Abstract
There has been considerable interest in pursuing phospholamban as a putative therapeutic target for overcoming depressed calcium handling in human heart failure. Studies predominantly done in mice have shown that phospholamban is a key regulator of sarcoplasmic reticulum calcium cycling and cardiac function. However, mice differ significantly from humans in how they regulate calcium, whereas rabbits better recapitulate human cardiac function and calcium handling. To investigate phospholamban’s role in the rabbit heart, transgenic rabbits that overexpressed wild-type phospholamban in the ventricular cardiomyocytes and slow-twitch skeletal muscles were generated. Rabbits expressing high levels of phospholamban were not viable due to severe skeletal muscle wasting, the onset of cardiac pathology and early death. A viable transgenic line exhibited a 30% increase in PLN protein levels in the heart. These animals showed isolated foci of cardiac pathology, but cardiac function as well as the response to β-adrenergic stimulation were normal. SR-calcium uptake measurements showed that the transgenic hearts had the expected reduced affinity for calcium. The data show that phospholamban-overexpressing transgenic rabbits differ markedly in phenotype from analogous transgenic mice in that rabbits are quite sensitive to alterations in phospholamban levels. Exceeding a relatively narrow window of phospholamban expression results in significant morbidity and early death.
Keywords: Phospholamban, Calcium, Heart Failure, Transgenic rabbits
Introduction
While gain of function and loss of function approaches in mice have yielded valuable data directed at understanding the mechanisms underlying heart disease, the relevance of these data to human cardiac physiology/pathology can, in some cases, be problematic because of the many differences that exist between the species. Compared to the mouse, the rabbit offers significant advantages as a model for cardiovascular research. The larger size and slower heart rate of the rabbit are advantageous for physiological analyses such as echocardiography and cardiac catheterization. As in human hearts, the “slow” β-myosin heavy chain isoform (MyHC) is the predominant heavy chain expressed in the ventricle while the mouse ventricle contains >95% α-MyHC (Swynghedauw 1986). This difference in myosin composition contributes to disparities in ATPase activities and force generation kinetics, which may alter the phenotype in response to other genetic alterations (Alpert et al. 2002). The rabbit heart also exhibits molecular changes in β-adrenergic signaling similar to those observed in human heart failure (Maurice et al. 1999). Importantly, mice and humans differ significantly in how they handle Ca2+ during contraction/relaxation and in how they respond to alterations in Ca2+ flux during heart failure, with the rabbit more accurately recapitulating human Ca2+ regulation (Bers 2002a; Bers 2002b). Relative to the mouse, rabbit and human cardiac muscle rely on the sarcolemmal sodium-calcium exchanger for cytosolic Ca2+-removal, with a lesser contribution from the sarcoplasmic reticulum (SR) Ca-ATPase (SERCA2a) (Bers 2000).
The Ca2+-pump in cardiac SR is under reversible regulation by phospholamban (PLN), a 52 amino acid phosphoprotein. Dephosphorylated PLN inhibits the apparent affinity of the SERCA pump for Ca2+ and this inhibition is reversed upon phosphorylation of PLN during β-agonist stimulation (Inui et al. 1986;MacLennan et al. 2003;Simmerman et al. 1986). This mechanism of PLN modulated inhibition of SERCA serves as a direct means to regulate cardiac contractility. Importantly, an inverse relationship between the relative PLN/SERCA2a ratio and cardiac SR Ca2+-uptake, as well as contractility, has been defined by genetically altered mouse models (Luo et al. 1996). Based on these and related data, it has been proposed that the increases in the PLN/SERCA ratio, which occur in end-stage human heart failure, lead to depressed SR Ca2+ handling and depressed cardiac function (Dash et al. 2001a;Hasenfuss 1998). Inhibition of PLN activity has major effects on morbidity and mortality in a number of genetic models of heart failure (Freeman et al. 2001;Minamisawa et al. 1999;Sato et al. 2001), leading to the hypothesis that targeting PLN inhibition will result in increased SERCA2 activity. This in turn would have a positive inotropic effect, restoring SR function and benefiting the heart failure patient (Freeman et al. 2001;Haghighi et al. 2003;Muller et al. 2003). Because of these considerations, there has been considerable interest in exploring the potential of PLN as a therapeutic target, as SERCA activity is reduced in failing myocardium (Beuckelmann et al. 1992;Morgan 1991).
Although much of the data justifying the use of PLN as a therapeutic target comes from the mouse, its relevance to the human remains untested and disparities in PLN-based regulation between the two species may be significant. For example, humans null for PLN develop dilated cardiomyopathy, whereas PLN ablation in mice is relatively benign, with the animals showing normal life spans and a lack of heart failure, even in old age (Haghighi et al. 2003;Hoit et al. 1995;Luo et al. 1994;Slack et al. 2001). It is clear, however, that alterations in the levels of both SERCA and PLN can have major effects on morbidity and mortality in a number of genetic models of heart failure (Freeman et al. 2001;Minamisawa et al. 1999;Sato et al. 2001). These and other discrepancies highlight the importance of studying the role of PLN in a mammalian heart that is more closely related to the human.
We hypothesize that regulation of the relative PLN/SERCA2 levels is critical for maintaining proper cardiac function and increases in the PLN/SERCA2 levels will result in increased inhibition of the SERCA2 Ca2+-affinity and contractile function, similar to that observed in heart failure. To assess the role of PLN expression in the rabbit heart, we generated transgenic (TG) rabbits overexpressing wild-type PLN under the control of the rabbit β-MyHC promoter, which drives transgene expression in the ventricular cardiomyocytes and slow type I muscle fibers. The rabbit β-MyHC promoter was chosen because it drives ventricular transcription in the neonate-adult, mimicking endogenous β-MyHC expression, the predominant isoform in the rabbit heart (Sanbe et al. 2005). The data show that the rabbit is surprisingly sensitive to modulation in PLN levels. Animals that expressed modest levels of PLN were not viable and only a single line of TG rabbits that showed a 30% increase in PLN levels was established. Measurements of SR-Ca2+ uptake revealed that TG hearts had reduced affinity for Ca2+, as was observed in the mouse models, but that cardiac function was normal. The data show that the rabbit hearts have a very narrow window within which PLN activity can be manipulated.
Materials & Methods
Transgenic rabbit generation and identification
Isolation of the rabbit β-MyHC promoter has been described previously (Sanbe et al. 2005). Rabbit PLN cDNA (GenBank Accession# Y00761) was isolated by the reverse transcription–polymerase chain reaction (RT-PCR). The cDNA was subcloned into the β-MyHC promoter cassette with a human growth hormone polyadenylation signal downstream (James et al. 2005), digested free of vector sequence with NotI, purified, and used to generate TG rabbits. Fertilized oocyte injections were performed on New Zealand White rabbits, and the TG rabbits identified using polymerase chain reactions. The first reaction detected the transgene with primers directed against the β-MyHC promoter (5′-TCTTCCAGTAATAGGACAAGCTCAGAC-3′) and PLN (5′-AGAATTCTTCAGAGAAGCATGACGATGATGCAG-3′). The second primer set was a template control, which detected β-MyHC (5′-GGATCCCTGGAGCAGGAGAAGAAGGTG-3′ and 5′-GATCTTGCTGTTCAGCTGACTGATG-3′) and thus served as an internal control for the PCR. Genomic DNA from tail clips was used for Southern Blots to confirm copy number, positive offspring and check that insertion occurred at a single site.
Cardiac function and β-adrenergic responsiveness
Rabbits were anesthetized with ketamine and acepromazine and then maintained with 2% isoflurane via inhalation for assessment of global cardiac function by transthoracic echocardiography and cardiac catheterization as described (James et al. 2005). Following the basal echocardiogram and measurement of aortic and left ventricular pressure, responsiveness to β-adrenergic stimulation was assessed by administering increasing doses of intravenous dobutamine (Bedford Laboratories, Bedford, OH) at 10, 20 and 40 mcg/kg/min. Ten min after each sequential increase in dobutamine dose, both the echocardiogram and invasive pressure measurements were repeated. After the final measurement, dobutamine infusion was discontinued, the catheters removed, anesthesia discontinued and the rabbit was allowed to recover in a pre-warmed incubator. Offline echocardiogram measurements of shortening fraction, wall thickness and velocity of circumferential fiber shortening (VCFc) at baseline and at each dobutamine dose were made by an investigator blinded to genotype. Invasive hemodynamic measurements of aortic blood pressure, left ventricular systolic and end diastolic pressure and ±dP/dt were likewise recorded. After 14 days in order to allow the effects of β-adrenergic stimulation to subside, the rabbits were euthanized with pentobarbital after sedation with ketamine and xylazine. Heart and skeletal muscles were taken from 3 rabbits of each genotype and saved for histological examination. Tissues from the remaining rabbits were snap-frozen in liquid nitrogen and stored at −80°C for RNA and protein analyses.
Histology
Hearts were perfused with 50 mM KCl, 5% dextrose in 1X PBS cardiac relaxation buffer while still beating from deeply anesthetized rabbits. Upon removal, hearts were immediately dropped into Histochoice fixative (Amresco, Solon, OH), bisected longitudinally and fixed for 48 h. Skeletal muscles were bisected longitudinally or transversely and fixed as well. Tissues were processed and embedded in paraffin. Serial sections (5 μm) were taken for staining with Harris modified hematoxylin & eosin Y alcoholic solution with phloxine (Fisher Scientific, Pittsburgh, PA) or with Gomori’s one-step trichome (PolyScientific, Bay Shore, NY). Stained sections were viewed with an Olympus BX60 microscope (Olympus, Center Valley, PA) and images were captured with SPOT camera and software (Diagnostic Instruments, Inc., Sterling Heights, MI).
RNA and protein analyses
Ventricular RNA was isolated using TRI-Reagent (Molecular Research Center, Cincinnati, OH), according to the manufacturer’s protocol and purified over RNeasy columns (Qiagen, Valencia, CA). Northern blots confirmed PLN transcript size and probe specificity. Expression levels of PLN, SERCA2 and GAPDH were determined thereafter by RNA dot blot with γ32P-labeled probes.
For protein analysis, ventricular free wall was homogenized at 4°C in 50 mM potassium phosphate buffer (pH = 7.0), 10 mM NaF, 1 mM EDTA, 0.3 M sucrose, 0.3 mM PMSF, and 0.5 mM DTT. Protein concentration of the homogenates was determined using the BioRad Protein Assay (BioRad Laboratories, Hercules, CA). Proteins were separated by 12% SDS-PAGE and transferred to nitrocellulose membranes. Nonspecific binding was blocked for 1 h at room temperature using 5% dried milk in Tris-buffered saline (pH 7.4) containing 0.1% Tween 20. Membranes were probed overnight at 4°C with specific primary monoclonal antibodies (Affinity Bioreagents Inc., Golden, CO) to PLN, SERCA2a and Na/Ca exchanger (NCX). Protein loading was normalized to endogenous GAPDH levels, using a specific primary monoclonal antibody (Abcam Inc., Cambridge, MA). A mouse IgG, horseradish peroxidase-linked whole antibody (GE Healthcare, Buckinghamshire, U.K.) was used as a secondary antibody and incubation time was 1.5 h at room temperature. The ECL Western blotting detection system (GE Healthcare, Buckinghamshire, U.K.) was used for detection of the signal and the optical density of the bands was determined by ImageQuant 5.2 software (GE Healthcare, Buckinghamshire, U.K.).
SR Ca2+ uptake
Ventricular free wall from NTG and TG rabbits was homogenized at 4°C in 50 mM potassium phosphate buffer (pH = 7.0), 10 mM NaF (to inhibit phosphatase activity), 1 mM EDTA (to inhibit protein kinase activity), 0.3 M sucrose, 0.3 mM PMSF, and 0.5 mM DTT. Cardiac homogenates were centrifuged at 2,000 rpm for 5 min to remove connective tissue. Protein concentration of the homogenates was determined by using the BioRad Protein Assay (BioRad Laboratories, Hercules, CA). Oxalate-supported Ca2+ uptake in cardiac homogenates was measured by a modified Millipore filtration technique using CaCl2 (Luo et al. 1994). Briefly, 100–250 μg of homogenate were incubated at 37°C in reaction buffer containing 40 mM Imidazole (pH = 7.0), 95 mM KCl, 5 mM NaN3, 5 mM MgCl2, 0.5 mM EGTA, and 5 mM K2C2O4. The initial uptake rates were determined over a wide range of calcium values (pCa 5 to 8). Calcium uptake into cardiomyocytes was initiated by addition of 5 mM ATP, and aliquots were filtered through a 0.45 μm Millipore filter after 0, 30, 60, and 90 s to terminate the reaction. The data were analyzed by nonlinear regression using Origin 6.1 software (OriginLab Corporation, Northampton, MA).
Immunohistochemistry
Paraffin-embedded sections of heart and skeletal muscle were deparaffinized and dehydrated through a standard series of xylene and alcohol washes prior to antigen retrieval. Slides were microwaved in a 0.1 M glycine antigen retrieval solution (pH 3.5) until boiling (approximately 2 min) then kept at a slow boil for 25 min and cooled to 21°C over approximately 30 min. The slides were then rinsed in PBS and incubated in blocking solution (1% BSA, 0.1% cold water fish skin gelatin, 0.1% Tween-20, and 0.005% NaN3 in PBS), for one h at 21°C. The slides were probed with a PLN mouse monoclonal antibody (1:150, Upstate Biotechnology/Millipore, Charlottesville, VA) in blocking solution overnight at 4°C. They were then incubated with a goat anti-mouse Alexa Flour 488 antibody (1:100, Invitrogen, Carlsbad, CA) in blocking solution for one h at 21 ºC and subsequently counterstained with a desmin polyclonal antibody (1:100, Biomeda, Foster City, CA) in blocking solution for one h at 21°C, followed by incubation with goat anti-rabbit Alexa Flour 568 antibody (1:100, Invitrogen, Carlsbad, CA) in blocking solution for 1 h. The slides were coverslipped and mounted in Vectashield Hard Set (Vector Laboratories, Burlingame, CA). Sections were examined by confocal microscopy (PCM 2000, Nikon, Melville, NY) and images captured with Simple PCI version 4 (Compix Inc., Sewickley, PA).
Transmission electron microscopy
Rabbits were anesthetized and the hearts perfused with cardioplegic buffer (100 mM KCl, 5% dextrose in 1X PBS) by isolating the aorta, suturing it over a needle connected to a peristaltic pump and pumping 6% glutaraldehyde in 0.1 M cacodylate buffer (Electron Microscopy Sciences, Ft. Washington, PA) for 5 min at 8 ml/min. Following perfusion, the heart was dissected and fixed in 3.5% gluteraldehyde/0.1 M cacodylate buffer. The heart fragments selected for analysis were cut into 1 mm cubes and fixed for 24 h. The cubes were rinsed in cacodylate buffer 0.1 M (pH 7.6) for 30 min and post-fixed in 2% OsO4/cacodylate buffer (Electron Microscopy Sciences, Ft. Washington, PA) for 2 h on ice. The specimens were then dehydrated in a series of acetone washes and embedded in Embed 812 (Electron Microscopy Sciences, Ft. Washington, PA). The embedded samples were cut into ultra-thin sections, counterstained in uranyl acetate and lead citrate (Electron Microscopy Sciences, Ft. Washington, PA) and examined with a Hitachi 7600 transmission electron microscope and digital camera.
Statistical analyses
Data are expressed as mean ± SEM and analyzed using Student’s t-test or one-way analysis of variance, where a significant difference was p < 0.05, following a Tukey’s post-hoc adjustment.
Results
Generation and analyses of transgenic rabbits expressing PLN
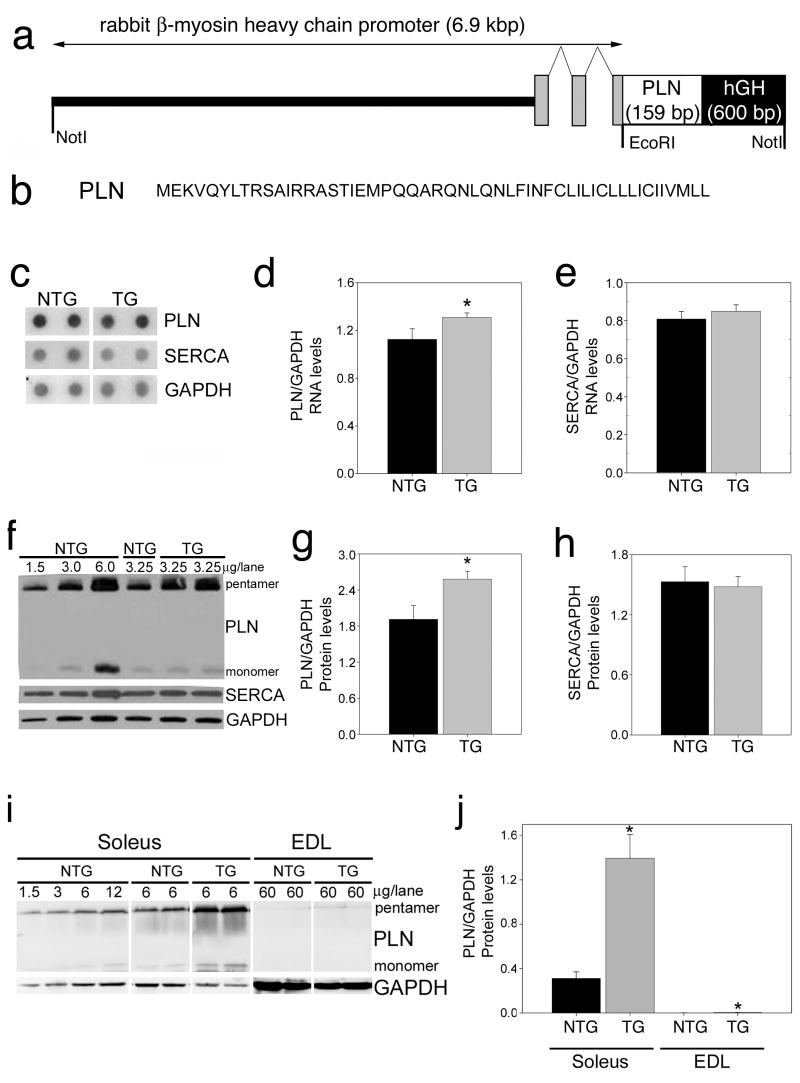
Considering the therapeutic interest in modulating PLN activity, we wished to understand the physiological effects of enhanced PLN levels. The rabbit β-MyHC promoter faithfully reflects the expression pattern of the endogenous β-MyHC gene (Sanbe et al. 2005), driving ventricular-and slow-type skeletal muscle-restricted expression in the rabbits. The promoter construct consisted of 6000 bp of upstream sequence from the transcriptional start site and a unique EcoRI site into which the rabbit PLN transgene sequences were inserted. Downstream of the cloning site, we placed a polyadenylation signaling sequence derived from the human growth hormone gene (Fig. 1a).
Figure 1.
Transgenic modulation of phospholamban in the rabbit heart. (a) Construct design. The rabbit β-myosin heavy chain (β-MyHC) promoter region was used to drive the rabbit phospholamban coding sequence, flanked by a human growth hormone polyadenylation signal (hGH polyA). The exon-intron structure composing the 5′-untranslated sequence of β-MyHC is denoted by grey rectangles/line respectively. (b) Amino acid sequence of the wild-type phospholamban construct. (c) Representative RNA dot blots of phospholamban and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) expression from two of six different rabbits are shown. Each dot represents an individual rabbit and shows the reproducibility of phospholamban expression within a line. Ventricular RNA isolated from 6-month old rabbits of both genders was probed for phospholamban and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase. Duplicate blots were done and the data normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) RNA expression. (d) Total phospholamban levels normalized to glyceraldehyde 3-phosphate dehydrogenase expression (n = 6/genotype). *P < 0.05 vs. nontransgenic (NTG) based Student’s t-test. (e) Normalized sarcoplasmic/endoplasmic reticulum Ca2+-ATPase expression was unchanged by phospholamban overexpression. (f) Representative immunoblot of cardiac homogenates isolated from six month old nontransgenic and transgenic rabbit ventricles showing pentameric and monomeric phospholamban separated by SDS-PAGE and probed with specific monoclonal antibodies. Sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 2a and glyceraldehyde 3-phosphate dehydrogenase levels were also determined. (g) phospholamban expression in the transgenic rabbit hearts was significantly increased relative to the nontransgenic hearts. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. *, P < 0.05 vs. nontransgenic. (h) Quantitation of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase levels revealed that the expression in the transgenic rabbit hearts was not changed compared to levels seen in nontransgenic rabbit hearts. (i, j) Skeletal muscle expression of phospholamban in the viable transgenic founder. Shown are multiple dilutions of protein derived from the indicated muscles. Note the robust expression in the slow type soleus and lack of expression in the extensor digitorum longus (EDL) fast twitch muscle.
To define the role(s) of PLN expression in the rabbit heart, we generated TG rabbits expressing wild-type PLN (Fig. 1b). Overexpression of PLN causes increased inhibition of SERCA2 in TG mice (Kadambi et al. 1996). From a total of 738 pronuclear injections, 11 TG founders were obtained, but surprisingly on the basis of our previous experience with creating PLN TG mice, only one founder was both viable and able to transmit the transgene through the germline, allowing us to establish a single stable TG line (Table 1). A majority of the rabbits that were initially identified as transgenic on the basis of both PCR and Southern analysis of earclips appeared to be mosaic (n = 7), as each rabbit was subject to at least 3 breeding attempts and >32 offspring from each potential founder rabbit were generated and tested for transgene transmission. All offspring from those 7 rabbits were negative for the transgene, at which point the breeding programs for those animals were terminated because of the apparent lack of the transgene in the eggs or sperm.
Table 1.
Generation of transgenic phospholamban rabbits. The number of attempts to generate transgenic phospholamban rabbits versus the number of founders able to transmit the transgene through the germline.
| PLN-TG | |
|---|---|
| Eggs implanted | 738 |
| Total pups born | 98 |
| Confirmed TG founders F0 | 11 |
| # of lines with TG offspring | 1 |
| Non-viable TG founders | 3 |
Of the remaining 4 unique lines, 3 became too ill to breed or died before reaching breeding age. At 6–7 months of age, when rabbits reach sexual maturity, transgene RNA levels in hearts derived from the viable founder line were elevated only 1.18 fold relative to NTG hearts (Fig. 1c and d). TG PLN expression had no effect on either SERCA2 RNA or protein levels (Fig. 1e and h). Quantitative immunoblotting of ventricular homogenates revealed 1.34 fold over-expression at the protein level for TG rabbit hearts compared with NTG rabbit hearts (Fig. 1f). There was no alteration in the relative degree of PLN phosphorylation in the TG hearts (data not shown). Quantitative immunoblotting to determine whether any compensatory changes in SERCA2a levels had occurred showed no differences in SERCA2a levels between the NTG (1.53 ± 0.15 SERCA2a/GAPDH ratio) and TG (1.48 ± 0.10 SERCA2a/GAPDH ratio) hearts (Fig. 1f). Furthermore, there were no alterations in the sodium-calcium exchanger’s protein expression levels (data not shown).
As expected on the basis of endogenous promoter activity (β-MyHC is also the slow skeletal muscle isoform), there was substantial expression of the transgene in slow skeletal fibers and no expression in fast skeletal fibers such as are found in the extensor digitorum longus (EDL) muscle (Fig. 1i, j).
Founder animals that became visibly ill exhibited symptoms reminiscent of a muscular dystrophy: they were unable to walk without significant effort as they grew older with some large muscles exhibiting severe atrophy. When PLN is expressed in the soleus muscles of mice, only a mild pathology results, characterized by reduced muscle mass and compromised function (Song et al. 2004). These effects were much more pronounced in the rabbits, with the overtly ill founder lines exhibiting dramatic muscle wasting and fatty accumulations in the slow-twitch muscle fibers in which the transgenically-encoded PLN is expressed. In an effort to assess PLN overexpression, RNA was isolated from the residual soleus muscle from a TG animal that could not breed and was essentially immobile by 6 months of age. The RNA showed a 2.2 fold increase in PLN RNA expression (Fig. 2a), but this is likely an underestimate of the initial -fold overexpression because of the extensive loss of PLN-expressing fibers. When euthanized for humane reasons, the slow-twitch fibers in which transgene expression occurred showed severe wasting and replacement of the muscle with fatty deposits, while muscles that contain both slow and fast fibers, such as the gastrocnemius, were less affected (Fig. 2b). Viable TG rabbits exhibited no skeletal muscle pathology. Attempts to increase PLN expression by breeding the healthy founder line to homozygosity were unsuccessful with no live births of homozygotes observed despite >10 attempts with different male and female F1’s. There are several potential explanations for the failure in generating the homozygotes. First, when TG animals are made homozygous they are susceptible to insertional mutagenic effects. Second, gene expression is not necessarily linear, but is often dependent on chromosomal context. Thus a double dose could have led to more than a doubling of the TG protein level. Taken together, these data highlight the sensitivity of rabbit muscle to PLN modulation.
Figure 2.
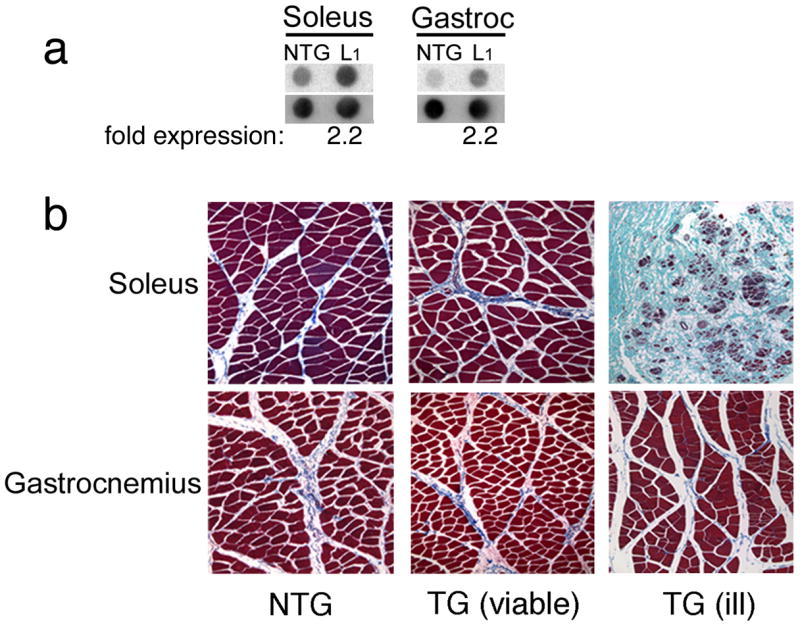
Skeletal muscle histology of phospholamban rabbits. (a) RNA blots from nontransgenic and an ill, transgenic rabbit at 6 months. An effort was made to obtain samples from overtly healthy tissue in order to avoid titrating RNA levels present at a site in which substantial fatty infiltrates were present. Only modest levels of overexpression in both the soleus and gastrocnemius were detected. (b) Skeletal muscle samples were collected from 6 month old rabbits. No gender differences presented. All sections were stained with Masson’s trichrome and images captured at 10X magnification. An example of an ill F0 animal is also shown. The slow-twitch skeletal muscle in which phospholamban was driven exhibited a dystrophic-like phenotype, as evidenced by the severe muscle wasting in the soleus muscle and, to a lesser extent, in the gastrocnemius, reflecting the mixed fiber-types present in that muscle. This animal had 2.2-fold overexpression of phospholamban at the RNA level in the soleus (data not shown); protein levels were not determined because of the extensive wasting that was present.
We then analyzed cardiac histology in the viable and ill TG rabbits. Sections derived from the TG line that expressed low levels of PLN appeared to be, for the most part, indistinguishable from NTG sections (Fig. 3a). However, cardiac sections taken from the overtly ill founder, analyzed above (Fig. 2), showed significant cardiac pathology, with increased interstitial spacing and internal vacuolization (Fig. 3a). We then carried out a more complete survey of the NTG versus healthy TG hearts and were, in the latter, able to detect widely scattered regions of myocyte dropout, vacuolization and fibrosis (Fig. 3b). Thus, the muscle pathology present in the skeletal fibers of the overtly ill animal was recapitulated to some extent in the cardiac fibers as well although the wasting was not nearly as severe and, even in the healthy animals, isolated foci of cardiac muscle pathology could be detected.
Figure 3.
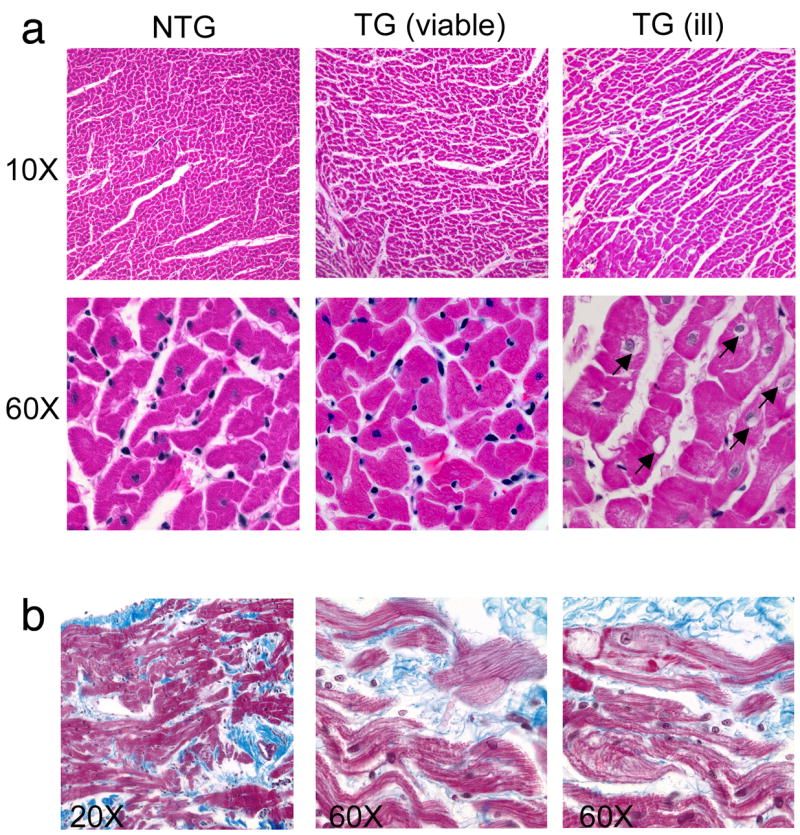
Cardiac histology of viable and ill transgenic lines. (a) All right ventricular sections were collected from 6 month old rabbits and stained with Masson’s trichrome. Ventricular myofibers from founder rabbits who were overtly ill exhibited a vacuolated appearance that was not observed in nontransgenic sections. Vacuoles are indicated by arrows. (b) Right ventricular sections taken from 6 month transgenic rabbits (viable line). Shown are areas of myocyte dropout, vacuolization and fibrosis that were not apparent in the nontransgenic hearts.
To investigate whether PLN was mis-localized in the TG hearts, we subjected cardiac tissue to immunohistochemical analysis with an anti-PLN antibody. Confocal microscopy revealed that PLN is primarily perinuclear in location, as described previously in porcine muscle (Fig. 4) (de Jonge et al. 2006). No differences in staining pattern could be distinguished between the NTG and TG hearts and cardiomyocyte ultrastructure also confirmed that TG hearts were indistinguishable from NTG hearts (Fig. 4a and b). In contrast with these data, sections taken from the overtly ill founder analyzed above showed severe pathology and alterations in the staining pattern of PLN (Fig. 5). The perinuclear staining apparent in Fig. 4 is absent and the entire intra and inter fiber structure is aberrant as revealed by staining of the intermediate filament, desmin. The slow type soleus skeletal muscle shows significant fiber loss and the normal cellular anatomy of the remaining fibers is perturbed.
Figure 4.
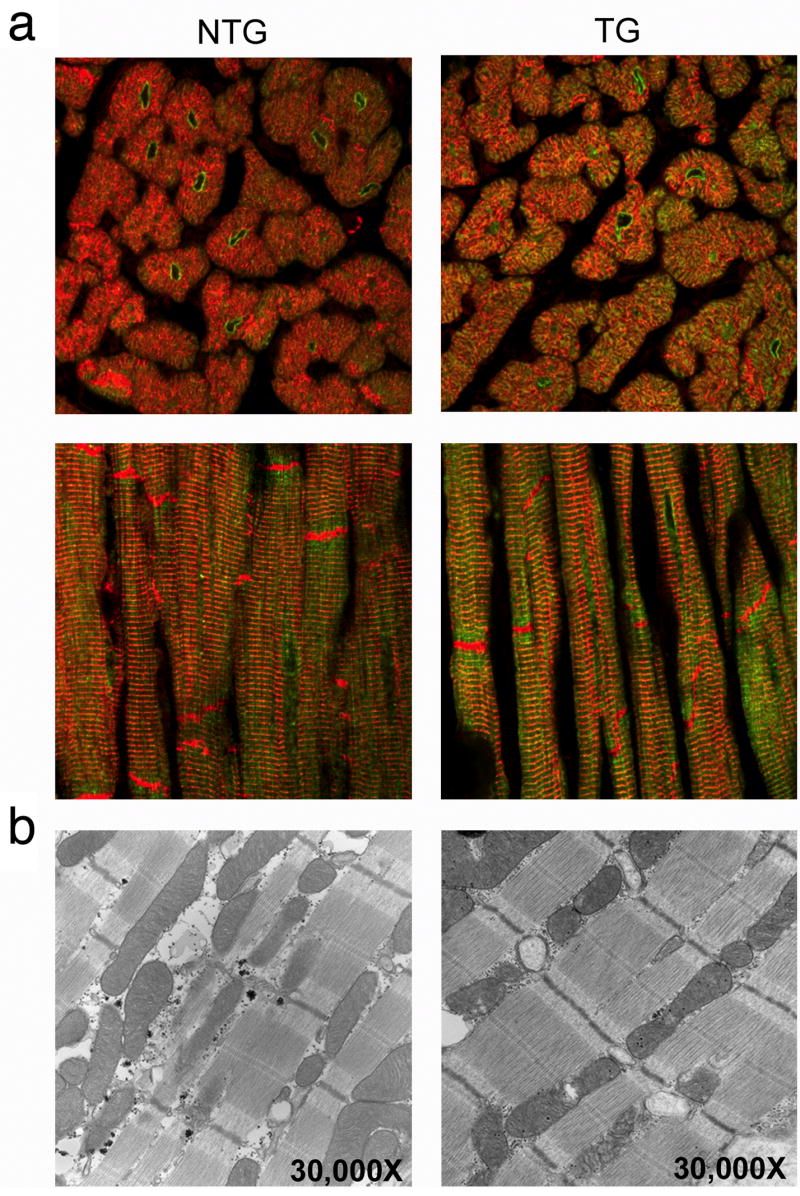
Phospholamban localization and ultrastructural analyses. (a) Whole heart sections from six month old rabbits were stained with phospholamban antibody (green) and counterstained with desmin antibody (red) to identify cardiac myofibers. No gender-specific differences were observed. Cross-sectional and longitudinal views of phospholamban staining show concentrated perinuclear accumulations in both the nontransgenic and transgenic sections. Magnification; 60X. (b) nontransgenic and transgenic hearts appeared to be identical at the ultrastructural level.
Figure 5.
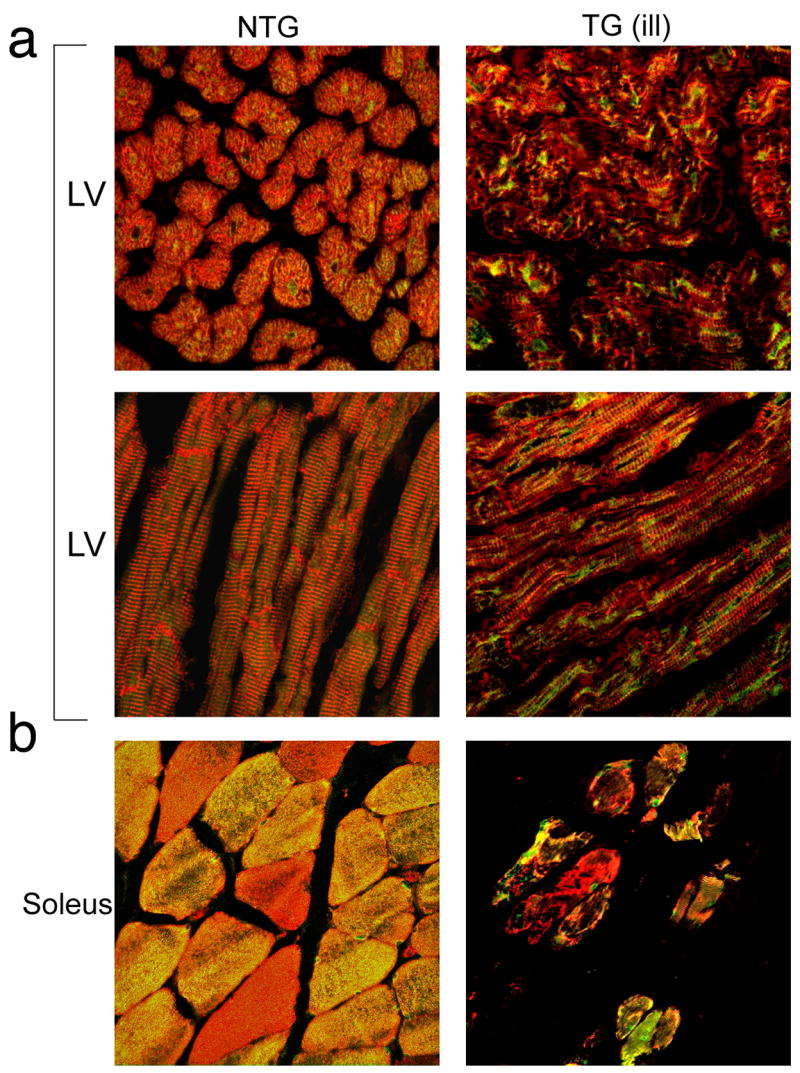
Phospholamban staining of an ill transgenic rabbit. Sections of left ventricle and soleus muscle were taken from an ill, 6 month old transgenic F0 rabbit that was exhibiting overt signs of distress and had to be euthanized, and a 6 month old nontransgenic age-matched control. Sections were stained with phospholamban antibody (green) and counterstained with desmin antibody (red) to identify cardiac myofibers. (a) Both the left ventricle and soleus show signs of pathology. Left ventricle samples show disarray while the soleus exhibits severe muscle wasting. Cross-sectional and longitudinal views of the left ventricle show phospholamban staining to be clumped in the transgenic cardiomyocytes, in contrast to the more evenly distributed pattern in the nontransgenic heart. (b) Few soleus myofibers remained in the ill transgenic rabbit. Phospholamban staining varied widely from fiber to fiber in both the transgenic and nontransgenic soleus. Magnification; 20X.
Prior experiments in which PLN levels were manipulated in genetically modified mouse hearts showed that profound effects occurred in the β-adrenergic response (Dash et al. 2001b;Luo et al. 1994). To determine if a rabbit model, in which only small changes in PLN levels were apparently tolerated also showed a differential response, we measured cardiac function during dobutamine infusion. The TG and NTG hearts showed identical responses to increasing dobutamine concentrations (Fig. 6) and the complete cardiac contractile parameters are shown in Table 2.
Figure 6.
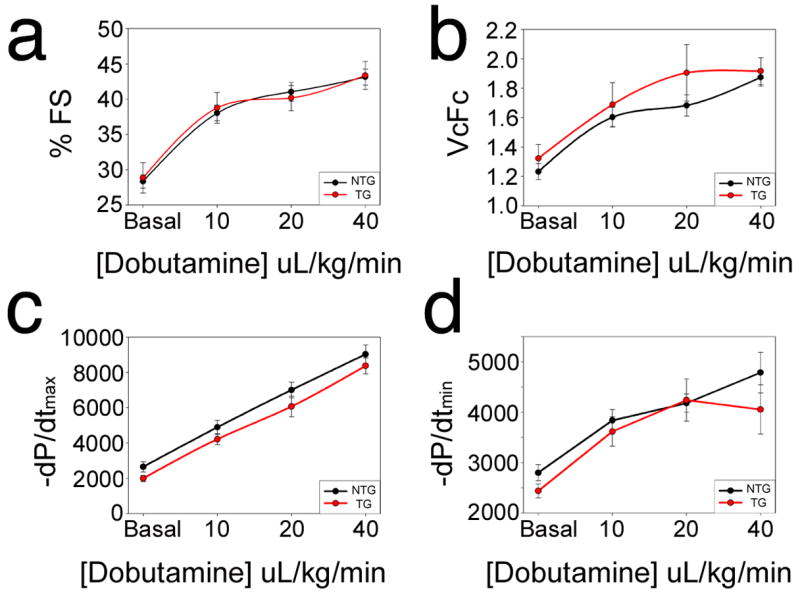
Cardiac function. Serial echocardiograms were performed on six month old nontransgenic and transgenic rabbits from both genders (n = 8–14/genotype) exposed to increasing doses of dobutamine. (a) Percent fractional shortening (% FS) showed no differences at baseline or in response to increasing levels of dobutamine. (b) Velocity of circumferential fiber shortening (VCFc) was not different between transgenic and nontransgenic hearts. (c, d) Cardiac catheterization also showed no differences between the transgenic and nontransgenic hearts.
Table 2.
Invasive Hemodynamics
| Dob. | N | HR (bpm) | LVSP (mm Hg) | LVEDP (mm Hg) | +dP/dt max | −dP/dt min | |
|---|---|---|---|---|---|---|---|
| NTG | 0 | 15 | 260 ± 10 | 63.7 ± 3.0 | 4.7 ± 0.9 | 2651 ± 286 | 2799 ± 161 |
| TG | 0 | 8 | 231 ± 11 | 61.6 ± 1.9 | 6.7 ± 0.9 | 1992 ± 170 | 2437 ± 139 |
| NTG | 10 | 15 | 279 ± 9 | 76.4 ± 3.2 | 4.6 ± 1.0 | 4893 ± 402 | 3837 ± 216 |
| TG | 10 | 8 | 251 ± 13 | 76.2 ± 3.3 | 6.6 ± 0.7 | 4216 ± 314 | 3616 ± 292 |
| NTG | 20 | 15 | 292 ± 7 | 85.7 ± 3.1 | 5.5 ± 1.2 | 7007 ± 442 | 4181 ± 181 |
| TG | 20 | 8 | 268 ± 13 | 82.9 ± 2.9 | 7.0 ± 0.5 | 6071 ± 586 | 4240 ± 419 |
| NTG | 40 | 11 | 303 ± 8 | 95.4 ± 3.0 | 7.4 ± 1.0 | 9033 ± 532 | 4786 ± 402 |
| TG | 40 | 4 | 294 ± 17 | 86.7 ± 2.7 | 7.7 ± 1.1 | 8373 ± 451 | 4055 ± 488 |
Dob, dobutamine dose in μg/kg/min; LVSP, peak LV systolic pressure; LVEDP, LV end-diastolic pressure.
The lack of an overt phenotype in the viable line of TG rabbits might reflect the very low level of PLN expression. To determine if the elevated PLN levels had any effect at the cellular level in terms of Ca2+ flux, rabbit hearts were assessed by oxalate-supported SR Ca2+ transport activity in cardiac homogenates at varying Ca2+ concentrations. The initial rates of SR Ca2+ transport indicated that PLN overexpression resulted in a significant increase in the EC50 of SERCA2a for Ca2+ (0.494 ± 0.011 μM), compared to NTG rabbits (0.346 ± 0.012 μM) (Fig. 7). There were no significant differences in the maximal velocity of Ca2+ uptake between the two groups (NTG: 9.89 ± 0.89 nmol/mg/min; TG: 10.23 ± 1.96 nmol/mg/min). These data suggest that overexpression of PLN did result in decreased affinity of SERCA2a for Ca2+, consistent with the inhibitory properties of PLN, but this decrease was not manifested by functional changes in global cardiac mechanics or hemodynamics (Fig. 6).
Figure 7.
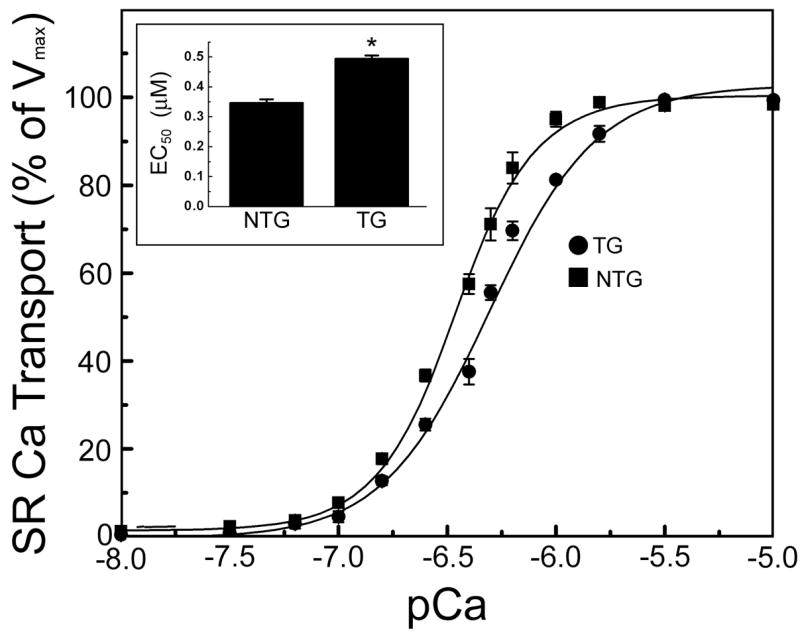
Measurements of calcium flux. Sarcoplasmic reticulum calcium (SR Ca2+) uptakes were measured from homogenates isolated from six month old nontransgenic and transgenic rabbit left ventricles. Sarcoplasmic reticulum calcium uptake in nontransgenic (squares, n = 3) and transgenic (circles, n = 3) hearts. Maximal velocity of Ca2+ uptake did not differ between transgenic and nontransgenic samples (10.23 ± 1.96 and 9.89 ± 0.89 nmol/mg/min, respectively). Inset represents EC50 (μM) of sarcoplasmic reticulum calcium uptake. Data were analyzed by nonlinear regression with Microcal Origin software (version 6.1).
Discussion
Although we were able to generate a large number of rabbits that were initially scored as transgenic, only 1 survived to breeding age and demonstrated germline transmission. This is a much lower percentage than we observe with our mouse transgenics, which normally average 70–80% germline transmission with the potential founder lines. The mechanism(s) underlying these differences are unclear although we have noted a much lower incidence of germline transmission in our other rabbit constructs as well, using both innocuous reporters (James et al. 2000) or cDNAs encoding other sarcomeric proteins (Sanbe et al. 2005). The mechanistic or technical bases for these differences remain unresolved and these limitations certainly make the experiments more difficult and costly. Practically, they limit the TG rabbit approach to those questions that cannot be effectively asked via murine transgenesis.
Depressed SR Ca2+ handling (Dash et al. 2001a;Hasenfuss 1998), reduced SERCA activity and elevated PLN:SERCA2 ratios are often observed in human heart failure and there has been considerable interest in targeting PLN for therapeutic intervention. However many of the most persuasive data substantiating the use of PLN as a therapeutic target come from murine-based gain and loss of function studies and disparities in PLN-based regulation between the two species are significant (Haghighi et al. 2003;Hoit et al. 1995;Luo et al. 1994). Thus, the TG rabbit is the more appropriate model, compared to mouse, to study the effects of PLN manipulation. Our data report the first non-murine TG model to overexpress PLN in heart and slow-twitch skeletal muscle. Because the rabbit heart more closely reflects human cardiac function, it therefore poses a more relevant model for testing the therapeutic efficacy and consequences of manipulating PLN activity.
We initially hypothesized that rabbits and humans might be less sensitive to alterations in PLN expression and phosphorylation, due to the decreased reliance on SERCA and increased dependence on the sodium-calcium exchanger for Ca2+ sequestration in larger mammalian hearts (Bers 2002b). This does not appear to be the case and the rabbits are surprisingly sensitive to modulation of PLN levels. That is, all founders except one with very low levels of TG PLN expression were not able to be maintained and propagated into lines. Founders expressing levels of PLN that might be considered modest in the mouse (eg, 2.2-fold overexpression) developed severe skeletal muscle wasting in the rabbit and exhibited noticeable cardiac pathology. Interestingly, in C. elegans, loss of function studies showed that SERCA was required for muscle development and function (Zwaal et al. 2001) and loss of a single copy of SERCA2 in mouse hearts decreased SR Ca2+ uptake by 35% and impaired both contraction and relaxation (Periasamy et al. 1999). The limited TG expression and skeletal muscle effects are PLN-specific, as these phenotypes were not observed in previously generated TG rabbits using the same promoter (Sanbe et al. 2005). Thus, our observation of severe skeletal muscle wasting and inability to generate rabbits with higher levels of PLN expression may reflect a role for PLN in skeletal muscle development and function.
In mice, two-fold cardiac overexpression of wild-type PLN had no effect on whole organ function, but resulted in depressed contractility and relaxation in isolated cardiomyocytes (Kadambi et al. 1996), which could be overcome by β-agonist stimulation. TG mice with 4-fold PLN overexpression exhibited increased inhibition of SERCA2 and elevated PLN phosphorylation (Dash et al. 2001b). Therefore, we predicted that TG rabbit hearts would have impaired cardiac function. Cardiac histology from a founder that showed 2.2-fold overexpression was clearly abnormal and we were unable to obtain functional measurements from the ill rabbits because of their sensitivity to anesthesia. The lack of hemodynamic alterations is consistent with lower levels of overexpression, as observed in TG mice. We believe these data underscore a very narrow window for increasing PLN activity in the rabbit cardiomyocyte and/or slow type skeletal muscle.
Relative to the rodent, the soleus muscles of rabbits and pigs contain significantly higher levels of PLN, along with reduced levels of SERCA (Vangheluwe et al. 2005), while PLN levels are very low to non-existent in rodent skeletal muscle but are easily detectable in rabbits and are present at even higher levels in human muscle (Damiani et al. 2000). The soleus muscles of rabbits and humans contain significantly higher levels of PLN, along with reduced levels of SERCA (Vangheluwe et al. 2005), compared to the very low to non-existent PLN levels in rodent skeletal muscles (Damiani et al. 2000). PLN functions to inhibit the Ca2+ affinity of the SERCA pump (MacLennan et al. 2003) and its inhibitory effects are associated with disruption of PLN pentamers into monomers (Asahi et al. 2002). Thus, in both the rabbit and human, elevated levels of PLN coupled with reduced SERCA levels would result in an elevated PLN:SERCA ratio and superinhibition of SERCA, which might trigger a program of muscle wasting.
The marked differences in the mice versus rabbits that overexpress PLN highlight the importance of carrying out at least a subset of TG studies in higher organisms whose organ systems more closely resemble that of the human. We think that the rabbit model with slightly elevated levels of PLN in the heart and slow type skeletal muscle will be a useful complement for the mouse models for testing the effects of elevated PLN activity in the heart during both pharmacological and/or physical manipulations that, by themselves, affect cardiovascular function. As manipulation of PLN is being actively pursued as a therapeutic target in cardiovascular disease and heart failure, it will be important to test our animals’ responses to the cardiovascular abnormalities resulting from by surgically-induced pressure overload or chronic treatment with carvedilol or other β-blockers (Metra et al. 2002). The results of PLN manipulation in conjunction with commonly used therapeutic regimens for heart disease should be of value in the rabbit model.
Acknowledgments
This work was supported by NIH research grants R01 HL69799, HL60546, HL52318, HL60546, HL56370 (J.R.), HL26057, HL64018, HL77101 and the Leducq Foundation (E.G.K.), American Heart Association Post-Doctoral Fellowships (J.R.W. & J.S.P.), T32 HL07752 (J.S.P.) and F32 HL087478 (J.S.P.).
References
- Alpert NR, Brosseau C, Federico A, Krenz M, Robbins J, Warshaw DM. Molecular mechanics of mouse cardiac myosin isoforms. Am J Physiol Heart Circ Physiol. 2002;283:H1446–1454. doi: 10.1152/ajpheart.00274.2002. [DOI] [PubMed] [Google Scholar]
- Asahi M, Kurzydlowski K, Tada M, MacLennan DH. Sarcolipin inhibits polymerization of phospholamban to induce superinhibition of sarco(endo)plasmic reticulum Ca2+-ATPases (SERCAs) J Biol Chem. 2002;277:26725–26728. doi: 10.1074/jbc.C200269200. [DOI] [PubMed] [Google Scholar]
- Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res. 2000;87:275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002a;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac Na/Ca exchange function in rabbit, mouse and man: what’s the difference? J Mol Cell Cardiol. 2002b;34:369–373. doi: 10.1006/jmcc.2002.1530. [DOI] [PubMed] [Google Scholar]
- Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992;85:1046–1055. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- Damiani E, Sacchetto R, Margreth A. Variation of phospholamban in slow-twitch muscle sarcoplasmic reticulum between mammalian species and a link to the substrate specificity of endogenous Ca(2+)-calmodulin-dependent protein kinase. Biochim Biophys Acta. 2000;1464:231–241. doi: 10.1016/s0005-2736(00)00153-x. [DOI] [PubMed] [Google Scholar]
- Dash R, Frank KF, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium. J Mol Cell Cardiol. 2001a;33:1345–1353. doi: 10.1006/jmcc.2001.1394. [DOI] [PubMed] [Google Scholar]
- Dash R, Kadambi V, Schmidt AG, Tepe NM, Biniakiewicz D, Gerst MJ, Canning AM, Abraham WT, Hoit BD, Liggett SB, et al. Interactions between phospholamban and beta-adrenergic drive may lead to cardiomyopathy and early mortality. Circulation. 2001b;103:889–896. doi: 10.1161/01.cir.103.6.889. [DOI] [PubMed] [Google Scholar]
- de Jonge HW, van der Wiel CW, Eizema K, Weijs WA, Everts ME. Presence of SERCA and calcineurin during fetal development of porcine skeletal muscle. J Histochem Cytochem. 2006;54:641–648. doi: 10.1369/jhc.5A6812.2006. [DOI] [PubMed] [Google Scholar]
- Freeman K, Lerman I, Kranias EG, Bohlmeyer T, Bristow MR, Lefkowitz RJ, Iaccarino G, Koch WJ, Leinwand LA. Alterations in cardiac adrenergic signaling and calcium cycling differentially affect the progression of cardiomyopathy. J Clin Invest. 2001;107:967–974. doi: 10.1172/JCI12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, Fan GC, Tsiapras D, Hahn HS, Adamopoulos S, et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest. 2003;111:869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res. 1998;37:279–289. doi: 10.1016/s0008-6363(97)00277-0. [DOI] [PubMed] [Google Scholar]
- Hoit BD, Khoury SF, Kranias EG, Ball N, Walsh RA. In vivo echocardiographic detection of enhanced left ventricular function in gene-targeted mice with phospholamban deficiency. Circ Res. 1995;77:632–637. doi: 10.1161/01.res.77.3.632. [DOI] [PubMed] [Google Scholar]
- Inui M, Chamberlain BK, Saito A, Fleischer S. The nature of the modulation of Ca2+ transport as studied by reconstitution of cardiac sarcoplasmic reticulum. J Biol Chem. 1986;261:1794–1800. [PubMed] [Google Scholar]
- James J, Martin L, Krenz M, Quatman C, Jones F, Klevitsky R, Gulick J, Robbins J. Forced expression of alpha-myosin heavy chain in the rabbit ventricle results in cardioprotection under cardiomyopathic conditions. Circulation. 2005;111:2339–2346. doi: 10.1161/01.CIR.0000164233.09448.B1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J, Sanbe A, Yager K, Martin L, Klevitsky R, Robbins J. Genetic manipulation of the rabbit heart via transgenesis. Circulation. 2000;101:1715–1721. doi: 10.1161/01.cir.101.14.1715. [DOI] [PubMed] [Google Scholar]
- Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW, 2nd, Walsh RA, Kranias EG. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J Clin Invest. 1996;97:533–539. doi: 10.1172/JCI118446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- Luo W, Wolska BM, Grupp IL, Harrer JM, Haghighi K, Ferguson DG, Slack JP, Grupp G, Doetschman T, Solaro RJ, Kranias EG. Phospholamban gene dosage effects in the mammalian heart. Circ Res. 1996;78:839–847. doi: 10.1161/01.res.78.5.839. [DOI] [PubMed] [Google Scholar]
- MacLennan DH, Asahi M, Tupling AR. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann N Y Acad Sci. 2003;986:472–480. doi: 10.1111/j.1749-6632.2003.tb07231.x. [DOI] [PubMed] [Google Scholar]
- Maurice JP, Shah AS, Kypson AP, Hata JA, White DC, Glower DD, Koch WJ. Molecular beta-adrenergic signaling abnormalities in failing rabbit hearts after infarction. Am J Physiol. 1999;276:H1853–1860. doi: 10.1152/ajpheart.1999.276.6.H1853. [DOI] [PubMed] [Google Scholar]
- Metra M, Nodari S, D’Aloia A, Muneretto C, Robertson AD, Bristow MR, Dei Cas L. Beta-blocker therapy influences the hemodynamic response to inotropic agents in patients with heart failure: a randomized comparison of dobutamine and enoximone before and after chronic treatment with metoprolol or carvedilol. J Am Coll Cardiol. 2002;40:1248–1258. doi: 10.1016/s0735-1097(02)02134-4. [DOI] [PubMed] [Google Scholar]
- Minamisawa S, Hoshijima M, Chu G, Ward CA, Frank K, Gu Y, Martone ME, Wang Y, Ross J, Jr, Kranias EG, et al. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999;99:313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- Morgan JP. Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N Engl J Med. 1991;325:625–632. doi: 10.1056/NEJM199108293250906. [DOI] [PubMed] [Google Scholar]
- Muller OJ, Lange M, Rattunde H, Lorenzen HP, Muller M, Frey N, Bittner C, Simonides W, Katus HA, Franz WM. Transgenic rat hearts overexpressing SERCA2a show improved contractility under baseline conditions and pressure overload. Cardiovasc Res. 2003;59:380–389. doi: 10.1016/s0008-6363(03)00429-2. [DOI] [PubMed] [Google Scholar]
- Periasamy M, Reed TD, Liu LH, Ji Y, Loukianov E, Paul RJ, Nieman ML, Riddle T, Duffy JJ, Doetschman T, et al. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. J Biol Chem. 1999;274:2556–2562. doi: 10.1074/jbc.274.4.2556. [DOI] [PubMed] [Google Scholar]
- Sanbe A, James J, Tuzcu V, Nas S, Martin L, Gulick J, Osinska H, Sakthivel S, Klevitsky R, Ginsburg KS, et al. Transgenic rabbit model for human troponin I-based hypertrophic cardiomyopathy. Circulation. 2005;111:2330–2338. doi: 10.1161/01.CIR.0000164234.24957.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Kiriazis H, Yatani A, Schmidt AG, Hahn H, Ferguson DG, Sako H, Mitarai S, Honda R, Mesnard-Rouiller L, et al. Rescue of contractile parameters and myocyte hypertrophy in calsequestrin overexpressing myocardium by phospholamban ablation. J Biol Chem. 2001;276:9392–9399. doi: 10.1074/jbc.M006889200. [DOI] [PubMed] [Google Scholar]
- Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem. 1986;261:13333–13341. [PubMed] [Google Scholar]
- Slack JP, Grupp IL, Dash R, Holder D, Schmidt A, Gerst MJ, Tamura T, Tilgmann C, James PF, Johnson R, et al. The enhanced contractility of the phospholamban-deficient mouse heart persists with aging. J Mol Cell Cardiol. 2001;33:1031–1040. doi: 10.1006/jmcc.2001.1370. [DOI] [PubMed] [Google Scholar]
- Song Q, Young KB, Chu G, Gulick J, Gerst M, Grupp IL, Robbins J, Kranias EG. Overexpression of phospholamban in slow-twitch skeletal muscle is associated with depressed contractile function and muscle remodeling. Faseb J. 2004;18:974–976. doi: 10.1096/fj.03-1058fje. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev. 1986;66:710–771. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- Vangheluwe P, Schuermans M, Zador E, Waelkens E, Raeymaekers L, Wuytack F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J. 2005;389:151–159. doi: 10.1042/BJ20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal RR, Van Baelen K, Groenen JT, van Geel A, Rottiers V, Kaletta T, Dode L, Raeymaekers L, Wuytack F, Bogaert T. The sarco-endoplasmic reticulum Ca2+ ATPase is required for development and muscle function in Caenorhabditis elegans. J Biol Chem. 2001;276:43557–43563. doi: 10.1074/jbc.M104693200. [DOI] [PubMed] [Google Scholar]