Abstract
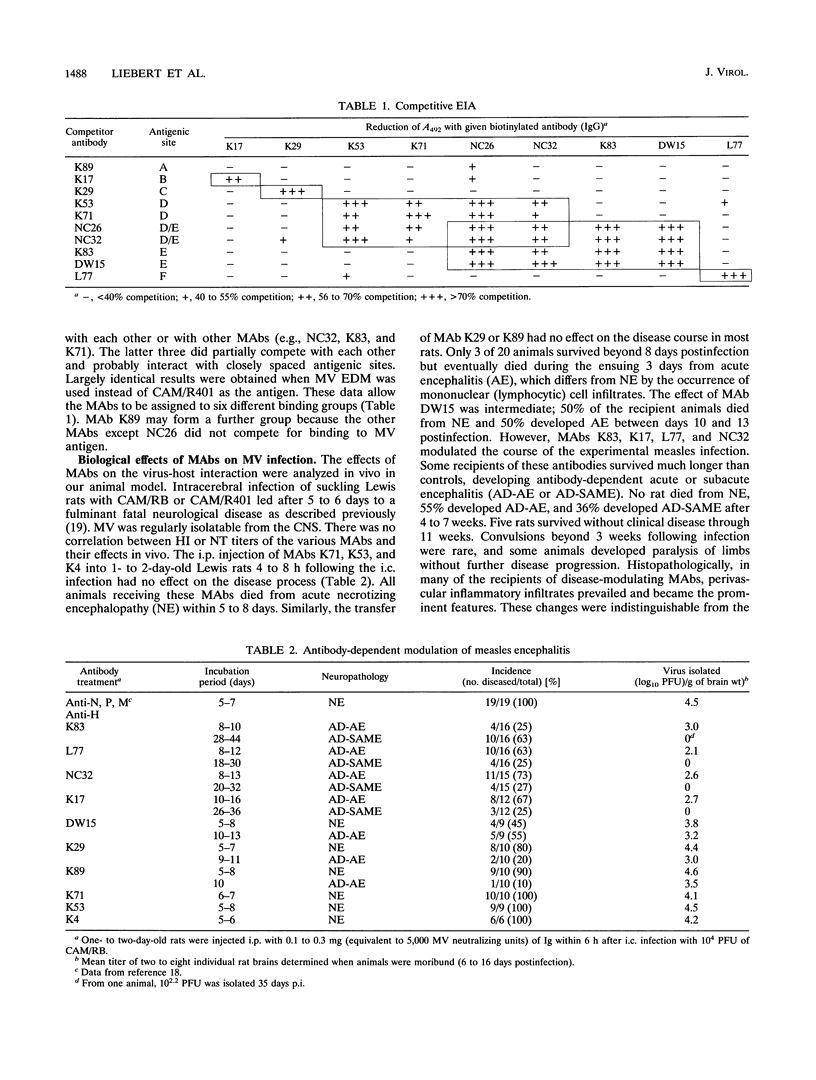
The biological activity of monoclonal antibodies specific for the hemagglutinin protein of measles virus strain CAM recognizing six epitope groups according to their binding properties to measles virus strain CAM/R401 was investigated in vivo in our rat model of measles encephalitis. When injected intraperitoneally into measles virus-infected suckling rats, some monoclonal antibodies modified the disease process and prevented the necrotizing encephalopathy seen in untreated animals. The analysis of measles virus brain isolates revealed emergence of variants that resisted neutralization with the passively transferred selecting monoclonal antibody but not with other monoclonal antibodies. Monoclonal antibody escape mutants were also isolated in vitro, and their neurovirulence varied in the animal model. Sequence data from the hemagglutinin gene of measles virus localize a major antigenic surface determinant of the hemagglutinin protein between amino acid residues 368 and 396, which may be functionally important for neurovirulence. The data indicate that the interaction of antibodies with the measles virus H protein plays an important role in the selection of neurovirulent variants. These variants have biological properties different from those of the parent CAM virus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkhatib G., Briedis D. J. The predicted primary structure of the measles virus hemagglutinin. Virology. 1986 Apr 30;150(2):479–490. doi: 10.1016/0042-6822(86)90312-0. [DOI] [PubMed] [Google Scholar]
- Baczko K., Lampe J., Liebert U. G., Brinckmann U., ter Meulen V., Pardowitz I., Budka H., Cosby S. L., Isserte S., Rima B. K. Clonal expansion of hypermutated measles virus in a SSPE brain. Virology. 1993 Nov;197(1):188–195. doi: 10.1006/viro.1993.1579. [DOI] [PubMed] [Google Scholar]
- Baczko K., Liebert U. G., Billeter M., Cattaneo R., Budka H., ter Meulen V. Expression of defective measles virus genes in brain tissues of patients with subacute sclerosing panencephalitis. J Virol. 1986 Aug;59(2):472–478. doi: 10.1128/jvi.59.2.472-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Pardowitz I., Rima B. K., ter Meulen V. Constant and variable regions of measles virus proteins encoded by the nucleocapsid and phosphoprotein genes derived from lytic and persistent viruses. Virology. 1992 Sep;190(1):469–474. doi: 10.1016/0042-6822(92)91236-n. [DOI] [PubMed] [Google Scholar]
- Barrett P. N., Koschel K., Carter M., ter Meulen V. Effect of measles virus antibodies on a measles SSPE virus persistently infected C6 rat glioma cell line. J Gen Virol. 1985 Jul;66(Pt 7):1411–1421. doi: 10.1099/0022-1317-66-7-1411. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Billeter M. A. Mutations and A/I hypermutations in measles virus persistent infections. Curr Top Microbiol Immunol. 1992;176:63–74. doi: 10.1007/978-3-642-77011-1_5. [DOI] [PubMed] [Google Scholar]
- Curran M. D., Clarke D. K., Rima B. K. The nucleotide sequence of the gene encoding the attachment protein H of canine distemper virus. J Gen Virol. 1991 Feb;72(Pt 2):443–447. doi: 10.1099/0022-1317-72-2-443. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature. 1979 Jun 7;279(5713):529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu A., Sheshberadaran H., Norrby E., Kövamees J. Molecular characterization of epitopes on the measles virus hemagglutinin protein. Virology. 1993 Jan;192(1):351–354. doi: 10.1006/viro.1993.1042. [DOI] [PubMed] [Google Scholar]
- Kasel J. A., Frank A. L., Keitel W. A., Taber L. H., Glezen W. P. Acquisition of serum antibodies to specific viral glycoproteins of parainfluenza virus 3 in children. J Virol. 1984 Dec;52(3):828–832. doi: 10.1128/jvi.52.3.828-832.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka Y., Webster R. G. Interplay between carbohydrate in the stalk and the length of the connecting peptide determines the cleavability of influenza virus hemagglutinin. J Virol. 1989 Aug;63(8):3296–3300. doi: 10.1128/jvi.63.8.3296-3300.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobune K., Kobune F., Yamanouchi K., Nagashima K., Yoshikawa Y., Hayami M. Neurovirulence of rat brain-adapted measles virus. Jpn J Exp Med. 1983 Jun;53(3):177–180. [PubMed] [Google Scholar]
- Kövamees J., Rydbeck R., Orvell C., Norrby E. Hemagglutinin-neuraminidase (HN) amino acid alterations in neutralization escape mutants of Kilham mumps virus. Virus Res. 1990 Oct;17(2):119–129. doi: 10.1016/0168-1702(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Liebert U. G., Linington C., ter Meulen V. Induction of autoimmune reactions to myelin basic protein in measles virus encephalitis in Lewis rats. J Neuroimmunol. 1988 Jan;17(2):103–118. doi: 10.1016/0165-5728(88)90018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebert U. G., Schneider-Schaulies S., Baczko K., ter Meulen V. Antibody-induced restriction of viral gene expression in measles encephalitis in rats. J Virol. 1990 Feb;64(2):706–713. doi: 10.1128/jvi.64.2.706-713.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebert U. G., ter Meulen V. Virological aspects of measles virus-induced encephalomyelitis in Lewis and BN rats. J Gen Virol. 1987 Jun;68(Pt 6):1715–1722. doi: 10.1099/0022-1317-68-6-1715. [DOI] [PubMed] [Google Scholar]
- Matloubian M., Somasundaram T., Kolhekar S. R., Selvakumar R., Ahmed R. Genetic basis of viral persistence: single amino acid change in the viral glycoprotein affects ability of lymphocytic choriomeningitis virus to persist in adult mice. J Exp Med. 1990 Oct 1;172(4):1043–1048. doi: 10.1084/jem.172.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims C. A. The pathogenetic basis of viral tropism. Am J Pathol. 1989 Sep;135(3):447–455. [PMC free article] [PubMed] [Google Scholar]
- Mäkelä M. J., Lund G. A., Salmi A. A. Antigenicity of the measles virus haemagglutinin studied by using synthetic peptides. J Gen Virol. 1989 Mar;70(Pt 3):603–614. doi: 10.1099/0022-1317-70-3-603. [DOI] [PubMed] [Google Scholar]
- Reich A., Erlwein O., Niewiesk S., ter Meulen V., Liebert U. G. CD4+ T cells control measles virus infection of the central nervous system. Immunology. 1992 Jun;76(2):185–191. [PMC free article] [PubMed] [Google Scholar]
- Rota J. S., Hummel K. B., Rota P. A., Bellini W. J. Genetic variability of the glycoprotein genes of current wild-type measles isolates. Virology. 1992 May;188(1):135–142. doi: 10.1016/0042-6822(92)90742-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A., Cattaneo R., Billeter M. A. A procedure for selective full length cDNA cloning of specific RNA species. Nucleic Acids Res. 1987 May 26;15(10):3987–3996. doi: 10.1093/nar/15.10.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Schaulies S., Liebert U. G., Segev Y., Rager-Zisman B., Wolfson M., ter Meulen V. Antibody-dependent transcriptional regulation of measles virus in persistently infected neural cells. J Virol. 1992 Sep;66(9):5534–5541. doi: 10.1128/jvi.66.9.5534-5541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Coulon P., Rollin P. E., Flamand A. Rabies virulence: effect on pathogenicity and sequence characterization of rabies virus mutations affecting antigenic site III of the glycoprotein. J Virol. 1985 Mar;53(3):926–934. doi: 10.1128/jvi.53.3.926-934.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers B. A., Greisen H. A., Appel M. J. Possible initiation of viral encephalomyelitis in dogs by migrating lymphocytes infected with distemper virus. Lancet. 1978 Jul 22;2(8082):187–189. doi: 10.1016/s0140-6736(78)91924-4. [DOI] [PubMed] [Google Scholar]
- Taylor M. J., Godfrey E., Baczko K., ter Meulen V., Wild T. F., Rima B. K. Identification of several different lineages of measles virus. J Gen Virol. 1991 Jan;72(Pt 1):83–88. doi: 10.1099/0022-1317-72-1-83. [DOI] [PubMed] [Google Scholar]
- Tsukiyama K., Yoshikawa Y., Yamanouchi K. Fusion glycoprotein (F) of rinderpest virus: entire nucleotide sequence of the F mRNA, and several features of the F protein. Virology. 1988 Jun;164(2):523–530. doi: 10.1016/0042-6822(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Weinmann-Dorsch C., Koschel K. Coupling of viral membrane proteins to phosphatidylinositide signalling system. FEBS Lett. 1989 Apr 24;247(2):185–188. doi: 10.1016/0014-5793(89)81330-4. [DOI] [PubMed] [Google Scholar]
- Zinnheimer-Dreikorn J., Koschel K. P. Antigenic modulation of measles subacute sclerosing panencephalitis virus in a persistently infected rat glioma cell line by monoclonal anti-haemagglutinin antibodies. J Gen Virol. 1990 Jun;71(Pt 6):1391–1394. doi: 10.1099/0022-1317-71-6-1391. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Löffler S., Carter M. J., Stephenson J. R. Antigenic characterization of measles and SSPE virus haemagglutinin by monoclonal antibodies. J Gen Virol. 1981 Dec;57(Pt 2):357–364. doi: 10.1099/0022-1317-57-2-357. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Tierney E. L., Hall S. L., London W. T., Kim H. W., Chanock R. M., Murphy B. R. Antibody responses of humans and nonhuman primates to individual antigenic sites of the hemagglutinin-neuraminidase and fusion glycoproteins after primary infection or reinfection with parainfluenza type 3 virus. J Virol. 1990 Aug;64(8):3833–3843. doi: 10.1128/jvi.64.8.3833-3843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]




