Abstract
A 4.3-kb rat aquaporin-5 (Aqp5) promoter that directs lung and salivary cell-specific expression in vitro directs low level expression of a GFP reporter in lungs of transgenic mice. Alignment of rat, mouse and human AQP5 genomic sequences identified a highly conserved region in the 3′ portion of intron 1, here termed ci1. To investigate the role of ci1 in Aqp5 expression, transient transfections were undertaken in AQP5-expressing mouse lung epithelial (MLE-15) and rat salivary (Pa-4) cells and AQP5-non-expressing NIH/3T3 cells. A 536 bp ci1 fragment enhanced transcriptional activity of the rat Aqp5 minimal promoter specifically in MLE-15 cells in an orientation-independent manner. Enhancer activity was Aqp5 promoter-specific, since no increase in activity was detected with the TK promoter. These results suggest that expression of transgenes in mouse lungs under direction of the 4.3 kb rat Aqp5 promoter may be augmented by inclusion of ci1 in transgenic constructs.
Keywords: transcriptional regulation, gene expression, transient transfections, cell lines, aquaporin water channels
INTRODUCTION
The Aqp5 gene encodes the water channel protein aquaporin-5 (AQP5) and is highly expressed in lung, salivary glands and lacrimal tissue [1–8]. In adult rat lung alveolar epithelium, AQP5 is selectively expressed on the apical surface of alveolar epithelial type I cells [2,4,5]. We have previously shown that a 4.3 kb upstream promoter fragment of Aqp5 is capable of driving high levels of expression of a luciferase reporter specifically in cell lines derived from lung (MLE-15), and salivary gland (Pa-4) [9]. However, efforts to direct transgenic expression of a green fluorescent protein (GFP) reporter using the same 4.3 kb upstream fragment of Aqp5 have resulted in low levels of expression in lung, despite the fact that expression in salivary gland is high [10]. These findings suggest that transcriptional regulation of the Aqp5 gene in vivo differs between lung and salivary gland, where sequences located outside the 4.3 kb fragment are needed for high level expression in lung.
One approach to identify potentially important regulatory elements is to perform multi-species alignments of genomic DNA. Evolutionary conservation of non-coding sequences suggests important regulatory (including enhancer) functions. In an effort to identify sequences of gene regulatory importance outside the Aqp5 promoter, we performed alignments of rat, mouse and human genomic AQP5 DNA sequences using the VISTA computational tools at http://genome.lbl.gov/vista/index.shtml [11,12]. We found a high level of conservation in intron 1, suggesting the presence of an intronic enhancer, possibly of importance for expression in lung. We investigated the role of this conserved intron 1 fragment (here called ci1) in regulating Aqp5 gene expression by performing transient transfection assays. We used both AQP5-expressing (MLE-15 and Pa-4) and non-expressing (NIH/3T3) cell lines to determine whether any potential enhancer activity was correlated to endogenous Aqp5 expression. By using one lung-derived cell line (MLE-15) and one salivary gland-derived cell line (Pa-4), we could determine whether any potential enhancer function of ci1 could be attributed more specifically to the lung. Additionally, luciferase reporter constructs harboring either the homologous rat Aqp5 or heterologous thymidine kinase (TK) minimal promoter were generated in order to test whether any enhancer activity was specific to the Aqp5 minimal promoter or if activity also could be detected in the context of a heterologous promoter.
Materials and methods
Maintenance of cell lines and transient transfection assays
MLE-15 cells (a generous gift from J. Whitsett, University of Cincinnati) were cultivated in 25 cm2 culture flasks (BD Biosciences, San Jose, CA) in HITES medium [9]. Pa-4 cells (a generous gift from D. O. Quissell, University of Colorado) were maintained in 25 cm2 culture flasks as described [9]. NIH/3T3 cells were obtained from ATCC and maintained in DMEM (Dulbecco’s Modified Eagles’ medium) supplemented with 2 mM glutamine and 10% fetal bovine serum (FBS). Transient transfection assays were performed in triplicate on 24-well Primaria plates (BD Biosciences). Briefly, 1μg of test plasmid DNA was added per well together with 25 ng of phRL-TK plasmid (Promega, San Luis Obispo, CA) expressing Renilla luciferase used for normalization of transfection efficiency. Corrections were made for plasmid size differences to ensure that the same molar amounts of the test vectors were added. MLE-15 cells were plated at 60,000 cells/well and NIH/3T3 cells at 80,000 cells/well 24 hr prior to transfection. Both cell lines were transfected using 6 μl/well of Superfect reagent (Qiagen, Valencia, CA). Pa-4 cells were plated at 100,000 cells/well, and transfected 24 hr later using 1.6 μl/well of Lipofectamine (Invitrogen, Carlsbad, CA). Cells were harvested 48 hr after transfection and the Dual-Luciferase Reporter Assay System (Promega) was used to measure firefly luciferase activity from the test plasmid and Renilla luciferase activity from phRL-TK.
Construction of luciferase reporter plasmids
Vectors constructed for transient transfection experiments were all based on the firefly luciferase reporter plasmid pGL2-Basic (Promega). The rat Aqp5 minimal promoter (bp -173 to -6, relative to the ATG start codon) was generated by exonuclease deletion of a larger Aqp5 genomic fragment that had previously been cloned upstream of the luciferase gene [9], creating the vector pGL2-Aqp5-Pmin. The conserved intron 1 (ci1) fragment of the rat Aqp5 gene was amplified by PCR and in the same process equipped with flanking restriction enzyme sites appropriate for vector construction as described below. The ci1 fragment includes 523 bp of genomic sequence, corresponding to nucleotides 478–1001 in the 1033 bp rat Aqp5 intron 1 sequence. PCR products were subcloned into pCR-Blunt II-TOPO (Invitrogen) and verified by sequencing. The ci1 fragment was then cloned into the SmaI site 5′ of the Aqp5 minimal promoter in pGL2-Aqp5-Pmin, in both a forward and a reverse orientation, generating the constructs pGL2-Aqp5-Pmin/USF (upstream forward) and pGL2-Aqp5-Pmin/USR (upstream reverse). As positive control, we used a previously characterized construct (pGL2-180F-170) containing an additional 180 bp of rat Aqp5 promoter-enhancer sequence, located immediately 5′ of the -170 minimal promoter fragment [9]. The thymidine kinase (TK) promoter was obtained from the vector phRL-TK (Promega) as a BglII/HindIII fragment and was cloned into BglII/HindIII sites upstream of the luciferase gene in pGL2-Basic to generate pGL2-TK. The ci1 fragment was then cloned into the KpnI site 5′ of the TK promoter, in both directions generating pGL2-TK/USF and pGL2-TK/USR.
Statistical analysis
Data are shown as mean ± standard error of the mean. Statistical analysis was performed by two-way analysis of variance (ANOVA), followed by post-hoc comparisons of group means using the Bonferroni post-tests. p< 0.05 was considered significant.
Results
Identification of evolutionary conservation in Aqp5 intron 1
We performed alignments of rat, mouse and human genomic AQP5 DNA sequences using the VISTA browser (http://genome.lbl.gov/vista/index.shtml) and identified the presence of a highly conserved sequence within Aqp5 intron 1, potentially suggesting an intronic enhancer, possibly of importance for high level expression in lung. Intron 1 of the rat Aqp5 gene includes 1033 base pairs, and the evolutionarily conserved sequence resides in the 3′ part of the intron. Maximum homology is 82% within the region corresponding to nucleotides 556–882 in the rat intron 1 sequence.
Subcloning of conserved intron 1 and generation of constructs for transient transfections
The conserved intron 1 fragment (ci1) of the rat Aqp5 gene (Fig. 1) was amplified by PCR and included in constructs used in transient transfection assays. The amplified ci1 fragment contains 523 bp of genomic sequence, corresponding to nucleotides 478–1001 of rat intron 1. The firefly luciferase reporter plasmid pGL2-basic was used to make a series of constructs utilized in this study (Fig. 2). First, a construct was generated harboring the Aqp5 minimal promoter fragment (Pmin) immediately upstream of the luciferase reporter. In parallel, another construct harboring the TK promoter in the same position in pGL2-basic was engineered. The ci1 fragment was subsequently cloned upstream of the respective promoter, in both a forward and a reverse direction to test for enhancer activity. As a positive control in transient transfections, we used a previously characterized construct [9] here called 180F-170, containing an additional 180 bp of Aqp5 promoter-enhancer sequence, located immediately 5′ of the -170 minimal promoter fragment (Fig. 1 and 2). Activities of the vectors harboring only the Aqp5 minimal promoter (Pmin) and the TK promoter (TK), respectively, were used as baseline activities.
Figure 1.
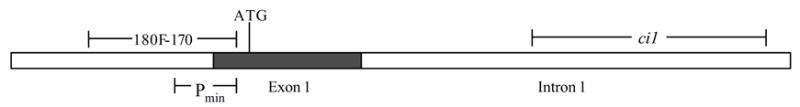
Schematic of Aqp5 minimal promoter, exon 1 and conserved intron 1. The minimal promoter (Pmin) from the rat Aqp5 gene (nucleotides −6 to −173 relative to the ATG start codon) is shown, along with the location of the conserved intron 1 (ci1) fragment and the extended 180F-170 Aqp5 promoter fragment (nucleotides −353 to −6 relative to the ATG start codon). These fragments were cloned into pGL2-Basic vector to generate the constructs shown in Figure 2.
Figure 2.
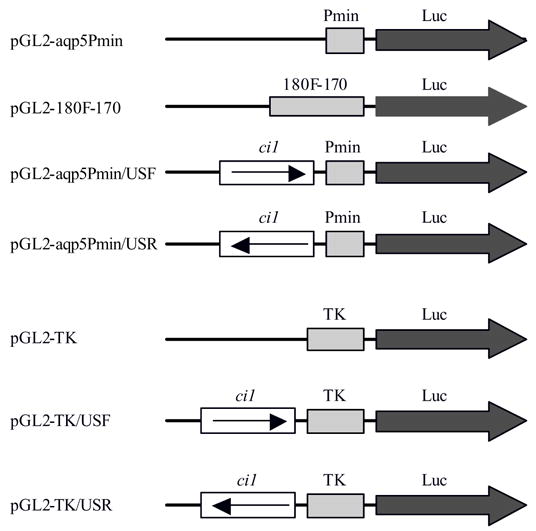
Constructs used in transient transfection assays. Luc represents the firefly luciferase reporter gene, Pmin the Aqp5 minimal promoter, 180F-170 an extended fragment of the Aqp5 promoter, ci1 the conserved intron 1 fragment, and TK the herpes simplex thymidine kinase promoter. In the transfection experiments, pGL2-Aqp5-Pmin and pGL2-TK served as reference (baseline) vectors. The pGL2-180F-170 vector was used as positive control, while all vectors containing the ci1 fragment were test vectors. The ci1 fragment was tested for enhancer activity by placing it in either a forward or a reverse position upstream of the promoters.
Transient transfections
In transient transfection experiments, we used three different cell lines: MLE-15 (mouse lung epithelial) cells [13], Pa-4 (rat parotid acinar) cells [14], and NIH/3T3 (mouse embryo fibroblast) cells [15]. Both MLE-15 and Pa-4 cells express the Aqp5 gene [9], and were used in this study to represent lung and salivary gland, respectively, two AQP5-expressing organs. NIH/3T3 cells, of mouse embryo fibroblast origin [15], do not express AQP5 [9] and were used to represent an AQP5-non-expressing cell type.
In transient transfections in MLE-15 cells, the Pmin/USF and Pmin/USR vectors directed substantial increases in luciferase activity, ~8- and 11-fold, respectively, relative to Pmin (Fig. 3). These results demonstrate that ci1 harbors enhancer activity, since activity is independent of the direction of the ci1 insert. The 180F-170 control vector showed a robust 14-fold increase in luciferase activity compared to Pmin (Fig. 3). When MLE-15 cells were transfected with the TK promoter constructs a small (1.4-fold) but statistically significant increase was observed with TK/USF, but not with TK/USR (Fig. 4).
Figure 3.
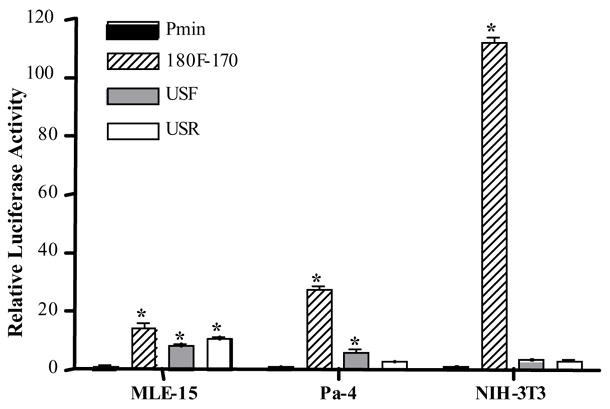
Transient transfections in MLE-15, Pa-4 and NIH/3T3 cells with constructs harboring the Aqp5 minimal promoter. * = luciferase activity significantly different from Pmin, p<0.001.
Figure 4.
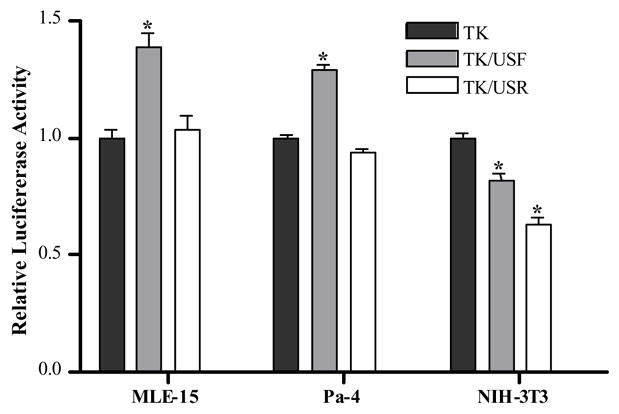
Transient transfections in MLE-15, Pa-4 and NIH/3T3 cells with constructs harboring the TK promoter. * = luciferase activity significantly different from Pmin, p<0.001.
In Pa-4 cells, transfections with the Pmin/USF and Pmin/USR constructs resulted in increased reporter expression of ~6- and 3-fold, respectively (Fig. 3). However, only the Pmin/USF construct directed an increase that was statistically significant, indicating that in Pa-4 cells ci1 does not strictly meet the criteria for enhancer function. In Pa-4 cells, the 180F-170 control vector directed a 27-fold increase in luciferase activity compared to Pmin (Fig. 3). Similar to MLE-15 cells, transfection of Pa-4 cells with the TK/USF vector, but not TK/USR, resulted in a small (1.3-fold) but significant increase in luciferase reporter activity (Fig. 4). In NIH/3T3 cells, very high luciferase activity was evident after transfection with the 180F-170 positive control vector, an approximate 112-fold increase in reporter activity (Fig. 3). Pmin/USF and Pmin/USR both resulted in ~3-fold increases which were not statistically significant. Transfection of NIH/3T3 cells with TK/USF and TK/USR, resulted in small but statistically significant decreases in luciferase activity (Fig. 4). Altogether, the transfection results demonstrate that ci1 meets the criteria for an enhancer in the context of the homologous Aqp5 minimal promoter, but not the TK promoter. Statistically significant enhancer activity was observed in MLE-15 cells only, not in Pa-4 and NIH/3T3 cells. Activities of the Aqp5-Pmin reporter constructs in all three cell lines are summarized in Table 1, where activity of the pGL2-Aqp5-Pmin construct is specified as 1.00.
Table 1.
Summary of transfection results with the Aqp5-Pmin constructs
| Cell line | Pmin | 180F-170 | USF | USR |
|---|---|---|---|---|
| MLE-15 | 1.00 | 14.3 | 8.33 | 10.7 |
| Pa-4 | 1.00 | 27.3 | 5.73 | 2.75 |
| NIH/3T3 | 1.00 | 112 | 3.02 | 2.85 |
Numbers indicate fold induction of luciferase activity relative to reference vector Pmin
Discussion
In this study, we identified enhancer activity in an evolutionarily conserved region of intron 1 in the rat aquaporin 5 (Aqp5) gene. The conserved part of intron 1 (ci1), was able to enhance luciferase reporter activity in the lung cell line MLE-15 when placed in either direction upstream of the Aqp5 minimal promoter. However, in Pa-4 cells, ci1 was only able to increase reporter expression significantly when placed in a forward direction, thus not meeting the requirements for true enhancer activity. In NIH/3T3 cells, neither of the constructs Pmin/USF and Pmin/USR was able to direct a statistically significant increase in luciferase activity. In the context of the heterologous TK promoter, ci1 did not fulfill the criteria for an enhancer in MLE-15 or Pa-4 cells since significantly increased reporter activity was detected only when ci1 was placed in a forward orientation. In NIH/3T3 cells, ci1 had a small but statistically significant repressor effect on activity of TK constructs. These results demonstrate that ci1 exhibits significant enhancer activity only in the context of the Aqp5 minimal promoter in MLE-15 cells, suggesting a more important role for ci1 in regulating AQP5 expression in lung.
Activity of the proximal enhancer present immediately upstream of the Aqp5 minimal promoter in the 180F-170 positive control construct was extremely high in NIH/3T3 cells, despite the fact that this cell line does not express endogenous Aqp5. Previous transfection experiments with luciferase constructs containing the more extended 4.3 kb upstream fragment of Aqp5 have shown very low activity in NIH/3T3 cells [9]. One possible interpretation of these observations is that, in contrast to the 180F-170 construct, the 4.3 kb fragment contains upstream elements that bind repressors present in NIH/3T3 cells only, eliminating activity of the proximal enhancer in these non-AQP5-expressing cells.
We analyzed the rat ci1 fragment for potential transcription factor binding sites using MatInspector (Genomatix). Among others, three potential sites for GATA-binding factors and one site for Sp1 were found in ci1 (data not shown). These transcription factors have been shown to be important regulators of the Aqp5 promoter [16,17] and might also be of importance for Aqp5 gene regulation through binding to the ci1 enhancer fragment. The role of these and other transcription factors in enhancing gene expression through interactions with consensus sites in ci1 will be characterized in future studies.
Intronic enhancers have previously been identified in a number of genes, an example of which is the immunoglobulin heavy chain locus [18–20] which is only active in antibody producing B-cells. Another example of a tissue-specific intronic enhancer is found in the cystic fibrosis transmembrane regulator, where an element in intron 1 has been demonstrated to have an important role in regulating expression in the small intestine, but not lung, in transgenic mice [21].
Aquaporin-5 (AQP5) is a member of a family of water channel proteins [22, 23] and is expressed at high levels in the lung, salivary gland and lacrimal tissues [1–8]. In the human, mouse and rat genome, the Aqp5 gene is located in a closely spaced tandem arrangement with Aqp2 and Aqp6 [24]. All three genes are similar in genomic organization with four exons and, interestingly, multispecies VISTA alignment reveals that they all have intronic sequences that have been conserved during evolution. In the Aqp2 gene, the conserved intronic sequence is located in intron 1, while in Aqp6 it is located in intron 3. Furthermore, evolutionary analysis of aquaporins has shown that these genes have evolved by several rounds of gene duplication [25]. The most recent gene duplication gave rise to Aqp2 and Aqp5, and it is interesting that both these genes show evolutionary conservation of the 3′ portion of intron 1. This structural similarity is compelling and suggests that an important function in intron 1 of both Aqp2 and Aqp5 has been retained through gene duplication and further evolution.
Adult alveolar epithelium is comprised of two morphologically and functionally distinct cell types, type I (AT1) and type II (AT2) cells. AT2 cells have been extensively characterized with regard to their role in surfactant production, whereas AT1 cells have been presumed to provide the major surface for gas exchange across the lung. However, the relative importance of each cell type for certain homeostatic functions within the alveolar epithelium (e.g., ion transport) remains unclear. One approach to establishing the importance of a given cell type for a specific function in vivo is to perform cell-specific (conditional) gene knockouts using the Cre/loxP recombination system. However, this approach requires availability of a cell-specific promoter to drive expression of Cre. Mice are available for this purpose to make AT2 cell-specific knockouts [26,27], but no transgenic mouse line yet exists that expresses Cre specifically in AT1 cells. In adult lung alveolar epithelium, AQP5 is selectively expressed in alveolar type I cells [2,4,5,9], suggesting that the Aqp5 promoter is potentially useful to direct expression of Cre in AT1 cells. In an attempt to characterize promoter elements of the Aqp5 gene required to direct expression specifically in AT1 cells in vivo, we generated transgenic mice in which a GFP reporter was driven by a 4.3 kb upstream fragment from the rat Aqp5 gene which includes the transcriptional start site and upstream promoter sequences. Despite the fact that this 4.3 kb fragment directs salivary and lung-specific gene expression in vitro, and is capable of directing easily detectable expression of GFP in salivary gland, only low levels of GFP expression were found in lungs of transgenic mice, suggesting that additional regulatory sequences outside the 4.3 kb fragment are necessary for efficient expression in lung (and in particular AT1) cells. Based on our current observations, it is possible that expression in lungs of transgenic mice may be augmented by inclusion of ci1 (and possibly other) conserved genomic elements, together with the 4.3 kb upstream fragment, in transgenic constructs. To unequivocally establish the importance of the ci1 fragment in the full genomic context in vivo will likely require a transgenic strategy similar to that used by Stemmler et al. [28] to delineate the function of intron 2 in regulation of the E-cadherin gene. In this approach, a reporter gene (e.g., LacZ) is inserted at the ATG start codon of the Aqp5 gene with conditional deletion of the ci1 fragment using the Cre/loxP recombination system. Quantitation of LacZ reporter gene expression before and after deletion of ci1 should provide important information regarding the regulatory role of this intronic fragment in Aqp5 expression in different tissues.
In summary, we have demonstrated that an evolutionarily conserved fragment of intron 1 in the rat Aqp5 gene harbors enhancer activity. Statistically significant enhancer activity was only observed in the context of the Aqp5 promoter in the lung cell line MLE-15, but not in Pa-4 cells, despite the fact that the latter also expresses the endogenous Aqp5 gene. These results suggest that ci1 may be particularly important for enhancing Aqp5 gene expression in the lung, and that its inclusion in transgenic constructs may lead to higher levels of expression in lung epithelial cells.
Acknowledgments
This work was supported by the Hastings Foundation and National Institutes of Health Research Grants HL-38578, HL-38621, HL-38658, HL-62659, HL-64365, HL56590, HL73471, DE-10742 and DE-14183. E.D. Crandall is Hastings Professor and Kenneth T. Norris Jr. Chair of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raina S, Preston GM, Guggino WB, Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995;270:1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol. 1997;273:C1549–C1561. doi: 10.1152/ajpcell.1997.273.5.C1549. [DOI] [PubMed] [Google Scholar]
- 3.Ishida H, Hirai SI, Mita S. Immunolocalization of aquaporin homologs in mouse lacrimal glands. Biochem Biophys Res Commun. 1997;238:891–895. doi: 10.1006/bbrc.1997.7396. [DOI] [PubMed] [Google Scholar]
- 4.Funaki H, Yamamoto FH, Koyama Y, Kondo D, Yaoita E, Kawasaki K, Kobayashi H, Sawaguchi S, Abe H, Kihara I. Localization and expression of AQP5 in cornea, serous salivary glands, and pulmonary epithelial cells. Am J Physiol. 1998;275:C1151–C1157. doi: 10.1152/ajpcell.1998.275.4.C1151. [DOI] [PubMed] [Google Scholar]
- 5.Borok Z, Lubman RL, Danto SI, Zhang XL, Zabski SM, King LS, Lee DM, Agre P, Crandall ED. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. Am J Respir Cell Mol Biol. 1998;4:554–561. doi: 10.1165/ajrcmb.18.4.2838. [DOI] [PubMed] [Google Scholar]
- 6.Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K. Aquaporin-5 (AQP5), a water channel protein, in the rat salivary and lacrimal glands: immunolocalization and effect of secretory stimulation. Cell Tissue Res. 1999;95:513–521. doi: 10.1007/s004410051257. [DOI] [PubMed] [Google Scholar]
- 7.Nejsum LN, Kwon T-H, Jensen UB, Fumagalli O, Frokiaer J, Krane CM, Menon AG, King LS, Agre PC, Nielsen S. Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc Natl Acad Sci. 2002;99:511–515. doi: 10.1073/pnas.012588099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borok Z, Verkman AS. Role of aquaporin water channels in fluid transport in lung and airways. J Appl Physiol. 2002;93:2199–2206. doi: 10.1152/japplphysiol.01171.2001. [DOI] [PubMed] [Google Scholar]
- 9.Borok Z, Li X, Fernandes VF, Zhou B, Ann DK, Crandall ED. Differential regulation of rat aquaporin-5 promoter/enhancer activities in lung and salivary epithelial cells. J Biol Chem. 2000;275:26507–26514. doi: 10.1074/jbc.M910007199. [DOI] [PubMed] [Google Scholar]
- 10.Zhou B, Ann DK, Liebler JM, Li X, Crandall ED, Borok Z. 4.3-kb Aquaporin-5 promoter/enhancer directs preferential expression in lung and salivary glands of transgenic mice. Am J Respir Crit Care Med. 2003;167:A809. [Google Scholar]
- 11.Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004;32:W273–W279. doi: 10.1093/nar/gkh458. (Web Server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubchak I, Ryaboy DV. VISTA family of computational tools for comparative analysis of DNA sequences and whole genomes. In: Bina Minou., editor. Gene Mapping, Discovery and Expression, Methods and Protocols (Methods in Molecular Biology) Vol. 338. Humana Press; 2006. pp. 69–89. [DOI] [PubMed] [Google Scholar]
- 13.Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, Whitsett JA. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci. 1993;90:11029–11033. doi: 10.1073/pnas.90.23.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quissell DO, Barzen KA, Redman RS, Camden JM, Turner JT. Development and characterization of SV40 immortalized rat parotid acinar cell lines. In Vitro Cell Dev Biol Anim. 1998;34:58–67. doi: 10.1007/s11626-998-0054-5. [DOI] [PubMed] [Google Scholar]
- 15.Jainchill JL, Aaronson SA, Todaro GJ. Murine sarcoma and leukemia viruses: Assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969;4:549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Honghua, Lu Min Min, Zhang Lili, Whitsett Jeffrey A, Morrisey Edward E. GATA6 regulates differentiation of distal lung epithelium. Development. 2002;129:2233–2246. doi: 10.1242/dev.129.9.2233. [DOI] [PubMed] [Google Scholar]
- 17.Nomura J, Horie I, Seto M, Nagai K, Hisatsune A, Miyata T, Isohama Y. All-trans retinoic acid increases expression of aquaporin-5 and plasma membrane water permeability via transactivation of Sp1 in mouse lung epithelial cells. Biochem Biophys Res Commun. 2006;351:1048–53. doi: 10.1016/j.bbrc.2006.10.159. [DOI] [PubMed] [Google Scholar]
- 18.Neuberger MS. Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells. EMBO J. 1983;2:1373–1378. doi: 10.1002/j.1460-2075.1983.tb01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillies SD, Morrison SL, Oi VT, Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983;33:717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- 20.Banerji J, Olson L, Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983;33:729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- 21.Rowntree RK, Vassaux G, McDowell TL, Howe S, McGuigan A, Phylactides M, Huxley C, Harris A. An element in intron 1 of the CFTR gene augments intestinal expression in vivo. Hum Mol Genet. 2001;10:1455–1464. doi: 10.1093/hmg/10.14.1455. [DOI] [PubMed] [Google Scholar]
- 22.Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- 23.King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 24.Ma T, Yang B, Umenishi F, Verkman AS. Closely spaced tandem arrangement of AQP2, AQP5, and AQP6 genes in a 27-kilobase segment at chromosome locus 12q13. Genomics. 1997;43:387–389. doi: 10.1006/geno.1997.4836. [DOI] [PubMed] [Google Scholar]
- 25.Zardoya R, Villalba S. A phylogenetic framework for the aquaporin family in eukaryotes. J Mol Evol. 2001;52:391–404. doi: 10.1007/s002390010169. [DOI] [PubMed] [Google Scholar]
- 26.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 2002;11:21–29. doi: 10.1023/a:1013986627504. [DOI] [PubMed] [Google Scholar]
- 28.Stemmler MP, Hecht A, Kemler R. E-cadherin intron 2 contains cis-regulatory elements essential for gene expression. Development. 2005;132:965–976. doi: 10.1242/dev.01662. [DOI] [PubMed] [Google Scholar]


