus to the development of atherosclerosis: a role for endoplasmic reticulum stress and glycogen synthase kinase-3. Can J Physiol Pharmacol. 2006;84:39–48. doi: 10.1139/Y05-142. [DOI] [PubMed] [Google Scholar]
- 30.Cercek B, Fishbein MC, Forrester JS, et al. Induction of insulin-like growth factor-I messenger RNA in rate aorta after balloon denudation. Circ Res. 1990;66:1755–1760. doi: 10.1161/01.res.66.6.1755. [DOI] [PubMed] [Google Scholar]
- 31.Khorsandi MJ, Fagin JA, Giannella-Neto D, et al. Regulation of insulin-like growth factor-I and its receptor in rat aorta after balloon denudation. Evidence for local bioactivity. J Clin Invest. 1992;90:1926–1931. doi: 10.1172/JCI116070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu B, Zhao G, Witte DP, et al. Targeted overexpression of IGF-I in smooth muscle cells of transgenic mice enhances neointimal formation through increased proliferation and cell migration after intraarterial injury. Endocrinol. 2001;142:3598–3606. doi: 10.1210/endo.142.8.8331. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M, Smith EP, Kuroda H, et al. Targeted expression of a protease-resistant IGFBP-4 mutant in smooth muscle of transgenic mice results in IGFBP-4 stabilization and smooth muscle hypotrophy. J Biol Chem. 2002;277:21285–21290. doi: 10.1074/jbc.M112082200. [DOI] [PubMed] [Google Scholar]
- 34.Hayry P, Myllarniemi M, Aavik E, et al. Stabile D-peptide analog of insulin-like growth factor-1 inhibits smooth muscle cell proliferation after carotid ballooning injury in rat. FASEB J. 1995;9:1336–1344. doi: 10.1096/fasebj.9.13.7557024. [DOI] [PubMed] [Google Scholar]
- 35.Campbell M, Allen WE, Silversides JA, Trimble ER. Glucose-induced phosphatidylinositol 3-kinase and mitogen-activated protein kinase-dependent upregulation of the platelet-derived growth factor-beta receptor potentiates vascular smooth muscle cell chemotaxis. Diabetes. 2003;52:519–526. doi: 10.2337/diabetes.52.2.519. [DOI] [PubMed] [Google Scholar]
- 36.Holmes DIR, Wahab NA, Mason RM. Identification of glucose-regulated genes in human mesangial cells by mRNA differential display. Biochem and Biophys Res Comm. 1997;238:179–184. doi: 10.1006/bbrc.1997.7265. [DOI] [PubMed] [Google Scholar]
- 37.Kawamura H, Yokote K, Asaumi S, et al. High glucose-induced upregulation of osteopontin is mediated via Rho/Rho kinase pathway in cultured rat aortic smooth muscle cells. Aterioscler Thromb Vas Biol. 2004;24:276–281. doi: 10.1161/01.ATV.0000112012.33770.2a. [DOI] [PubMed] [Google Scholar]
- 38.Stenina OI, Krukovets I, Wang K, et al. Increased expression of thrombospondin-1 in vessel wall of diabetic zucker rat. Circ. 2003;107:3209–3215. doi: 10.1161/01.CIR.0000074223.56882.97. [DOI] [PubMed] [Google Scholar]
- 39.Maile LA, Busby WH, Sitko K, et al. Insulin-like growth factor-I signaling in smooth muscle cells is regulated by ligand binding to the 177CYDMKTTC184 sequence of the beta3-subunit of the alphaVbeta3. Mol Endocrinol. 2006;20:405–413. doi: 10.1210/me.2005-0241. [DOI] [PubMed] [Google Scholar]
- 40.Maile LA, Busby WH, Sitko K, et al. The heparin binding domain of vitronectin is the region that is required to enhance insulin-like growth factor-I signaling. Mol Endocrinol. 2005;20:881–892. doi: 10.1210/me.2005-0382. [DOI] [PubMed] [Google Scholar]