Abstract
Mammalian cells utilize multiple signaling mechanisms to protect against the osmotic stress that accompanies plasma membrane ion transport, solute uptake, and turnover of protein and carbohydrates (Schliess, F., and Haussinger, D. (2002) Biol. Chem. 383, 577-583). Recently, osmotic stress was found to increase synthesis of bisdiphosphoinositol tetrakisphosphate ((PP)2-InsP4), a high energy inositol pyrophosphate (Pesesse, X., Choi, K., Zhang, T., and Shears, S. B. (2004) J. Biol. Chem. 279, 43378-43381). Here, we describe the purification from rat brain of a diphosphoinositol pentakisphosphate kinase (PPIP5K) that synthesizes (PP)2-InsP4. Partial amino acid sequence, obtained by mass spectrometry, matched the sequence of a 160-kDa rat protein containing a putative ATP-grasp kinase domain. BLAST searches uncovered two human isoforms (PPIP5K1 (160 kDa) and PPIP5K2 (138 kDa)). Recombinant human PPIP5K1, expressed in Escherichia coli, was found to phosphorylate diphosphoinositol pentakisphosphate (PP-InsP5) to (PP)2-InsP4 (Vmax = 8.3 nmol/mg of protein/min; Km = 0.34 μm). Overexpression in human embryonic kidney cells of either PPIP5K1 or PPIP5K2 substantially increased levels of (PP)2-InsP4, whereas overexpression of a catalytically dead PPIP5K1D332A mutant had no effect. PPIP5K1 and PPIP5K2 were more active against PP-InsP5 than InsP6, both in vitro and in vivo. Analysis by confocal immunofluorescence showed PPIP5K1 to be distributed throughout the cytoplasm but excluded from the nucleus. Immunopurification of over expressed PPIP5K1 from osmotically stressed HEK cells (0.2 m sorbitol; 30 min) revealed a persistent, 3.9 ± 0.4-fold activation when compared with control cells. PPIP5Ks are likely to be important signaling enzymes.
The phosphorylated inositol moiety is viewed as a fundamental signaling entity that the cell utilizes to generate combinatorially complex arrays of communication pathways with multiple functions (1). To list just a few examples, a 1,4,5-trisphosphate configuration yields a molecule (Ins(1,4,5)P3)2 that gates intracellular Ca2+ channels (2). The 3,4,5,6-tetrakisphosphate of inositol (Ins(3,4,5,6)P4) inhibits Cl- channel conductance (3). An inositol ring with six phosphates (InsP6) enhances the activity of Dbp5, a key component of a molecular ratchet that winches mRNA out of the nucleus (4). Many additional biological activities have been attributed to these and other inositol phosphates (5-7).
In the early 1990s, two groups working independently discovered a novel subgroup of the inositol phosphate signaling family in which diphosphate groups are added to Ins(1,3,4,5,6)P5 and InsP6 (8, 9), forming compounds that are now generally described as “inositol pyrophosphates” or “diphosphoinositol polyphosphates” (see Fig. 1) (10). These molecules have been reported to regulate vesicle trafficking (11), transcription (12), chemotaxis (13), telomere maintenance (14, 15), apoptosis (16, 17), and DNA repair (18) and to mediate environmental stress responses (19-22). One of these inositol pyrophosphates, PP-InsP5, has been shown to directly phosphorylate-specific target proteins in a kinase-independent manner (23).
FIGURE 1. The inositol pyrophosphate metabolic pathway.
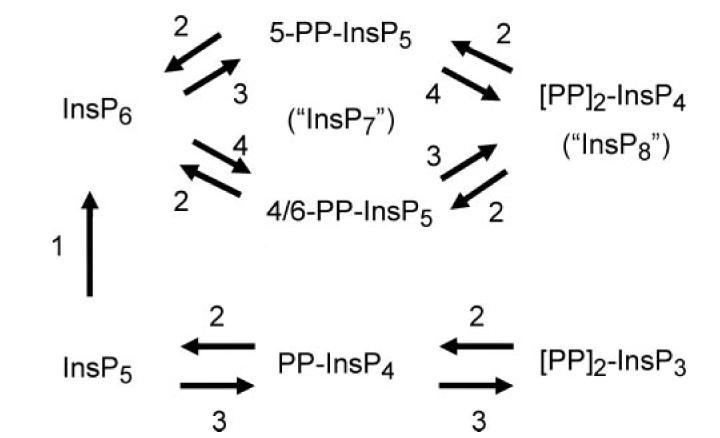
The diagram describes the enzymes that are responsible for synthesizing inositol pyrophosphates: 1, Ins(1,3,4,5,6)P5 2-kinase (57); 2, DIPP (29); 3, IP6K (24); 4, PPIP5K/Vip1 (this study and Ref. 33). It should be noted that the major isomer of PP-InsP5 to accumulate in mammalian cells is 5-PP-InsP5 (36).
The importance of inositol pyrophosphates has meant that the molecular identification of all of the enzymes that synthesize and metabolize this group of molecules has been one of the key goals of this field of research in recent years. For example, the family of kinases that synthesize PP-InsP4 and PP-InsP5 (see Fig. 1) has already been cloned (24-26). Specific phosphatases that hydrolyze the inositol pyrophosphates have also been cloned (27-29). However, the molecular identity of PPIP5K (E.C. 2.7.1.155; see Fig. 1), the enzyme that phosphorylates PP-InsP5 to (PP)2-InsP4,3 has eluded researchers for 14 years. It has been observed in mammalian cell extracts (8, 9, 30, 31) but has not previously been cloned. Yet (PP)2-InsP4 is a molecule of particular interest as a signaling entity because, alone among the inositol pyrophosphates, its levels are acutely regulated, for example, by thermal stress (19), osmotic shock (21), and cAMP (in a cAMP-dependent protein kinase-independent manner) (32). Thus, there is considerable interest in determining both the molecular nature and the modes of regulation of the enzyme(s) that synthesize (PP)2-InsP4.
In the current study, we describe the purification and sequencing of PPIP5K from the rat. We also characterize two human isoforms of PPIP5K. During the final stages of this study, York and colleagues (33) described the characterization of a novel InsP6 kinase from Saccharomyces cerevisiae, Vip1. The 4/6-PP-InsP5 that is produced from InsP6 by Vip1 regulates the transcriptional activity of a cyclin/cyclin-dependent kinase complex that controls cellular phosphate accumulation (34). Vip1 was shown to additionally phosphorylate PP-InsP5 in vitro, although it was not reported whether this reaction occurs in vivo (33). We demonstrate here that mammalian PPIP5K1 is a homologue of Vip1 that functions as an PP-InsP5 kinase both in vitro and in intact cells. Moreover, we show that this enzyme is acutely activated by osmotic stress. We conclude that PPIP5Ks are important enzymes in signal transduction.
EXPERIMENTAL PROCEDURES
Inositol Phosphate Kinase Assays
PP-InsP5 kinase activity during its purification was assayed at 37 °C in 100 μl of buffer containing 50 mm KCl, 20 mm HEPES (pH 7.2), 20 mm phosphocreatine, 10 mm NaF, 10 mm ATP, 12 mm MgSO4,1 mm EDTA, 1 mm DTT, 0.2 mg/ml bovine serum albumin, and 0.01 mg/ml phosphocreatine kinase, plus 10,000 dpm of PP-[3H]InsP5; the latter was generated in situ by preincubation for 60 min with [3H]InsP6 plus InsP6 kinase (murine type 1 (35)). Reactions were quenched, neutralized, and analyzed by Partisphere SAX HPLC as described previously (21), except that the eluate was mixed on-line with 3 volumes of Mono-flow-4 scintillant (National Diagnostics, Atlanta, GA), and radioactivity was detected by a radiometric D515 flow scintillation analyzer (Packard Instrument Co., Meriden, CT). The PP-InsP5 kinase activity of the recombinant enzyme was assayed using ∼1000 dpm of PP-[3H]InsP5, and, where indicated, the assays were supplemented with non-radioactive PP-InsP5 (36). In these experiments, assays were quenched with 50 μl of 40 mm EDTA followed by 40 μl of 2 m PCA. Neutralization was as described previously (21). Samples were then chromatographed on a 250 × 4.6-mm 5-μm Q100 column (Thompson Instruments, Clear Brook, VA) held at 40 °C using a column heater (Bio-Rad). Samples were eluted at a flow rate of 1 ml/min using a gradient generated from buffer A (1 mm EDTA) and buffer B (2 m NH4H2PO4, pH 3.35, with H3PO4/1 mm EDTA) as follows: 0-10 min, 0% buffer B, 10-11 min, 0-50% buffer B, 11-65 min = 50-64% buffer B.
In some experiments, we assayed the extent of kinase activity against [3H]InsP6, [3H]Ins(1,3,4,5,6)P5, PP-[3H]InsP4, or (PP)2-[3H]InsP3, using the kinase assay buffer described above. These reactions were analyzed by Partisphere SAX HPLC (35).
Purification of PP-InsP5 Kinase
All procedures were conducted at 0-4 °C. The starting material was 22 frozen stripped rat brains (Pel-Freeze Biologicals, Rogers, AR). These were homogenized in 80 ml of homogenization buffer comprising 20 mm HEPES (pH 6.8), 4 mm 3-((3-cholamidopropyl)dimethylammonio)-1-propanesulfonate, 1 mm DTT, and the following mixture of protease inhibitors: 100 μm 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride and 1 mg/ml each of pepstatin, aprotinin, and leupeptin. A 100,000 × g supernatant was prepared, filtered (0.45 μm), and diluted 1:1 (v/v) with medium A (20 mm HEPES, pH 6.8, 2 mm MgSO4,1 mm DTT, plus protease inhibitor mixture (see above)). This was applied to a 23 × 2.2-cm DEAE-Sepharose Fast Flow column (Amersham Biosciences, Uppsala, Sweden). The column was eluted at a flow rate of 1 ml/min with a gradient generated by mixing medium A with medium B (medium A plus 1 m NaCl) as follows: 4 CV, 0% medium B; 7.7 CV, 0-35% medium B; 0.2 CV, 35-100% medium B; 2.5 CV, 100% medium B; 15-ml fractions were collected.
The fractions that were saved after the DEAE column were dialyzed twice (2 h each) against medium C (20 mm HEPES, pH 6.8, 2 mm MgSO4), filtered (0.45 μm), and loaded onto a 16.2 × 2.2-cm heparin-agarose column (Sigma). The column was eluted at a flow rate of 1 ml/min with a gradient generated by mixing medium C with medium D (medium C plus 1 m NaCl) as follows: 2 CV, 0% medium D; 6 CV, 0-35% medium D; 3 CV, 35% medium D; 2 CV, 35-100% medium D; 2 CV, 100% D; 8-ml fractions were collected.
The fractions that were saved after the heparin-agarose column were diluted 1:1 with medium E (20 mm HEPES, pH 7.8, 2 mm MgSO4,1 mm DTT, 15 mm imidazole, 0.5 m NaCl) and loaded onto a 12.5 × 2.2-cm high performance nickel-Sepharose column (Amersham Biosciences). The column was eluted at a flow rate of 0.9 ml/min with a gradient generated by mixing medium E with medium F (medium E + 0.5 m NaCl) as follows: 2.5 CV, 0% medium F; 3 CV, 100% medium F; 2 CV, 0% medium F. Next, the column was eluted with a gradient generated by mixing medium E with medium G (medium E plus 0.5 m imidazole, pH 7.8) as follows: 6 CV, 0-20% G, 0.2 CV, 20-100% G, 2 CV, 100% G; 9-ml fractions were collected.
The single fraction that was saved after the nickel-Sepharose column was split into six aliquots, each of which was overlaid onto a 10-ml sucrose gradient (5-20% (w/v)), which was then centrifuged at 150,000 × g for 17h in a SW 45 Tirotor (Beckman). The fractions that were saved were dialyzed twice (2 h each) in 20 mm HEPES, pH 7.6, 2 mm MgSO4, 50 mm NaCl) and then concentrated to ∼60 μl, initially using an Amicon Ultra and subsequently using a Microcon (Ultracel YM-10; Amicon). Then, 3 μl of 5% SERVA Blue G dye (Serva, Heidelberg, Germany) in 0.2 m Bis-Tris buffer (pH 7.0) was added. The sample was split into two aliquots, each of which were loaded onto a blue native-PAGE gel (Invitrogen catalog number BN2001) in a native PAGE buffer system (Invitrogen catalog number BN2002). Then, 0.3-cm gel strips were manually cut from the gel and added directly to the kinase assay described above. The duplicated gel slices were analyzed by mass spectrometry (see below).
Mass Spectrometry
Lanes from the native polyacrylamide gel were digested using a Progest robotic digester (Genomic Solutions, Ann Arbor, WI). Minced gel bands were incubated twice for 15 min in 100 μl of 25mm ammonium bicarbonate/50% (v/v) acetonitrile. The gel was then dehydrated by a 20-min incubation in 100 μl of acetonitrile followed by drying under a nitrogen stream. Then, 250 ng of trypsin (Promega) was added followed by an 8-h incubation at 37 °C. The supernatants from the digests was saved. The gel was re-extracted three times: once with 50 μl of water for 20 min and twice with 20-min incubations in 50 μl of 5% (v/v) formic acid/50% (v/v) acetonitrile. Supernatants from each gel fraction were pooled, lyophilized, and resuspended in 35 μl of 0.1% formic acid.
NanoLC-ESI-MS/MS analyses were performed using an Agilent 1100 nanoLC system on-line with an Agilent XCT Ultra ion trap mass spectrometer with the chip cube interface. Briefly, 20 μl of the peptide digest was loaded onto an Agilent C18 chip (75 μm × 43 mm) followed by a 15-min wash with 5% (v/v) acetonitrile/0.1% (v/v) formic acid (buffer A). Peptides were eluted with a gradient generated from a linearly increasing concentration of acetonitrile (in 0.1% (v/v) formic acid) as follows: 0-45 min, 5-50% (v/v) acetonitrile, 45-50 min, acetonitrile increased to 95% (v/v), 50-60% acetonitrile maintained at 95%. The mass spectrometer was used in the positive ion, standard enhanced mode and included settings of a mass range from 200 to 2200 m/z, an ionization potential of 2.1 kV, an ion change control smart target (number of ions in the trap prior to scan out) of 100,000 or 200 ms of accumulation, and a 1.0-V fragmentation amplitude. MS/MS data were acquired using a data-dependent acquisition format with the six most abundant ions from each MS scan further interrogated by MS/MS. The automated switching for MS/MS required a threshold of 5000 counts.
Peak lists were generated from the data obtained from each nanoLC-ESI-MS/MS analysis using the data extractor feature of the SpectrumMill software from Agilent. The data extractor settings included limiting the data search to deconvolved ions observed between 300 and 5000 Da and a retention time between 10 min and 60 min. MS scans with the same precursor mass (±1.5 m/z) and retention time within 30 s were merged. Moreover, of the remaining MS/MS spectra, only spectra that contained sequence tag information greater than 2 residues were submitted for data base searching. The resulting extracted data were then searched against the NCBInr data base using the MS/MS Search function in the SpectrumMill software. Search settings included a trypsin specificity with up to two missed cleavages allowed, a precursor ion mass tolerance of 1.5 Da, a product ion mass tolerance of 1.0 Da, variable methionine oxidation, and a minimum matched spectral intensity of 60%. Sequence assignments of MS/MS spectra were manually validated.
Plasmid Construction
The IMAGE cDNA clone (ID 6142663; GenBank™ ID BC057395.1) encoding human PPIP5K1 was amplified by PCR using as primers 5′-CACCATGTGGTCATTGACGGCCAGTGAG-3′ and 5′-CCCTCCCTAATTTATCTCCTCAGGGACCTCCTG-3′. The PCR product was cloned into the pENTR/TEV/D-TOPO vector (Invitrogen) to construct pENTR/TEV/D-TOPO-PPIP5K1, which was subsequently used to construct pDEST527-PPIP5K1 to express recombinant protein in E. coli and also to construct pDEST501-PPIP5K1 and pDEST515-PPIP5K1 to express CFP-PPIP5K1 and FLAG tag PPIP5K1, respectively, in HEK cells.
A cDNA encoding a kinase-dead mutant, pDEST515-PPIP5K1D332A, was made using the GenTailor site-directed mutagenesis system (Invitrogen). The primers used were 5′-TGGTCATTCCTTTGTGTGTGCTGTCAATGGCTTTAG-3′ and 5′-CACACACAAAGGAATGACCATTGGCACGAAG-3′.
The IMAGE cDNA clone (ID 3913521; GenBank ID: BC024591) encoding human PPIP5K2 was amplified by PCR using primers 5′-CACCATGAGTGAAGCCCCCAGATTCTTC-3′ and 5′-CCCTCATTTCTTT TTCCCAGTGTTTTTTTTG-3′. The PCR product was cloned into the vectors described above for transfection of FLAG tag PPIP5K2 in mammalian cells. All plasmid sequences were verified using the NIEHS sequencing core.
Expression and Purification of PPIP5K1 in E. coli
BL21(DE3)STAR cells harboring pDEST527-PPIP5K1 were grown in LB medium containing ampicillin (100 μg/ml) at 30 °C to A600nm = 0.6, whereupon 0.25 mm insopropyl-1-thio-β-d-galactopyranoside was added. After 150 min of induction, cells were harvested by centrifugation (15 min at 5000 × g; 4 °C) and stored at -80 °C until used. Frozen cells were thawed and resuspended in three volumes of ice-cold lysis buffer (50 mm phosphate buffer, pH 7.5, 500 mm NaCl, 10% glycerol, 1 mm phenylmethylsulfonyl fluoride, two EDTA-free protease inhibitor mixture tablets (Roche Applied Science)). Samples were sonicated and cleared by centrifugation, and the supernatant was loaded onto a 1.2-ml TALON Superflow metal affinity column (BD Biosciences) that had been pre-equilibrated with lysis buffer. After 1 h, the column was washed with 60 ml of ice-cold lysis buffer and then eluted with 0.2- and 0.35-ml aliquots of the lysis buffer containing 50 and 300 mm imidazole, respectively. Finally, 30 μl of each fraction was loaded onto each lane of NuPAGE 4-12% precast gel (Invitrogen). After transfer to polyvinylidene difluoride membranes, samples were immunoblotted with His tag monoclonal antibody (Novagen catalog number 70796-4) and visualized with ECL Western blotting reagents (Amersham Biosciences).
Expression of PPIP5K1 and PPIP5K2 in HEK293T Cells
Cells were transfected with 4 μg of the appropriate plasmid (see above) using FuGENE and analyzed 2 days later. For some experiments, cells were harvested and lysed by vortexing for 3 min in buffer containing 25 mm HEPES, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 10% glycerol, 10 mm MgSO4,10 mm NaF, 1 mm Na3VO4, protease inhibitor mixture tablet (Roche Applied Science: catalog number 11873580001; 1 tablet/10 ml lysis buffer). The supernatant was cleared by centrifugation (15 min at 13,000 × g). Next, 2.5 mg of the cleared lysate was incubated with 50 μl of suspended volume of ANTI-FLAG® M2 affinity gel (Sigma: catalog number A2220) pre-equilibrated according to the manufacturer’s instructions. The resin was washed three times with ice-cold lysis buffer and resuspended in a final volume of 60 μl. Aliquots of this immunopurified enzyme were loaded onto SDS-PAGE gel, transferred onto polyvinylidene difluoride membrane, immunoblotted with FLAG-specific antibody (Sigma, catalog number F1804), and visualized with ECL Western blotting reagents (Amersham Biosciences). For some experiments, intact cells were also radiolabeled with 20 μCi/ml [3H] inositol (American Radiolabeled Chemicals, St. Louis, MO) for 4 days (with transfection performed on the second day of labeling). [3H]Inositol phosphates were then extracted with perchloric acid, neutralized, and analyzed by Partisphere SAX HPLC as described previously (21).
Other Materials
PP-[3H]InsP4, PP-[3H]InsP5, and (PP)2-[3H]InsP3 were prepared as described previously (35). Recombinant human Ins(1,3,4,5,6)P5 2-kinase was prepared as described previously (37).
RESULTS
The Pathway of (PP)2-InsP4 Synthesis
Some previously elusive steps in inositol phosphate synthesis have eventually been discovered to be unexpected additional activities of kinases that had previously been held responsible for different reactions. For example, the Ins(3,4,5,6)P4 1-kinase was discovered to be an additional activity of an enzyme previously designated as the Ins(1,3,4)P3 5/6-kinase (38). Later, Ins(1,3,4,6)P4 5-kinase activity was eventually found to be catalyzed by an enzyme (inositol polyphosphate multikinase) previously designated an Ins(1,4,5)P3/Ins(1,3,4,5)P4 3/6-kinase (39). We therefore investigated whether (PP)2-InsP4 might be synthesized from (PP)2-InsP3 (Fig. 1) by phosphorylation of the latter’s free 2-OH by the Ins(1,3,4,5,6)P5 2-kinase. The recombinant human Ins(1,3,4,5,6)P5 2-kinase that was used in these experiments completely phosphorylated [3H]Ins(1,3,4,5,6)P5 to [3H]InsP6 (Fig. 2A). However, there was no phosphorylation of (PP)2-[3H]InsP3 or even PP-[3H]InsP4 (Fig. 2, B and C).
FIGURE 2. Substrate specificity of the Ins(1,3,4,5,6)P5 2-kinase.
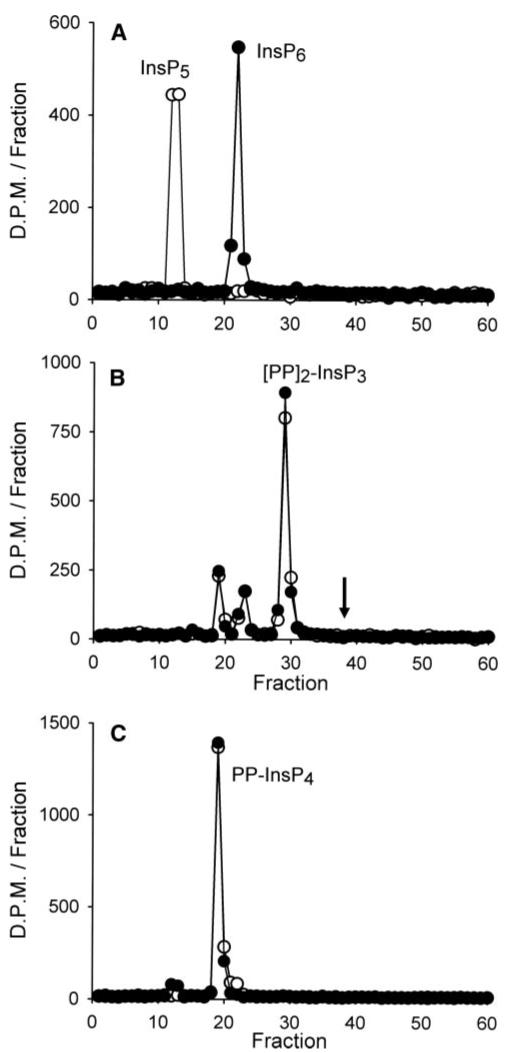
Recombinant human Ins(1,3,4,5,6)P5 2-kinase (0.5 μg) was incubated at 37 °C for either 0 min (open circles)or 30 min(closed circles)in 200 μl of kinase assay buffer (see “Experimental Procedures”) supplemented with [3H]Ins(1,3,4,5,6)P5 (A), (PP)2-[3H]InsP3 (B), or PP-[3H]InsP4 (C). Reactions were analyzed by Partisphere SAX HPLC. The arrow in panel B marks the elution position of (PP)2-InsP4.
Another multifunctional enzyme that we considered as a potential candidate for synthesizing (PP)2-InsP4 is inositol polyphosphate multikinase since we have previously shown that it can add a diphosphate group to Ins(1,3,4,5,6)P5 (40). However, when inositol polyphosphate multikinase was individually incubated either with PP-[3H]InsP5 or with 10 μm of each of the six isomers of PP-InsP5 plus [γ-32P]ATP, no (PP)2-InsP4 was formed (data not shown).
In view of the data described above, the most significant route of (PP)2-InsP4 synthesis in mammalian cells would appear to involve phosphorylation of PP-InsP5 (Fig. 1) by a novel kinase activity (30). We therefore set out to purify and sequence this enzyme.
Purification of PP-InsP5 Kinase
We purified a PPIP5K from rat brain. The PP-[3H]InsP5 used during this purification procedure was generated from [3H]InsP6 using InsP6 kinase (see “Experimental Procedures”). Brains were homogenized, and a 100,000 × g supernatant was prepared, which was then fractionated by DEAE ion-exchange chromatography (Fig. 3A). Two peaks of activity were routinely eluted with 250-350 mm NaCl. We initially surmised that the two peaks reflected the presence of two different proteins. However, when an aliquot of one of the kinase peaks (Fig. 3A, fraction 69) was co-incubated with an aliquot from a fraction (Fig. 3A, fraction 67), taken from the trough between the two peaks, all of the PP-InsP5 kinase activity was inhibited (compare Fig. 3, B and C). That is, the trough contains an inhibitor of the PP-InsP5 kinase. We found that this inhibitor was heat-stable (Fig. 3, C and D). However, the inhibitor was sensitive to incubation with avian MINPP (Fig. 3E), which is a very active InsP6 phosphatase (41). This experiment indicates that the inhibitor may be InsP6, or possibly, another MINPP substrate. Indeed, further experiments confirmed that InsP6 inhibits PP-InsP5 kinase activity (Fig. 3F). However, note that only trace amounts of PP-[3H]InsP5 substrate were present in these assays (Fig. 3). 1-2 μm levels of PP-InsP5 are present in intact cells (42); thus, InsP6 may be less potent as an inhibitor in vivo than is suggested by Fig. 3F. Since the presence of the inhibitor in the trough between the two peaks (Fig. 3A, fractions 66-68) likely masks the PP-InsP5 kinase activity in these fractions, we pooled the trough and the two peaks. We also anticipate that endogenous InsP6 in unfractionated 100,000 × g supernatant would also inhibit PP-InsP5 kinase activity. Thus, the purification parameters in Table 1 must be considered approximate.
FIGURE 3. DEAE anion-exchange chromatography of rat brain PP-InsP5 kinase activity, and an endogenous kinase inhibitor.
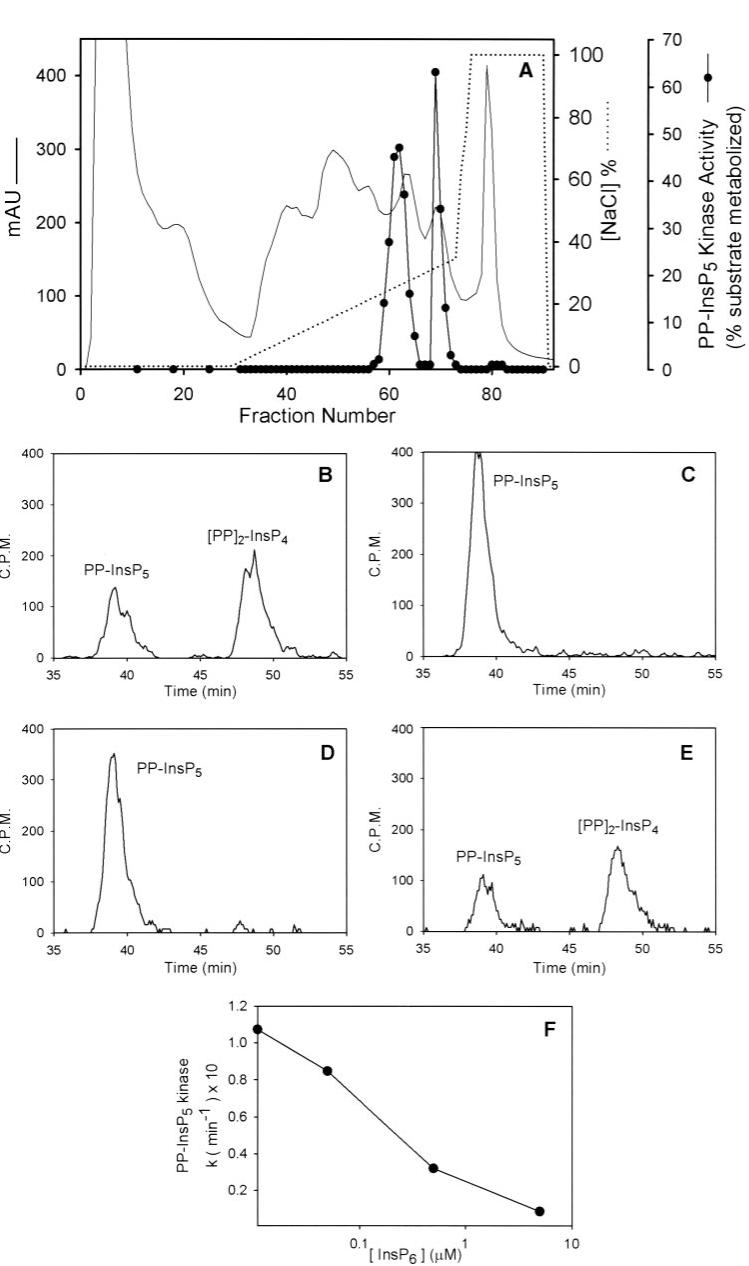
A, a 100,000 × g brain supernatant was applied to a DEAE column, and fractions were assayed for PP-InsP5 kinase activity (see “Experimental Procedures”). B,6 μl of fraction 69 (see panel A) was incubated for 15 min in kinase assay buffer as described under “Experimental Procedures.” These assays were supplemented with 10 μl of either vehicle (B)or 10-μl aliquots of fraction 67 (i.e. from between the two kinase activity peaks shown in A), which had been boiled (C, D, and E), boiled and incubated overnight at 37 °C (D), or boiled and incubated overnight at 37 °C with 5 μl of avian MINPP (E). In F, the indicated concentration of InsP6 was added to the PP-InsP5 kinase assay. Note that only trace amounts of PP-[3H]InsP5 substrate were present; thus, InsP6 is expected to be less potent as an inhibitor in vivo (see “Results”). mAU, milliabsorbance units.
TABLE 1. Purification of PPIP5K from rat brain.
The enzyme was purified as described in Figs. 3 and 4 and assayed under first-order conditions (k-1 is the first-order rate constant). The data for -fold purification and % recovery should be considered approximate because the enzyme co-purifies with an inhibitor (probably InsP6; see “Results” for details). Purification parameters for the native gel electrophoresis experiment are not available as the protein concentration in each gel slice was not determined.
| Protein | Volume | Total activity | Specific activity | Purification | Recovery | |
|---|---|---|---|---|---|---|
| mg/ml | ml | k-1/mg/min | k-1/mg/min | -fold | % | |
| Supernatant | 4.2 | 160 | 1,632 | 2.4 | 1 | 100 |
| DEAE | 1.2 | 193 | 1060 | 4.6 | 2 | 65 |
| Heparin | 0.075 | 60 | 653 | 145 | 60 | 40 |
| Ni | 0.1 | 9 | 870 | 967 | 402 | 53 |
| Sucrose | 0.04 | 3 | 429 | 3575 | 1490 | 26 |
The pooled fractions from the DEAE column were next fractionated by heparin-agarose affinity chromatography (Fig. 4A). Kinase activity was eluted from the heparin-agarose column with 0.7-0.9 m NaCl (Fig. 4A). The active fractions were combined again and further fractionated using nickel-Sepharose resin. The column was washed with 0.5 mNaCl/15 mm imidazole, and a single peak of PP-InsP5 kinase activity was eluted from the column with ∼60 mm imidazole (Fig. 4B).
FIGURE 4. Further purification of PP-InsP5 kinase from rat brain.
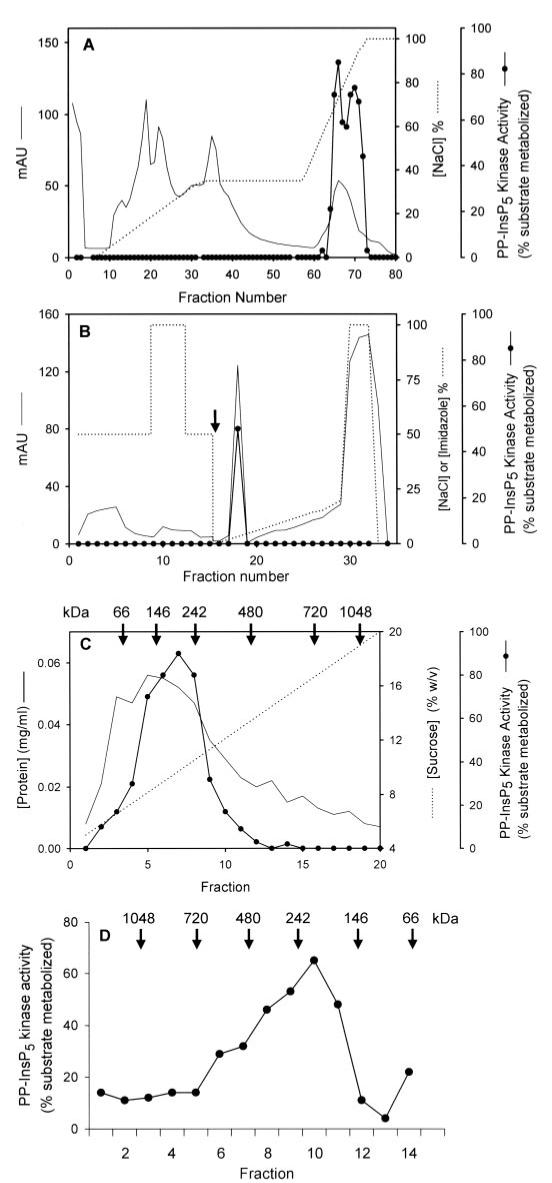
Fractions 58-72 that were eluted from the DEAE column (Fig. 2) were pooled and further purified by the following steps: heparin agarose affinity chromatography (A), nickel-Sepharose chromatography (B; the arrow designates the switch from the buffer E/F gradient to the buffer E/G gradient, see “Experimental Procedures”), sucrose density gradient centrifugation (C), and non-denaturing gel electrophoresis (D). The protein standards (Invitrogen) used for molecular mass estimations were IgM pentamer (1,048 kDa), apoferritin band 1 (720 kDa), apoferritin band 2 (480 kDa), B-phycoerythrin (242 kDa), lactate dehydrogenase (146 kDa), and bovine serum albumin (66 kDa). mAU, milliabsorbance units.
Size-exclusion chromatography (Superdex 200 10/300 GL column) indicated that the size of the PP-InsP5 kinase was ∼400 ± 50 kDa (data not shown); we took advantage of this large apparent size of the protein to further purify the active peak that eluted from the nickel-Sepharose column. First, the preparation was fractionated by sucrose density gradient centrifugation. A single peak of enzyme activity was obtained, with an apparent size of ∼200 kDa (Fig. 4C). The observation of an apparently larger enzyme during size-exclusion chromatography (see above) may indicate that dimers can form under certain conditions. The kinase activity peak was concentrated and further resolved by non-denaturing polyacrylamide gel electrophoresis, and activity was monitored with in-gel kinase assays (Fig. 4D).
During the purification of this kinase, we observed that the PP-InsP5 kinase activity co-purified with a weaker InsP6 kinase activity (data not shown). However, we cannot determine to what extent these two activities were performed by the same enzyme because we subsequently ascertained that our preparations also contained some genuine InsP6 kinase protein (see below).
Sequencing of the PP-InsP5 Kinase and Catalytic Characterization of Recombinant Protein
The peak fractions from the native gel (Fig. 4D) were sequenced by mass spectrometry. The highest scoring protein that was identified was a 160-kDa rat protein (accession number, NP_001074252). The summed unique MS/MS score of this protein was 380 with 126 distinct MS/MS spectra; 26 unique and non-overlapping peptides were assigned to this protein (Fig. 5). Note the separate, small peak of PP-InsP5 kinase activity in fraction 14. This fraction was contaminated with the InsP6 kinase protein (the summed unique MS/MS score was 29). It has previously been shown that the InsP6 kinase weakly phosphorylates PP-InsP5 (35).
FIGURE 5. Alignment of the amino acid sequences of Vip1 from yeast with the human and rat PPIP5Ks.


The alignment was created with PepTool (version 2; BioTools). The human (Hs; GenBank accession number BC057395) and rat (Rn; GenBank accession number NP_001074252) proteins are annotated in the GenBank data base as “Histidine acid phosphatase” (types 2A and 1) but are renamed here as PPIP5K1 and PPIP5K2 (for PP-InsP5 kinase types 1 and 2; see “Results” for details). The initial 128 residues of Vip1 from S. cerevisiae are omitted from the figure as they did not align with the mammalian sequences. Residues that are conserved in at least three of the proteins are highlighted in bold font. The residues that are underscored were identified in the purified native rat protein by mass spectrometry. York and colleagues (33) identified Asp-487 as a catalytically essential residue in Vip1; the above alignment suggested that Asp-332 was the equivalent residue in the human enzyme.
Molecular threading of the rat 160-kDa protein sequence using the PHYRE server (the successor to 3D-PSSM (43)) identified a “phosphoglycerate mutase-like” sequence from residues 393-917, which includes the RHG catalytic motif (residues 401-403) that typically defines members of the histidine acid phosphatase family. These include MINPP (44) and some phytases (45), which actively hydrolyze InsP6 and other inositol phosphates. Additionally, molecular threading indicates that residues 1-365 comprise a potential ATP-grasp domain, which has previously been identified in two other inositol phosphate kinases, the Ins(1,4,5)P3 3-kinase (46) and ITPK1 (47). This analysis suggested that NP_001074252 describes a protein that might be responsible for the PP-InsP5 kinase activity that we have purified. Indeed, while this work was in progress, York and colleagues (33) identified a novel InsP6 kinase, Vip1, in S. cerevisiae. This protein also phosphorylated PP-InsP5, at least in vitro (33). Vip1 has 29% amino acid identity to the sequence of the rat PPIP5K (Fig. 5).
A BLAST search identified a candidate 160-kDa human homologue of our rat protein (90% amino acid identity; 97% within the putative kinase domain; Fig. 5). We have named this protein PPIP5K1. The BLAST search also uncovered a second human isoform, PPIP5K2, a 138-kDa protein that is 66% identical to human and rat PPIP5K1 (most of the divergence of the type 1 and 2 kinases occurs in their C termini; Fig. 5).
A 5669-nucleotide cDNA that encodes human PPIP5K1 was available as an IMAGE clone (ID: 6142663; GenBank accession number BC057395.1). The 3′-non-translated region of this cDNA terminates with a poly[A] tail. The putative start codon, which begins at nucleotide 151, lies within a weak Kozak consensus (GGAGGGATGT). An in-frame stop codon lies 21 nucleotides upstream of the start codon. The full-length version of human PPIP5K1 was expressed in E. coli (which lacks endogenous PP-InsP5 kinase) with an N-terminal poly(His) tag, and then we purified the protein using nickel-agarose. The recombinant protein phosphorylated PP-InsP5 to (PP)2-InsP4 (Fig. 6A). To perform kinetic experiments on the recombinant enzyme, the mass amounts of PP-InsP5 that were required were chemically synthesized. The PP-InsP5 isomer that was prepared has the diphosphate attached to the 5-carbon since this is the major isomer in mammalian cells (36). We estimate a Km value of 0.34 μm and a Vmax of 8.3 nmol/mg of protein/min for phosphorylation of PP-InsP5 (Fig. 6B). InsP6 was also phosphorylated by PPIP5K1 but more slowly (Vmax of 0.1 nmol/mg of protein/min). The kinase did not phosphorylate Ins(1,3,4,5,6)P5, PP-InsP4, or (PP)2-InsP3 (data not shown). Despite the presence of an RHG histidine acid phosphatase motif, neither Ins(1,3,4,5,6)P5, InsP6, PP-InsP5, nor (PP)2-InsP4 was dephosphorylated by this protein (Fig. 6A and data not shown). Thus, the functional significance of this phosphatase motif remains to be determined.
FIGURE 6. Catalytic activity of human PPIP5K1.
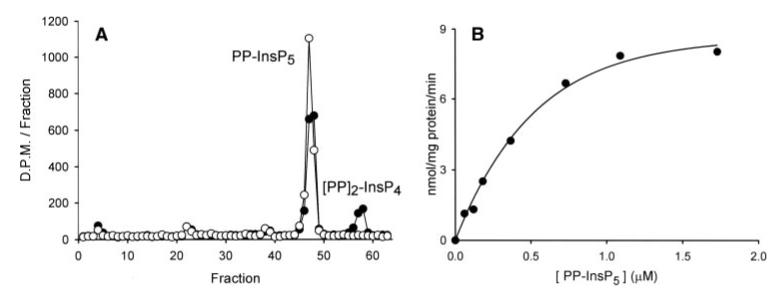
A, recombinant human PPIP5K1 (20 ng) was incubated at 37 °C in 100 μl of kinase assay buffer with PP-[3H]InsP5 for either 0 min (open circles)or 40 min(closed circles). Reactions were analyzed by Q100 HPLC. B, kinetic analysis of 5-PP-[3H]InsP5 phosphorylation using the indicated concentration of substrate. A representative experiment is shown, typical of three. The Km value = 0.34 μM, Vmax = 8.3 nmol/mg of protein/min (estimated by non-linear regression, using SigmaPlot, version 8.02).
Expression of PPIP5K1 and PPIP5K2 in HEK Cells
We examined the catalytic activities of human PPIP5K1 and PPIP5K2 in vivo by separately expressing the enzymes in HEK293T cells. In some experiments, the kinases were expressed with an N-terminal CFP tag. This allowed us to visualize the intracellular distribution of the enzyme and to verify transfection efficiency; PPIP5K1 was distributed throughout the cytosol but was excluded from the nucleus (Fig. 7). No nuclear immunofluorescence from CFP-PPIP5K1 was observed even after treatment of cells with a nuclear exportin inhibitor, leptomycin B (either 20 or 50 nm, for up to 6 h) (48). A similar intracellular distribution was observed for FLAG-tagged PPIP5K1 and CFP-PPIP5K2 (data not shown). The transfection efficiency was ∼30% in these experiments. This relatively low transfection efficiency was a consequence of our deliberately expressing the kinase at a relatively low level, after preliminary experiments revealed that high levels of expression were toxic to mammalian cells (data not shown).
FIGURE 7. The intracellular distribution of PPIP5K1.
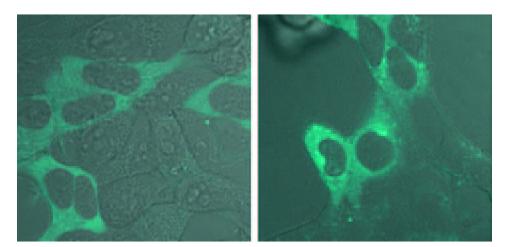
Cells were transfected with CFP-PPIP5K1; control and osmotically stressed (0.2 m sorbitol; 30 min) are shown. Confocal images were taken on a Zeiss LSM 510 META using a Plan-Apochromat ×63/1.4 oil objective. The 458-nm laser line from a krypton/argon laser was used for excitation of the CFP. A 505-nm long pass emission filter was used to collect the images with a pinhole setting of 1 airy unit.
There was a 9-fold increase in (PP)2-InsP4 levels following the overexpression of full-length FLAG-PPIP5K1 in HEK cells (Fig. 8 and Table 2). Since only 30% of these cells expressed exogenous PPIP5K1 (see above), we estimate that the actual increases in (PP)2-InsP4 levels in the successfully transfected cells were ∼30-fold. The cellular levels of (PP)2-InsP4 were increased less dramatically following overexpression of FLAG-PPIP5K2 (2.4-fold, see Table 2; 8-fold after correcting for transfection efficiency). These experiments demonstrate that PPIP5K1 and PPIP5K2 can phosphorylate PP-InsP5 in vivo. Neither isoform affected the cellular levels of any other inositol phosphates (Table 2). The latter result and our in vitro data (see above) indicate that neither PPIP5K1 nor PPIP5K2 is active as an inositol phosphate phosphatase, although the protein contains a histidine acid phosphatase motif.
FIGURE 8. The effect upon inositol phosphate levels in control and osmotically stressed HEK cells following overexpression of PPIP5K1.
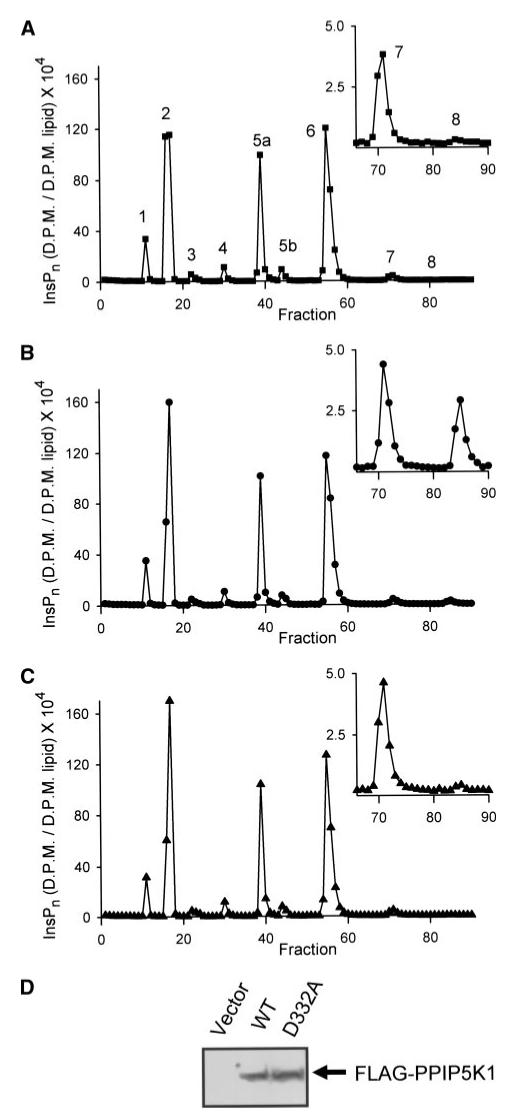
[3H]Inositol-radiolabeled HEK cells were transfected with vector (A), FLAG-PPIP5K1 (B), or FLAG-PPIP5K1D332A (C) as described under “Experimental Procedures.” The inositol phosphates were then extracted as described under “Experimental Procedures” and analyzed by Partisphere SAX HPLC. Fraction 1 corresponds to an elution time of 10 min. In panel A, each peak has a number that corresponds to the quantity of phosphates around the inositol ring. This is a representative experiment; the mean peak values from several experiments are listed in Table 2. In this HPLC gradient, there are two InsP5 peaks, 5a and 5b, corresponding to Ins(1,3,4,5,6)P5 and d/L-Ins(1,2,4,5,6)P5, respectively. Neither peak was altered by the experimental conditions used in the current study, and so are represented as total InsP5 in Table 2. PPIP5K1 expression was validated by Western analysis of 80 μg of cell lysate (D). WT, wild type.
TABLE 2. The levels of inositol phosphates in HEK cells and the effects of osmotic stress and overexpression of either PPIP5K1 or PPIP5K2.
Shown are inositol phosphate levels (104 × dpm/dpm lipids). The levels of each category of inositol phosphate (means and standard errors from 3-6 experiments) were determined as described in the legend for Fig. 8. Δ [PP]2-InsP4 refers to the change in [PP]2-InsP4 levels after subtracting the value in the control cells. *,p < 0.01 versus control (unpaired t test).
| Control | Sorbitol | PPIP5K1 | PPIP5K2 | PPIP5K1 + sorbitol | PPIP5K2 + sorbitol | PPIP5K1D332A | PPIP5K1D332A + sorbitol | |
|---|---|---|---|---|---|---|---|---|
| InsP1 | 32.7 ± 2.3 | 35.9 ± 5.4 | 34.7 ± 1.70 | 25.7 ± 4.6 | 31.5 ± 2.7 | 23.1 ± 2.8 | 34.0 ± 2.9 | 42.4 ± 7.3 |
| InsP2 | 197 ± 11.7 | 194 ± 9.5 | 195 ± 8.0 | 177 ± 12.6 | 183 ± 2.9 | 178 ± 8.5 | 209 ± 11.4 | 191 ± 6.6 |
| InsP3 | 11.1 ± 1.1 | 31.8 ± 1.2* | 10.5 ± 1.4 | 12.3 ± 1.2 | 30.5 ± 0.7* | 29.8 ± 0.8* | 12.6 ± 1.7 | 31.8 ± 0.9 |
| InsP4 | 10.1 ± 1.1 | 20.8 ± 2.7* | 10.4 ± 0.8 | 7.8 ± 1.4 | 20.2 ± 1.4* | 15.8 ± 0.9* | 12.9 ± 0.7 | 22.6 ± 2.7 |
| InsP5 | 115 ± 7.0 | 114 ± 6.0 | 114 ± 6.6 | 94.3 ± 4.8 | 109 ± 4.4 | 97.0 ± 3.8 | 121 ± 9.1 | 113.1 ± 2.7 |
| InsP6 | 217 ± 7.3 | 178 ± 4.4* | 218 ± 8.5 | 203 ± 11.2 | 176 ± 2.7 | 171 ± 10.3* | 225 ± 8.7 | 177 ± 2.3 |
| PP-InsP5 | 7.8 ± 0.4 | 4.5 ± 0.2* | 7.6 ± 0.5 | 7.9 ± 0.5 | 4.5 ± 0.2* | 5.1 ± 0.5* | 9.8 ± 0.2 | 5.2 ± 0.2 |
| [PP]2-InsP4 | 0.5 ± 0.04 | 6.8 ± 0.7* | 4.5 ± 0.5* | 1.2 ± 0.07* | 12.0 ± 1.2* | 10.0 ± 2.3* | 0.6 ± 0.1 | 5.3 ± 0.5 |
| Δ[PP]2-InsP4 | 6.3 | 4.0 | 0.7 | 11.5 | 9.5 | 0.1 | 4.8 |
In view of the relatively substantial effect of PPIP5K1 upon intracellular (PP)2-InsP4 levels, we investigated whether PPIP5K1 might be more active in the HEK cells than when it is expressed in E. coli. We therefore expressed FLAG-PPIP5K1 in HEK cells and then immunopurified the protein; the value of the Vmax was 17 ± 3.5 nmol/mg of protein/min (n = 3), which is only 2-fold higher than the activity of recombinant protein expressed in E. coli (see above).
We also transfected cells with a FLAG-PPIP5K1D332A mutant. This mutation is predicted to eliminate kinase activity (Fig. 5). The design of this mutant was based on the work of York and colleagues (33) with Vip1 (see the legend for Fig. 5). Indeed, the levels of inositol phosphates in cells transfected with the kinase-dead mutant (Fig. 8C; Table 2) were identical to the levels of inositol phosphates in cells transfected with vector (Fig. 8A; Table 2).
Activation of PPIP5K1 by Hyperosmotic Stress
The exposure of vector-transfected HEK cells to hyperosmotic stress (0.2 m sorbitol; 30 min) increased the levels of (PP)2-InsP4 ∼14-fold (Table 2). Quantitatively similar effects of osmotic stress have been reported previously (21). Levels of InsP3 and InsP4 also increased in osmotically stressed cells (∼3- and 2-fold, respectively; Table 2); this particular effect has previously been attributed to stimulation of phospholipase C-catalyzed hydrolysis of PtdIns(4,5)P2 to Ins(1,4,5)P3 (49). The stress-dependent activation of phospholipase C is independent of accelerated (PP)2-InsP4 synthesis (49). Hyperosmotic stress had one additional effect upon the inositol phosphate profile that we have not previously observed in other cell types: an 18% decrease in InsP6 levels (Table 2). Thus, the inositol phosphate family responds in several different ways to this particular environmental stress, suggesting a complex, multifunctional repertoire.
In osmotically stressed cells that also overexpressed FLAG-PPIP5K2, the increase in intracellular levels of (PP)2-InsP4 was consistently higher (by 35%) than would be expected if the individual effects of osmotic stress and overexpression were only additive (Table 2). One explanation for these data is that PPIP5K2 is an enzyme that is activated by osmotic stress. We further explored this possibility by immunopurification of FLAG-PPIP5K2 from control and sorbitol-stressed cells. The catalytic activity of the enzyme isolated from osmotically stressed cells was not significantly higher than the activity of the enzyme isolated from control cells (data not shown). However, in these experiments, it is possible that PPIP5K2 might be activated by a mechanism (such as by association with other proteins) that is not preserved during cell lysis and enzyme immunopurification.
An analysis of the effects of osmotic stress and overexpression of FLAG-PPIP5K1 upon cellular (PP)2-InsP4 levels (Table 2) is equivocal as to whether or not PPIP5K1 is activated by osmotic stress (possibly because cellular (PP)2-InsP4 levels might be near the asymptote of a hyperbolic relationship with PP-InsP5 kinase activity). Nevertheless, the PP-InsP5 kinase activity of FLAG-PPIP5K1 immunopurified from osmotically stressed cells was 3.9 ± 0.4-fold higher than enzyme purified from control cells (compare Fig. 9A with 9C). A similar degree of activation was observed when InsP6 was the substrate (compare Fig. 9B with 9D). These experiments verify that PPIP5K1 is an enzyme that is activated by osmotic stress. We also confirmed that the D332A mutant is catalytically inactive (Fig. 9, E and F) following its immunopurification (Fig. 9G).
FIGURE 9. Activation of PPIP5K1 by osmotic stress.
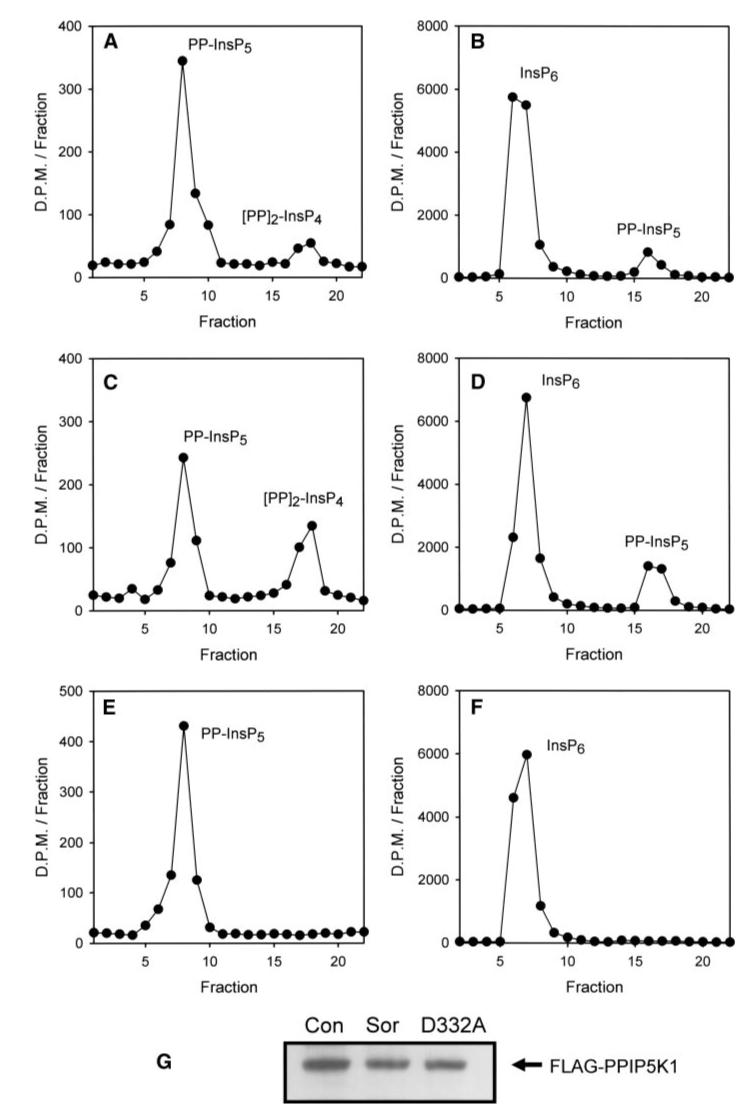
HEK cells were transfected for 48 h with either wild-type FLAG-PPIP5K1 (A-D) or kinase-dead FLAG-PPIP5K1D332A (E and F). The medium was then replaced with either control medium (A, C, and E) or medium containing 0.2 m sorbitol (B, D, and F) for 30 min. Next, cells were lysed, and the PPIP5K1 was immunopurified using anti-FLAG M2 Affinity gel. Kinase activity was then assayed under first-order conditions and analyzed by either Q100 HPLC (A, C, and E; PP-InsP5 kinase assayed using 2 μl of sample incubated for 20 min; fraction 1 corresponds to an elution time of 37 min) or Partisphere SAX HPLC (B, D, and F; InsP6 kinase assayed using 6 μl of sample for 60 min; fraction 1 corresponds to an elution time of 42 min). A representative experiment is shown, typical of three. The first-order rate constants in this experiment are as follows (h-1 μl-1): PP-InsP5 kinase, wild-type (WT) control (Con) = 0.16, WT + sorbitol (Sor) = 0.708, D332A + sorbitol = 0. InsP6 kinase, WT control = 0.017, WT + sorbitol = 0.04, D332A + sorbitol = 0. G shows the Western analysis of FLAG-PPIP5K1 after immunopurification.
DISCUSSION
Since 1993, it has been known that mammalian cells possess an enzyme activity, PPIP5K, that phosphorylates 5-PP-InsP5 to (PP)2-InsP34 (8, 9, 30, 31). Our current study finally reveals the molecular identities of the kinases that perform these reactions. We have discovered two PP-InsP5 kinases, which we name PPIP5K1 and PPIP5K2. The amino acid sequences of these enzymes do not show significant homology with other types of inositol phosphate kinases. However, molecular threading indicates that the kinase domain of PPIP5K1 and PPIP5K2 comprises an ATP-grasp structure (see “Results”). We can therefore anticipate that this portion of PPIP5Ks might have some structural similarities with either ITPK1 and/or the Ins(1,4,5)P3 3-kinases since the latter also have ATP-grasp kinase domains (46, 47, 50).
We have shown here that human PPIP5K1 is activated by osmotic stress in a manner that is preserved during its immunopurification from HEK cells (Fig. 9). It may not be widely appreciated that there are few previous demonstrations that any of the inositol phosphate kinases are acutely regulated (with the notable exception of the Ins(1,4,5)P3 3-kinases (51)). This novel finding concerning PPIP5K1 regulation also advances our earlier demonstration that intracellular (PP)2-InsP4 levels increase in response to osmotic stress (21); in that earlier study, we did not demonstrate an enzyme activity that might be responsible for this effect. This stress-dependent activation of PPIP5K1 may involve covalent modification, or instead, might be dependent upon associated regulatory proteins or cofactors. Experiments with intact cells indicated that PPIP5K2 might also be activated by osmotic stress (Table 2), but this effect was not observed following immunopurification of the enzyme (see “Results”). It is therefore possible that the regulation of PPIP5K1 and PPIP5K2 involve somewhat different mechanisms. Future studies in this area are likely to be significant because there is considerable interest in understanding the molecular processes by which cellular signaling processes help cells successfully adapt to changes in their osmotic environment. Unless the cell compensates quickly, alterations in cell hydration will prompt metabolic processes to become adversely affected, DNA will become damaged, and cell shrinkage will lead to apoptosis (52, 53). In higher organisms, kidney cells, endothelial cells, and lymphocytes routinely experience changes in extracellular osmolarity (52, 54). However, these and most other cells must also adapt to the alterations in intracellular osmolarity that inevitably accompany normal cellular activities: ion transport across the plasma membrane, solute uptake, and alterations in protein and carbohydrate turnover (53). Short term adaptations to osmotic imbalance, within milliseconds to minutes, are vital to cell survival. Perhaps (PP)2-InsP4 in part helps cells to survive osmotic stress because it facilitates repair of damaged DNA (18).
It remains to be determined whether or not (PP)2-InsP4, like PP-InsP5, can function by directly phosphorylating proteins (23). It is notable that this ability of PP-InsP5 is thought to arise out of it being a “high energy” polyphosphate (23). Since (PP)2-InsP4 is even more electrostatically and sterically congested, its own free energy of hydrolysis is predicted to be much higher than that of PP-InsP5 (23, 55), especially if, as now seems likely, (PP)2-InsP4 has vicinal diphosphates (33). The degree of protein phosphorylation by PP-InsP5, at least, is directly proportional to its concentration (23). Thus, stimulus-dependent changes in cellular levels of inositol pyrophosphates are thought to provide a mechanism to regulate protein phosphorylation. Other actions of (PP)2-InsP4 may also be controlled by the increases in its levels following osmotic stress.
Very recently, York and colleagues (33) reported that Vip1in S. cerevisiae is an active InsP6 kinase. Vip1 shares 28-29% amino acid identity with the human and rat PPIP5K1 and PPIP5K2 that we have characterized in the current study (Fig. 5). We have found that PPIP5K1 and PPIP5K2 also phosphorylate InsP6 (Fig. 9 and data not shown). However, both of these enzymes show higher activities when PP-InsP5 is the substrate, under our in vitro conditions (Fig. 9 and data not shown). Indeed, PPIP5K1 and PPIP5K2 overexpression only maintains steady-state levels of PP-InsP5, whereas there is up to a 30-fold increase in (PP)2-InsP4 levels (Table 2, and taking into account the 30% transfection efficiency). The latter observation is consistent with the in vitro data that indicated that the PP-InsP5 kinase activities of PPIP5K1 and PPIP5K2 are higher than their InsP6 kinase activity (Fig. 9).
Nevertheless, the fact that steady-state levels of the PP-InsP5 precursor did not decrease in response to elevated (PP)2-InsP4 levels following either PPIP5K1 or PPIP5K2 overexpression (Table 2) is indicative of an increased metabolic flux from InsP6 to PP-InsP5. Although this may be a mass action effect or the result of increased inherent activity of IP6K, it is also possible that the InsP6 kinase activity of PPIP5Ks contributes to this phenomenon.
The earlier work of the York laboratory (33), published just before the current study was completed, demonstrated that Vip1 from yeast phosphorylates PP-InsP5, at least in vitro. During the preparation of our study, we learned that the York laboratory has also determined that human PPIP5K1 and PPIP5K2 can phosphorylate PP-InsP5 and InsP6 (56). The latter study, together with our independent cloning of PPIP5K1 and PPIP5K2 and their expression inside cells, has provided the first molecular identification of enzymes that show PP-InsP5 kinase activity in vivo. The activation by osmotic stress of PPIP5K1 (Fig. 9), and probably also PPIP5K2 (Table 2), indicates that these enzymes are likely to be signaling entities with considerable regulatory significance.
Acknowledgments
We thank Tatsuya Sueyoshi for helpful suggestions and Jeff Tucker for advice with confocal imaging. We thank John York and colleagues for sharing unpublished data and for helpful discussions.
Footnotes
This work was supported by grants from the Intramural Research Program of the NIEHS/National Institutes of Health. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
- Ins(3,4,5,6)P4
- d-myo-inositol 3,4,5,6 tetrakisphosphate)
- CFP
- cyan fluorescent protein
- CV
- column volumes
- DTT
- dithiothreitol
- PP-InsP4
- diphosphoinositol tetrakisphosphate
- PP-InsP5
- diphosphoinositol pentakisphosphate (“InsP7”)
- (PP)2-InsP3
- bis-diphosphoinositol trisphosphate, (PP)2-InsP4, bis-diphosphoinositol tetrakisphosphate (“InsP8”)
- PPIP5K1 and PPIP5K2
- PP-InsP5 kinase types 1 and 2
- HPLC
- high pressure liquid chromatography
- Bis-Tris
- 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol
- HEK
- human embryonic kidney
- MS/MS
- tandem mass spectrometry
- nanoLC-ESI-MS/MS
- nano liquid chromatography-electrospray mass ionization-MS/MS
- WT
- wild-type
- SAX
- strong anion exchange
- MINPP
- multiple inositol polyphosphate phosphohydrolase.
In Ref. 9, the structure of (PP)2-InsP4 was not fully appreciated, and so it was denoted as “IP6X” (for unknown derivative of InsP6).
REFERENCES
- 1.York JD, Hunter T. Science. 2004;306:2053–2055. doi: 10.1126/science.1107225. [DOI] [PubMed] [Google Scholar]
- 2.Streb H, Irvine RF, Berridge MJ, Schulz I. Nature. 1983;306:67–68. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 3.Xie W, Kaetzel MA, Bruzik KS, Dedman JR, Shears SB, Nelson DJ. J. Biol. Chem. 1996;271:14092–14097. doi: 10.1074/jbc.271.24.14092. [DOI] [PubMed] [Google Scholar]
- 4.Alcázar-Román AR, Tran EJ, Guo S, Wente SR. Nat. Cell Biol. 2006;8:645–647. doi: 10.1038/ncb1427. [DOI] [PubMed] [Google Scholar]
- 5.Irvine RF, Schell M. Nat. Rev. Mol. Cell Biol. 2001;2:327–338. doi: 10.1038/35073015. [DOI] [PubMed] [Google Scholar]
- 6.Seeds AM, York JD. Biochem. Soc. Symp. 2007:183–197. doi: 10.1042/BSS0740183. [DOI] [PubMed] [Google Scholar]
- 7.Shears SB. Biochem. J. 2004;377:265–280. doi: 10.1042/BJ20031428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephens LR, Radenberg T, Thiel U, Vogel G, Khoo K-H, Dell A, Jackson TR, Hawkins PT, Mayr GW. J. Biol. Chem. 1993;268:4009–4015. [PubMed] [Google Scholar]
- 9.Menniti FS, Miller RN, Putney JW, Jr., Shears SB. J. Biol. Chem. 1993;268:3850–3856. [PubMed] [Google Scholar]
- 10.Bennett M, Onnebo SM, Azevedo C, Saiardi A. CMLS Cell. Mol. Life Sci. 2006;63:552–564. doi: 10.1007/s00018-005-5446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saiardi A, Sciambi C, McCaffery JM, Wendland B, Snyder SH. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14206–14211. doi: 10.1073/pnas.212527899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Alami M, Messenguy F, Scherens B, Dubois E. Mol. Microbiol. 2003;49:457–468. doi: 10.1046/j.1365-2958.2003.03562.x. [DOI] [PubMed] [Google Scholar]
- 13.Luo HR, Huang YE, Chen JC, Sairardi A, Iijima M, Ye K, Huang Y, Nagata E, Devreotes P, Snyder SH. Cell. 2003;114:559–572. doi: 10.1016/s0092-8674(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 14.York SJ, Armbruster BN, Greenwell P, Petes TD, York JD. J. Biol. Chem. 2005;280:4264–4269. doi: 10.1074/jbc.M412070200. [DOI] [PubMed] [Google Scholar]
- 15.Saiardi A, Resnick AC, Snowman AM, Wendland B, Snyder SH. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1911–1914. doi: 10.1073/pnas.0409322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagata E, Luo HR, Saiardi A, Bae BI, Suzuki N, Snyder SH. J. Biol. Chem. 2005;280:1634–1640. doi: 10.1074/jbc.M409416200. [DOI] [PubMed] [Google Scholar]
- 17.Morrison BH, Bauer JA, Kalvakolanu DV, Lindner DJ. J. Biol. Chem. 2001;276:24965–24970. doi: 10.1074/jbc.M101161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo HR, Saiardi A, Yu H, Nagata E, Ye K, Snyder SH. Biochemistry. 2002;41:2509–2515. doi: 10.1021/bi0118153. [DOI] [PubMed] [Google Scholar]
- 19.Choi K, Mollapour E, Shears SB. Cell. Signal. 2005;17:1533–1541. doi: 10.1016/j.cellsig.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Dubois E, Scherens B, Vierendeels F, Ho MWY, Messenguy F, Shears SB. J. Biol. Chem. 2002;277:23755–23763. doi: 10.1074/jbc.M202206200. [DOI] [PubMed] [Google Scholar]
- 21.Pesesse X, Choi K, Zhang T, Shears SB. J. Biol. Chem. 2004;279:43378–43381. doi: 10.1074/jbc.C400286200. [DOI] [PubMed] [Google Scholar]
- 22.Safrany S. Mol. Pharmacol. 2004;66:1585–1591. doi: 10.1124/mol.104.002667. [DOI] [PubMed] [Google Scholar]
- 23.Saiardi A, Bhandari A, Resnick R, Cain A, Snowman AM, Sny-der SH. Science. 2004;306:2101–2105. doi: 10.1126/science.1103344. [DOI] [PubMed] [Google Scholar]
- 24.Saiardi A, Erdjument-Bromage H, Snowman A, Tempst P, Snyder SH. Curr. Biol. 1999;9:1323–1326. doi: 10.1016/s0960-9822(00)80055-x. [DOI] [PubMed] [Google Scholar]
- 25.Saiardi A, Nagata E, Luo HR, Snowman AM, Snyder SH. J. Biol. Chem. 2001;276:39179–39185. doi: 10.1074/jbc.M106842200. [DOI] [PubMed] [Google Scholar]
- 26.Schell MJ, Letcher AJ, Brearley CA, Biber J, Murer H, Irvine RF. FEBS Lett. 1999;461:169–172. doi: 10.1016/s0014-5793(99)01462-3. [DOI] [PubMed] [Google Scholar]
- 27.Caffrey JJ, Safrany ST, Yang X, Shears SB. J. Biol. Chem. 2000;275:12730–12736. doi: 10.1074/jbc.275.17.12730. [DOI] [PubMed] [Google Scholar]
- 28.Hidaka K, Caffrey JJ, Hua L, Zhang T, Falck JR, Nickel GC, Carrel L, Barnes LD, Shears SB. J. Biol. Chem. 2002;277:32730–32738. doi: 10.1074/jbc.M205476200. [DOI] [PubMed] [Google Scholar]
- 29.Safrany ST, Caffrey JJ, Yang X, Bembenek ME, Moyer MB, Burkhart WA, Shears SB. EMBO J. 1998;17:6599–6607. doi: 10.1093/emboj/17.22.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shears SB, Ali N, Craxton A, Bembenek ME. J. Biol. Chem. 1995;270:10489–10497. doi: 10.1074/jbc.270.18.10489. [DOI] [PubMed] [Google Scholar]
- 31.Huang C-F, Voglmaier SM, Bembenek ME, Saiardi A, Snyder SH. Biochemistry. 1998;37:14998–15004. doi: 10.1021/bi981920l. [DOI] [PubMed] [Google Scholar]
- 32.Safrany ST, Shears SB. EMBO J. 1998;17:1710–1716. doi: 10.1093/emboj/17.6.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulugu S, Bai W, Fridy PC, Bastidas RJ, Otto JC, Dollins DE, Haystead TA, Ribeiro AA, York JD. Science. 2007;316:106–109. doi: 10.1126/science.1139099. [DOI] [PubMed] [Google Scholar]
- 34.Lee YS, Mulugu S, York JD, O’Shea EK. Science. 2007;316:109–112. doi: 10.1126/science.1139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saiardi A, Caffrey JJ, Snyder SH, Shears SB. J. Biol. Chem. 2000;275:24686–24692. doi: 10.1074/jbc.M002750200. [DOI] [PubMed] [Google Scholar]
- 36.Albert C, Safrany ST, Bembenek ME, Reddy KM, Reddy KK, Falck JR, Bröker M, Shears SB, Mayr GW. Biochem. J. 1997;327:553–560. doi: 10.1042/bj3270553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riley AM, Trusselle M, Kuad P, Borkovec M, Cho J, Choi JH, Qian X, Shears SB, Spiess B, Potter BV. ChemBioChem. 2006;7:1114–1122. doi: 10.1002/cbic.200600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X, Shears SB. Biochem. J. 2000;351:551–555. [PMC free article] [PubMed] [Google Scholar]
- 39.Chang S-C, Miller AL, Feng Y, Wente SR, Majerus PW. J. Biol. Chem. 2002;277:43836–43843. doi: 10.1074/jbc.M206134200. [DOI] [PubMed] [Google Scholar]
- 40.Zhang T, Caffrey JJ, Shears SB. FEBS Lett. 2001;494:208–212. doi: 10.1016/s0014-5793(01)02351-1. [DOI] [PubMed] [Google Scholar]
- 41.Cho J, Choi K, Darden T, Reynolds PR, Petitte JN, Shears SB. J. Biotechnol. 2006;126:248–259. doi: 10.1016/j.jbiotec.2006.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shears SB. Biochem. Soc. Symp. 2007:211–221. doi: 10.1042/BSS0740211. [DOI] [PubMed] [Google Scholar]
- 43.Kelley LA, MacCallum RM, Sternberg MJ. J. Mol. Biol. 2000;299:499–520. doi: 10.1006/jmbi.2000.3741. [DOI] [PubMed] [Google Scholar]
- 44.Craxton A, Caffrey JJ, Burkhart W, Safrany ST, Shears SB. Biochem. J. 1997;328:75–81. doi: 10.1042/bj3280075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mullaney EJ, Ullah AH. Biochem. Biophys. Res. Commun. 2003;312:179–184. doi: 10.1016/j.bbrc.2003.09.176. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez B, Schell MJ, Letcher AJ, Veprintsev DB, Irvine RF, Williams RL. Mol. Cell. 2004;15:689–701. doi: 10.1016/j.molcel.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Miller GJ, Wilson MP, Majerus PW, Hurley JH. Mol. Cell. 2005;18:201–212. doi: 10.1016/j.molcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 49.Pesesse X, Leyman A, Luyten T, Missiaen L, Erneux C. Biochem. Biophys. Res. Commun. 2005;336:157–162. doi: 10.1016/j.bbrc.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 50.Miller GJ, Hurley JH. Mol Cell. 2004;15:703–711. doi: 10.1016/j.molcel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Pattni K, Banting G. Cell. Signal. 2004;16:643–654. doi: 10.1016/j.cellsig.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Alfieri RR, Petronini PG. Pfluegers Arch. Eur. J. Physiol. 2007;454:173–185. doi: 10.1007/s00424-006-0195-x. [DOI] [PubMed] [Google Scholar]
- 53.Schliess F, Haussinger D. Biol. Chem. 2002;383:577–583. doi: 10.1515/BC.2002.059. [DOI] [PubMed] [Google Scholar]
- 54.Go WY, Liu X, Roti MA, Liu F, Ho SN. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10673–10678. doi: 10.1073/pnas.0403139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hand CE, Honek JF. Bioorg. Med. Chem. Lett. 2007;17:183–188. doi: 10.1016/j.bmcl.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 56.Fridy PC, Otto JC, Dollins E, York JD. J. Biol. Chem. 282:30754–30762. doi: 10.1074/jbc.M704656200. [DOI] [PubMed] [Google Scholar]
- 57.Verbsky JW, Chang SC, Wilson MP, Mochizuki Y, Majerus PW. J. Biol. Chem. 2005;280:1911–1920. doi: 10.1074/jbc.M411528200. [DOI] [PubMed] [Google Scholar]


