Abstract
Background
The aim of this study was to determine whether older disabled women with diabetes have an increased risk of falls compared to women without diabetes and to identify fall risk factors among this high-risk subgroup of patients.
Methods
Data are from the Women’s Health and Aging Study I (n = 1002, age ≥ 65 years), a prospective, population-based cohort study of the one third most disabled women in the Baltimore (MD) urban community-dwelling population. Participants were followed semiannually for 3 years. Falls were ascertained at each interview. Diabetes was ascertained by means of a standardized algorithm using multiple sources of information.
Results
Baseline prevalence of diabetes was 15.5%. Of the 878 women who participated in at least one follow-up visit and were able to walk at baseline, 64.9% fell at least once during the study and 29.6% experienced two or more falls during a follow-up interval. After adjustment for traditional risk factors, women with diabetes had a higher probability of any fall (odds ratio [OR] 1.38; 95% confidence interval [CI], 1.04–1.81) and of falling two or more times during a follow-up interval (OR 1.69; CI, 1.18–2.43), compared with women without diabetes. Among diabetic women, presence of widespread musculoskeletal pain (OR 5.58; CI, 1.89–16.5), insulin therapy (OR 2.02; CI, 1.10–3.71), overweight (OR 3.50; CI, 1.21–10.1), and poor lower-extremity performance (OR 7.76; CI, 1.03–58.8) were independently associated with increased likelihood of recurrent falls, after adjusting for major risk factors. There were synergistic effects of diabetes and lower-extremity pain and also diabetes and body mass index levels on the risk of falling (p for interactions < .05).
Conclusion
Even among disabled older women diabetes is associated with an increased risk of falling, independent of established fall risk factors. In this specific group of older women, pain, high body mass index, and poor lower-extremity performance are powerful predictors of falling.
Falls are a major cause of death and a significant source of morbidity and disability among elderly persons (1,2). Among the older population, falls are the most common cause of hospital admission for trauma and account for almost 90% of fractures (3). Furthermore, recurrent falls and fear of falling may severely reduce the quality of life; indeed, even noninjurious falls can result in a postfall syndrome characterized by anxiety, reduced social and physical activities, deconditioning, and increased risk of institutionalization (4).
In the last two decades, the search for potentially modifiable conditions associated with the risk of falling identified a number of sociodemographic, behavioral, and clinical characteristics (5,6). Among the latter, growing evidence suggests that diabetes mellitus may represent one of the major predictors of the risk of falling (7,8). Diabetes mellitus is highly prevalent in older people (9). Data from the Third National Health and Nutrition Survey suggest that more than 17% of women develop diabetes by the age of 75 (10). Older persons with diabetes might be at high risk of a broad spectrum of poor health outcomes, including physical disability and falls, because in this specific population traditional diabetes complications interact with age-related functional decline (11).
A number of diseases and impairments, including cardiovascular diseases, peripheral neuropathy, impaired gait and balance, overweight, visual deficit, and cognitive impairment are more prevalent in diabetics (12) and may explain the excess risk of falling associated with diabetes. Nevertheless, the specific mechanisms underlying the pathway between diabetes and falls have not so far been elucidated. For instance, previous studies reported that the relationship between diabetes and risk of falling is at least partially independent of major diabetes complications (13,14).
Using data from The Women’s Health and Aging Study (WHAS) (15), we evaluated the relationship between presence of diabetes and risk of falling over a 3-year follow-up. We sought to identify specific risk factors for falling among the subgroup of older women with diabetes.
METHODS
Study Population
The WHAS is an epidemiological study of the causes and course of disability among the one third most disabled women aged 65 years and older living in the community (15). Participants were recruited from an age-stratified random sample of community-dwelling Medicare beneficiaries residing in 12 contiguous ZIP codes in the Baltimore, Maryland area. Among 5316 noninstitutionalized women sampled, 1409 were eligible for the study because of a Mini-Mental State Examination (MMSE) (16) score ≥18 (17) and self-reported difficulty in performing one or more tasks in at least two of the following four domains of functioning: mobility/exercise tolerance, upper-extremity abilities, basic self-care, and higher functioning tasks of independent living. Eligibility criteria were assessed by a home interview administered to women who agreed to participate in the screening assessment. The screening questionnaire included batteries assessing disability status and cognitive functioning, both of which were used to determine study eligibility. Overall, 1002 women (71% of those eligible) agreed to participate in the study. Participants were re-evaluated over six semiannual in-home follow-up visits. The study was approved by the Johns Hopkins University Institutional Review Board, and all participants gave informed consent. For this specific analysis, women who died (n = 44) or dropped out (n = 14) within the first 6 months of the study, and those women who were unable to walk across a small room at baseline (n = 66), were excluded.
Assessment of Diabetes
Diabetes was ascertained at baseline using an algorithm specifically developed for this study. Disease-ascertainment algorithms used data from the baseline interview, the nurse’s examination, and the participant’s current medication list. The diabetes algorithm also used information from medical records, nonfasting blood test results, and a questionnaire sent to the participant’s primary care physician (12,15). Participants were asked whether a physician had ever told them they had any of 17 major diseases and were asked to show the interviewer their current medications. Women with a history of physician-diagnosed diabetes and who were taking oral hypoglycemic drugs or insulin were defined as diabetic. For those not taking diabetes medications or without history of diabetes, disease status was further evaluated by checking the value of total glycohemoglobin (HbA1), measured using low-pressure cation exchange chromatography (reference range 5%–8.6%, Ciba Corning 765 Glycomat; Palo Alto, CA). Because of refusal or missing data, this test was available for 65% of the participants; a value > 10% was considered diagnostic for diabetes. Only for eight participants the diagnoses were made using glycohemoglobin level. Because there is not consensus on the use of HbA1 for the diagnosis of diabetes, this cutoff was defined a priori to achieve good diagnostic specificity. For women with missing glycohemoglobin or with glycohemoglobin ≤10%, the absence of disease was confirmed by use of the questionnaire sent to the participant’s primary care physician or by direct contact with the physician.
Fall Ascertainment
At the baseline interview, women were asked about falls in the previous year. During the 3-year follow-up, the occurrence of falls in the previous 6 months was assessed during the six semiannual interviews. Participants were asked to report number of falls and the worst fall-related injury during each 6-month interval.
Risk Factors for Falls
Comorbidities and impairments
Medical conditions ascertained at baseline according to predefined criteria used in this analysis were stroke, knee osteoarthritis, and hypertension. Body mass index (BMI; kg/m2) was computed using measured height and weight. Women with BMI values between 25 and 29.9 were considered overweight, and those women with values ≥30 were considered obese (18). Peripheral arterial disease (PAD) was assessed by the ankle-brachial index (Doppler stethoscope Parks Model 841-A; Parks, Aloha, OR). An ankle-brachial index value < 0.9 was considered diagnostic (19). Large fiber sensory nerve function was quantified by measuring vibration perception threshold with the Vibratron II (Physitemp Instruments, Inc., Clifton, NJ). On the basis of previous WHAS analyses (20,21), participants were categorized in three groups according to detection of small vibratory stimuli, as follows: (1) normal function (< 3.43 vibration units), (2) mild to moderate dysfunction (3.43–< 6.31), or (3) severe dysfunction (≥6.31). Visual impairment was defined as a visual acuity worse than 20/40 (with corrective lenses, if used) (22); cognitive status was evaluated by means of the MMSE (16,17); depressive symptoms were assessed by the Geriatric Depression Scale (cut point ≥14) (23).
Pain was assessed according to location and severity of pain at a number of musculoskeletal sites, as previously described (24). Participants were queried about pain in the hand, wrist, back, and feet by asking whether they had pain in each site on most days for at least 1 month in the previous years. Referring to a 10-point numeric rating scale (NRS), participants were asked to rate the average pain in the past month from 0 to 10, with 0 indicating no pain and 10 indicating “severe or excruciating pain as bad as you can imagine.” For this specific analysis, four locations related to lower-extremity function were considered (back, hip, knee, and feet). Pain was categorized as follows: (a) no pain (defined as no pain or only mild pain [< 4 on NRS] in one site), (b) pain in two sites or moderate-to-severe pain (≥4 on NRS) in only one site, or (c) pain in three or four sites regardless of severity. Medications taken in the previous 2 weeks were coded to identify all active ingredients (25). Medications considered in this analysis included insulin, oral antihyperglycemics, psychotherapeutic agents (antidepressants, antipsychotics), anxiolytics (26), hypotensive agents, and analgesic medication.
Measures of functional status
Self-reported information included difficulty with five basic activities of daily living (ADLs), (bathing, transferring from bed to chair, using the toilet, dressing, and eating). Responses were coded as: none, a little, some, a lot, or unable to perform the task. ADL disability was defined as the presence of a lot of difficulty or inability in at least one ADL.
Performance-based measures of physical function included usual walking speed over 4 meters, five chair stands test, and balance test (15). Using the results of these three tests the summary performance score was calculated. The summary performance score is a global measure of leg functioning that predicts mobility loss, nursing home placement, and mortality among community-dwelling elderly individuals. A score (scale, 0–4) was assigned to performance on time to rise five times from a seated position, standing balance, and 4-meter walking velocity. Individuals received a score of 0 for each task they were unable to complete. Scores of 1–4 for each task were assigned based on quartiles of performance for more than 6000 participants in the Established Populations for the Epidemiologic Study of the Elderly (27). This well-tested score predicts disability, hospitalization, nursing home admission, and mortality in older persons (28). Maximal knee-extension strength was tested using a handheld dynamometer (Nichols Manual Muscle Tester; Fred Sammons, Inc., Burr Ridge, IL), with two trials for each leg. Tests were conducted with the participant seated comfortably in a hard chair. The dynamometer was placed a few inches above the right ankle between the medial and lateral malleolus for the knee extension. Participants were instructed to push against the dynamometer as hard as they could, and the examiner then pushed hard enough to break the contraction. The best performance of two trials was selected for each side, and the average of the left and right values were used in the analysis.
Statistical Analysis
Baseline characteristics of the study population were compared according to presence or absence of diabetes and by type of antidiabetic treatment. To study the association between diabetes and the risk of falling, two different outcomes were considered: (1) any fall during the follow-up, and (2) two or more falls during a 6-month follow-up interval. In addition, to explore the role of insulin therapy as an independent risk factor, analyses were stratified according to the type of therapy. Multivariate discrete time-survival analysis with logistic regression was used to estimate the association between diabetes and the likelihood of falling during the study. This method uses logistic regression to determine the relative odds of falling for women who had not previously fallen during the study. Each participant potentially contributed an observation for each 6-month follow-up interval (for a maximum of six). In fact, each woman contributed data up to the round at which she first reported the outcome, died, or was lost to follow-up and not thereafter (censored). Demographic and clinical characteristics hypothesized to be potential confounders or mediators of the association between diabetes and the risk of falling were progressively added to the models. Objective measures of physical function (summary performance score and knee-extension strength) were included in the logistic models as time-dependent covariates. To identify risk factors for recurrent falls specific to the diabetic population we constructed separate logistic models among the 136 women with diagnosis of diabetes. First, we identified factors associated with the risk of recurrent falls with adjustment only for age; then, to identify the independent predictors, all factors associated with the outcome at an α level of 0.1 were introduced in a backward selection logistic regression model (p for removal > 0.1).
RESULTS
Of 878 WHAS participants included in this analysis, 136 (15.5%) had an adjudicated diagnosis of diabetes at baseline, with an average self-reported duration of disease of 13.3 years (median = 11; range = <1–42). Compared to participants without diabetes (Table 1), those with diabetes were younger; after adjustment for age, they were more likely to be black and more likely to have cardiovascular conditions, peripheral nerve dysfunction, visual impairment, elevated BMI, lower MMSE score, and more depressive symptoms. Additionally, these women had poorer objective measures of lower-extremity function and were more likely to have ADL disability.
Table 1.
Selected Characteristics by Diabetes Status
| Without Diabetes
|
With Diabetes
|
||||
|---|---|---|---|---|---|
| Characteristics | (N = 742) | Diet/Oral Therapy (N = 97) | Insulin (N = 39) | All (N = 136) | p* |
| General | |||||
| Age, y | 79.1 ± 8.1 | 75.9 ± 6.7 | 74.0 ± 5.7 | 75.3 ± 6.5 | < .001 |
| African American, %† | 25.3 | 44.3 | 53.9 | 47.1 | < .001 |
| Education ≥ 12 y, % | 35.7 | 33.0 | 18.0 | 26.0 | .084 |
| Current smoker, % | 16.0 | 11.3 | 7.7 | 10.3 | .045 |
| Duration of diabetes | — | 11.9 ± 9.7 | 16.7 ± 8.7 | 13.3 ± 9.7 | — |
| Comorbidities, % | |||||
| Overweight (BMI 25–29.9) | 33.7 | 37.1 | 33.3 | 36.0 | < .05 |
| Obesity (BMI ≥ 30) | 33.2 | 43.3 | 56.4 | 47.1 | < .01 |
| Hypertension | 59.3 | 76.3 | 84.6 | 78.7 | < .001 |
| Stroke | 5.3 | 9.3 | 12.8 | 10.3 | < .05 |
| Peripheral arterial disease | 26.5 | 40.0 | 51.4 | 43.3 | < .001 |
| Peripheral nerve dysfunction | |||||
| Mild-moderate | 36.7 | 43.2 | 31.4 | 39.8 | |
| Severe | 18.9 | 14.8 | 31.4 | 19.5 | < .05 |
| Visual impairment | 24.3 | 27.3 | 21.2 | 25.6 | < .05 |
| Lower extremity pain | |||||
| 1–2 sites | 42.1 | 45.4 | 41.0 | 44.1 | .296 |
| 3–4 sites | 31.7 | 36.1 | 38.5 | 36.8 | .333 |
| Knee osteoarthritis | 44.5 | 53.6 | 51.3 | 52.9 | .131 |
| MMSE score | 26.5 ± 2.9 | 26.5 ± 3.1 | 25.6 ± 2.7 | 26.2 ± 3.0 | < .05 |
| Severe depression symptoms | 14.6 | 22.7 | 23.1 | 22.8 | < .001 |
| Functional status | |||||
| Walking speed, m/s | 0.66 ± 0.3 | 0.58 ± 0.3 | 0.59 ± 0.3 | 0.59 ± 0.3 | < .001 |
| Balance test score (0–4) | 2.17 ± 1.4 | 2.13 ± 1.4 | 1.90 ± 1.3 | 2.06 ± 1.4 | < .001 |
| Chair stand test (stand/s) | 0.28 ± 0.2 | 0.25 ± 0.2 | 0.21 ± 0.2 | 0.24 ± 0.2 | < .001 |
| Summary performance score | 6.45 ± 3.1 | 5.91 ± 3.3 | 5.38 ± 2.9 | 5.76 ± 3.2 | < .001 |
| Knee extension strength (kg/kg) | 0.20 ± 0.8 | 0.18 ± 0.7 | 0.17 ± 0.7 | 0.18 ± 0.7 | .012 |
| ADL disability, % | 24.7 | 34.0 | 33.3 | 33.8 | < .01 |
| Fall in past 12 mo | 33.8 | 23.7 | 33.3 | 26.5 | .217 |
| Medications, % | |||||
| Psychotherapeutic | 8.0 | 8.3 | 10.3 | 8.8 | .871 |
| Anxiolytics | 11.3 | 7.2 | 2.6 | 5.9 | .075 |
| Antihypertensive | 33.8 | 50.5 | 64.1 | 54.4 | < .001 |
| Daily analgesic use | 51.1 | 51.6 | 56.4 | 52.9 | .783 |
Note: Continuous variables are presented as mean ± standard deviation.
p values are for age-adjusted logistic or linear regression comparing women with (All) and without diabetes.
Racial groups were white = 626 (71.0%), African American = 250 (28.5%), and other = 2 (0.5%).
BMI = body mass index; MMSE = Mini-Mental State Examination; ADL = activity of daily living.
More than 60% of this cohort fell at least one time during the 3-year follow-up (Figure 1). There was a graded relationship between diabetes status and the risk of falling, with women using insulin therapy at highest risk (p < .001). The percentage of recurrent falls was also particularly high for women on insulin therapy (59%), whereas it was much lower for other diabetic women and for participants without diabetes (30.9% and 27.9%, respectively) (p <.001).
Figure 1.

Crude cumulative incidence of falling during follow-up according to diabetes status at baseline.
Multivariate analysis (Table 2) confirmed that disabled women with diabetes were at higher risk of both outcomes and that the association was independent of a number of potential confounders and mediators including several diabetes-related complications and time-dependent objective measures of lower-extremity physical performance. For example, women with diabetes had a 44% increased risk of falling during the follow-up, and the estimate was similar after full adjustment (OR 1.38; 95% CI, 1.04–1.81). Compared to women without diabetes, women with diabetes treated with insulin had almost a threefold increased risk of recurrent falls during the follow-up (adjusted OR 2.73; 95% CI, 1.61–4.63).
Table 2.
Adjusted Odds Ratios for Falls During Follow-Up According to Diabetes Status
| Proportion With Outcome, % | Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) | |
|---|---|---|---|---|
| Risk for fall | ||||
| Without diabetes | 63.5 | 1 | 1 | 1 |
| With diabetes, all sample | 72.8 | 1.44 (1.12–1.87) | 1.42 (1.08–1.86) | 1.38 (1.04–1.81) |
| Without diabetes | 63.5 | 1 | 1 | 1 |
| With diabetes | ||||
| Diet/oral therapy | 70.1 | 1.39 (1.03–1.88) | 1.39 (1.02–1.90) | 1.35 (0.99–1.84) |
| Insulin | 79.5 | 1.57 (1.02–2.40) | 1.47 (0.94–2.30) | 1.45 (0.92–2.27) |
| Risk for recurrent falls | ||||
| Without diabetes | 27.9 | 1 | 1 | 1 |
| With diabetes, all sample | 39.0 | 1.86 (1.33–2.60) | 1.77 (1.24–2.53) | 1.69 (1.18–2.43) |
| Without diabetes | 27.9 | 1 | 1 | 1 |
| With diabetes | ||||
| Diet/oral therapy | 30.9 | 1.44 (0.96–2.17) | 1.40 (0.91–2.15) | 1.34 (0.87–2.1) |
| Insulin | 59.0 | 3.09 (1.90–5.02) | 2.86 (1.72–4.85) | 2.73 (1.61–4.63) |
Notes: Model 1 was adjusted for age, race, education, and smoking. Model 2 was adjusted for factors in Model 1 plus overweight, obesity, hypertension, use of antihypertensive drugs, stroke, peripheral arterial disease, peripheral nerve dysfunction, knee osteoarthritis pain categories, visual impairment, Mini-Mental State Examination score, fall in 12 months before baseline interview, and activity of daily living disability. Model 3 was adjusted for factors in Model 2 plus physical performance score and knee strength included in the models as time-dependent covariates.
OR = odds ratio; CI = confidence interval.
In the analysis limited to women with diabetes, factors independently associated with the risk of recurrent falls included insulin therapy, overweight, lower-extremity pain, and a poor lower-extremity summary performance score (< 9 points). Obesity, adjudicated knee osteoarthritis, and stroke were also associated with the likelihood of falling, but the estimates were not statistically significant (Table 3).
Table 3.
Adjusted Odds Ratios for Recurrent Falls During Follow-Up Among 136 Women With Prevalent Diabetes at Baseline
| Risk Factors | Odds Ratio | 95% CI | p |
|---|---|---|---|
| Knee osteoarthritis | 1.78 | 0.93–3.39 | .082 |
| Stroke | 2.05 | 0.87–4.86 | .103 |
| Insulin therapy | 2.02 | 1.10–3.71 | .019 |
| Overweight (BMI 25–30) | 3.50 | 1.21–10.1 | .020 |
| Obesity (BMI ≥ 30) | 2.03 | 0.73–5.65 | .173 |
| Lower-extremity pain | |||
| 1–2 sites | 3.61 | 1.26–10.4 | .017 |
| 3–4 sites | 5.58 | 1.89–16.5 | .002 |
| Summary physical performance < 9 | 7.76 | 1.03–58.8 | .047 |
Notes: Odds ratios, 95% confidence intervals (CI), and p values are computed from a backward stepwise multivariate logistic regression analysis (p for selection < .1). Variables also included in the initial model were: previous fall in the last 12 months, Mini-Mental State Examination (MMSE) symptoms of depression, use of pain medications, use of hypotensive medications, and visual impairment. All the variables included in the initial logistic model were associated with the outcome at an α level of 0.1 after adjustment for age.
BMI = body mass index.
In the fully adjusted models, we found an interaction between diabetes status and lower-extremity pain and between diabetes and BMI categories (p values for the interaction terms < .05), suggesting that the excess risk of falling associated with diabetes was larger in women with pain and higher BMI compared to those women without these conditions. To illustrate this, analyses were done using pain/diabetes and BMI/diabetes categories (Figure 2). The upper graph shows that for every diabetes category there is a graded relationship between pain level and the risk of recurrent falls but the risk is particularly high among women on insulin therapy who had multiple-site pain (OR 6.5; 95% CI, 2.98–14.2). Of note, among women without pain, there was no relationship between diabetes status and risk of recurrent falls. Similar results were found after stratification for BMI categories (bottom graph), with overweight and obese diabetic women being at higher risk of recurrent falls during the follow-up.
Figure 2.
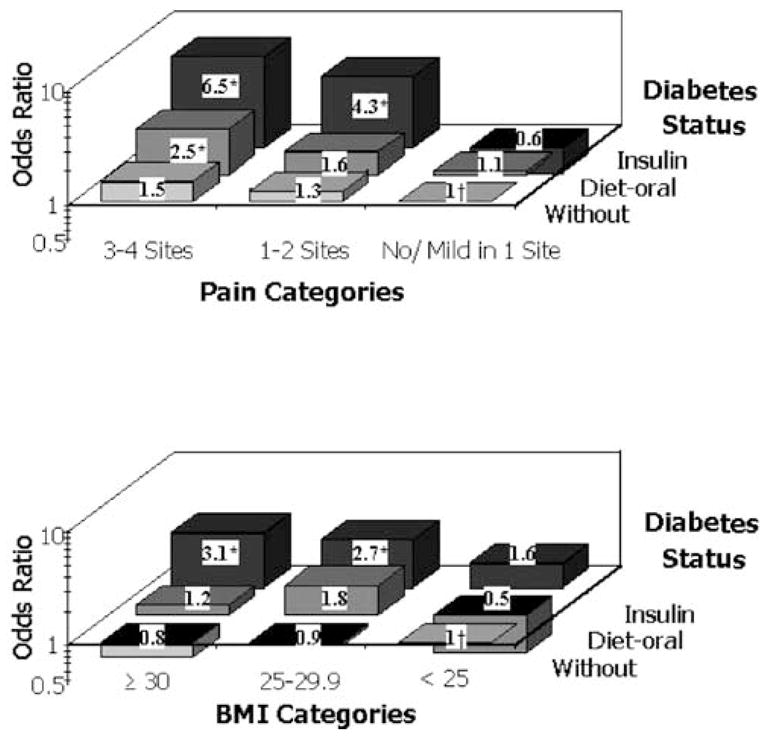
Odds ratios for recurrent falls during follow-up according to diabetes status with pain (top) and body mass (BMI) categories (bottom). Odds ratios are adjusted for age, race, presence of hand pain and chest pain, and lower-extremity performance score. *p < .05. †Reference group.
DISCUSSION
Women included in this study represent the approximately one third most disabled older women living in the community. Although the whole cohort had a very high risk of falls (64% absolute risk over the 3-year follow-up), we found that women with diabetes had a significant excess risk of falling that was independent of many major risk factors for falls and several diabetes complications. Among women with diabetes, insulin use, lower-extremity pain, and high BMI were the major risk factors for falls.
Our results, based on two different outcome definitions, reinforce the role of diabetes as a risk factor for falls, and particularly, recurrent falls, in older people (14,29). These findings also provide new insight into the specific mechanisms underlying the association between diabetes and falling. Diabetes has not been considered to be a risk factor for falls in older adults until recently (5,30,31), and the mechanisms responsible for such an association are not completely elucidated, particularly in older populations (32,33). For example, in the Study of Osteoporotic Fractures (13) insulin-treated diabetes was associated with a fourfold increased risk of recurrent falls. Nevertheless, adjustment for traditional risk factors explained only 30% of the increased risk.
Research to date has not explained the association between insulin therapy and increased fall risk (13,33). In our sample, diabetic women treated with insulin therapy had longer duration of disease and a greater prevalence of diabetes complications and other comorbidities, including peripheral nerve dysfunction, cardiovascular diseases, and poor lower-extremity performance, suggesting that insulin use should be regarded as a marker of disease severity. Of note, however, adjustment for a number of diabetes-related conditions and time-dependent objective measures of physical function, accounted for a small proportion (11%) of the association between insulin-treated diabetes and recurrent falling. Furthermore, additional adjustment for duration of disease, a variable strongly correlated with insulin therapy, did not modify the association between insulin use and risk of falling (data not shown). These findings are in agreement with previous studies (13,33,34) and suggest that other mechanisms, not evaluated thus far, need to be considered to elucidate the biological mechanisms underlying the association between insulin therapy and risk of falling. For example, use of insulin is the most important risk factor for hypoglycemia, and it is plausible that episodes of fainting and dizziness might also explain, at least in part, the excess risk of falls associated with diabetes.
Musculoskeletal pain has been recently highlighted as a powerful risk factor for falls in older women (35,36); nevertheless, its specific hazard in older diabetic patients has not been evaluated thus far. In older diabetic women, lower-extremity pain may have multiple and overlapping etiologies. Different diabetic neuropathies, including large- and small-fiber neuropathies and proximal motor neuropathy, are characterized by considerable pain and variable degrees of weakness and disability (37). Although our multivariate analyses were adjusted for objective measures of large-fiber sensory nerve dysfunction, other neuropathies might account for the interaction of diabetes with pain and falling. Multisite lower-extremity pain might also be considered a marker for the presence and severity of arthritis, a condition for which older diabetic women might be at particular risk as a consequence of obesity. Other causes of musculoskeletal pain in older adults might include fibromyalgia and myofascial pain.
Regardless of the cause of lower-extremity pain, a synergistic effect between diabetes and lower-extremity pain is of note. Because older diabetic women are characterized by a number of comorbidities and functional impairments predisposing to gait abnormalities and lower-extremity weakness (12), we hypothesize that, compared to their counterparts without diabetes, diabetic women might be less able to buffer and compensate for the pathophysiological and psychological factors associated with chronic pain including reflex inhibition, joint instability, fear of falling, and reduced attention. This pathway might be further enhanced in older individuals who are, independent of disease status, characterized by a multisystemic reduction of functional reserve (38). In a similar pathway, impaired gait and postural control and increased risk of falling have been reported in obese participants (39), supporting a biological explanation for the excess risk of falling observed in overweight and obese diabetic women.
A limitation of this study was that the algorithm used for diabetes ascertainment did not include a fasting glucose level. Moreover, glycohemoglobin was available for only 65% of the sample. Consequently, some women with fasting glucose level ≥126 mg/dl might have been classified as nondiabetic (40). Newly diagnosed older diabetics have greater prevalence of cardiovascular disease compared to persons without diabetes (41), and even short-term glycemic control has been associated with reduction in several symptoms including pain, dizziness, and fatigue (42). This evidence suggests that we might have underestimated the strength of the association between diabetes and the risk of falling. In addition, the estimates of risk for participants with diabetes are likely to be conservative because in this cohort the reference group was not representative of the general population without diabetes but, conversely, was characterized by participants with mild to severe disability. Finally, because the study population was disabled older women, our results cannot be generalized to all older women or to older men.
Older physically impaired women with diabetes have a high risk for falling. Besides traditional risk factors, insulin use, obesity, and lower-extremity pain identified diabetic older adults with the highest likelihood of falling, suggesting that multifactorial fall prevention programs (6,43) should be advocated in developing the standards of medical care for older patients with diabetes. Our results have important clinical implications and might be useful for better targeting preventive and therapeutic interventions to subgroups of patients who could benefit the most.
Acknowledgments
Address correspondence to Stefano Volpato, MD, MPH, Department of Clinical and Experimental Medicine, University of Ferrara, Via Savonarola, 9, 44100, Ferrara, Italy. E-mail: vlt@unife.it
References
- 1.Sattin RW. Falls among older persons: a public health perspective. Ann Rev Public Health. 1992;13:489–508. doi: 10.1146/annurev.pu.13.050192.002421. [DOI] [PubMed] [Google Scholar]
- 2.Fried L, Guralnik JM. Disability in older adults: evidence regarding significance, etiology and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 3.Fife D, Barancik JI. Northeastern Ohio Trauma Study III: incidence of fractures. Ann Emerg Med. 1985;14:244–248. doi: 10.1016/s0196-0644(85)80448-0. [DOI] [PubMed] [Google Scholar]
- 4.Cumming RG, Kelsey JL, Nevitt MC. Methodologic issues in the study of frequent and recurrent health problem. Falls in the elderly. Ann Epidemiol. 1990;1:49–56. doi: 10.1016/1047-2797(90)90018-n. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 6.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348:42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 7.Malmivaara A, Heliovaara M, Knekt P, Reunanen A, Aromaa A. Risk factors for injurious falls leading to hospitalization or death in a cohort of 19,500 adults. Am J Epidemiol. 1993;138:384–394. doi: 10.1093/oxfordjournals.aje.a116871. [DOI] [PubMed] [Google Scholar]
- 8.Miller DK, Lui LY, Perry HM, Kaiser FE, Morley JE. Reported and measured physical functioning in older inner-city diabetic African Americans. J Gerontol A Biol Sci Med Sci. 1999;54A:M230–M236. doi: 10.1093/gerona/54.5.m230. [DOI] [PubMed] [Google Scholar]
- 9.Harris M, editor. National Diabetes Data Group. Diabetes in America. 2. Bethesda, MD: National Institutes of Health; 1995. [Google Scholar]
- 10.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 11.Volpato S, Ferrucci L, Blaum C, et al. Progression of lower extremity disability in older women with diabetes: The Women’s Health and Aging Study. Diabetes Care. 2003;26:70–75. doi: 10.2337/diacare.26.1.70. [DOI] [PubMed] [Google Scholar]
- 12.Volpato S, Blaum C, Resnick H, Ferrucci L, Fried LP, Guralnik JM. Impairments and comorbidities explaining the association between diabetes mellitus and lower extremity disability: The Women’s Health and Aging Study. Diabetes Care. 2002;25:678–683. doi: 10.2337/diacare.25.4.678. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25:1749–1754. doi: 10.2337/diacare.25.10.1749. [DOI] [PubMed] [Google Scholar]
- 14.Gregg EW, Beckles GL, Williamson DF, et al. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23:1272–1277. doi: 10.2337/diacare.23.9.1272. [DOI] [PubMed] [Google Scholar]
- 15.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME, editors. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. [Last accessed: September 17, 2005]. NIH Pub. N0.95–4009. Available at: http://www.nia.nih.gov/HealthInformation/Publications/Reports/WomensHealthAgingStudy.htm. [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Obesity: preventing and managing the global epidemic. Geneva, Switzerland: World Health Organization; 1997. [PubMed] [Google Scholar]
- 19.Fowkes FGR. The measurements of atherosclerotic peripheral arterial disease in epidemiological surveys. Int J Epidemiol. 1988;17:248–283. doi: 10.1093/ije/17.2.248. [DOI] [PubMed] [Google Scholar]
- 20.Resnick HE, Vinik AI, Schwartz AV, et al. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: the Women’s Health and Aging Study. Diabetes Care. 2000;23:1642–1647. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- 21.Resnick HE, Vinik AI, Heimovitz HK, Brancati FL, Guralnik JM. Age 85 + years accelerates large-fiber peripheral nerve dysfunction and diabetes contributes even in the oldest-old: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2001;56:M25–M31. doi: 10.1093/gerona/56.1.m25. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. Geneva, Switzerland: World Health Organization; 1980. pp. 7–19. [Google Scholar]
- 23.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 24.Leveille SG, Ling S, Hochberg MC, et al. Widespread musculoskeletal pain and the progression of disability in older disabled women. Ann Intern Med. 2001;135:1038–1046. doi: 10.7326/0003-4819-135-12-200112180-00007. [DOI] [PubMed] [Google Scholar]
- 25.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 26.Passaro A, Volpato S, Romagnoni F, Manzoli N, Zuliani G, Fellin R. Benzodiazepines with different half-life and falling in a hospitalized population: The GIFA study. Gruppo Italiano di Farmacovigilanza nell’Anziano. J Clin Epidemiol. 2000;53:1222–1229. doi: 10.1016/s0895-4356(00)00254-7. [DOI] [PubMed] [Google Scholar]
- 27.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Study of Osteoporotic Features Research Group. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86:32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 30.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls: a prospective study. JAMA. 1989;261:2663–2668. [PubMed] [Google Scholar]
- 31.Nicodemus KK, Folsom A. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001;24:1192–1197. doi: 10.2337/diacare.24.7.1192. [DOI] [PubMed] [Google Scholar]
- 32.Wallace C, Reiber GE, LeMaster J, et al. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care. 2002;25:1983–1986. doi: 10.2337/diacare.25.11.1983. [DOI] [PubMed] [Google Scholar]
- 33.Ottenbacher KJ, Ostir GV, Peek MK, Goodwin JS, Markides KS. Diabetes mellitus as a risk factor for hip fracture in Mexican American older adults. J Gerontol A Biol Sci Med Sci. 2002;57A:M648–M653. doi: 10.1093/gerona/57.10.m648. [DOI] [PubMed] [Google Scholar]
- 34.Ivers RQ, Cumming RG, Mitchell P, Peduto AJ Blue Mountains Eye Study. Diabetes and risk of fracture: The Blue Mountains Eye Study. Diabetes Care. 2001;24:1198–1203. doi: 10.2337/diacare.24.7.1198. [DOI] [PubMed] [Google Scholar]
- 35.Nahit ES, Silman AJ, Macfarlane GJ. The occurrence of falls among patients with a new episode of hip pain. Ann Rheum Dis. 1998;57:166–168. doi: 10.1136/ard.57.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leveille SG, Bean J, Bandeen-Roche K, Jones R, Hochberg M, Guralnik JM. Musculoskeletal pain and risk for falls in older disabled women living in the community. J Am Geriatr Soc. 2002;50:671–678. doi: 10.1046/j.1532-5415.2002.50161.x. [DOI] [PubMed] [Google Scholar]
- 37.Winik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000;43:957–973. doi: 10.1007/s001250051477. [DOI] [PubMed] [Google Scholar]
- 38.Morley JE, Perry HM, Miller DK. Something about frailty. J Gerontol A Biol Sci Med Sci. 2002;57A:M698–M704. doi: 10.1093/gerona/57.11.m698. [DOI] [PubMed] [Google Scholar]
- 39.McGraw B, McClenaghan BA, Williams HG, Dickerson J, Ward DS. Gait and postural stability in obese and nonobese prepubertal boys. Arch Phys Med Rehabil. 2000;81:484–489. doi: 10.1053/mr.2000.3782. [DOI] [PubMed] [Google Scholar]
- 40.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 41.Wahl PW, Savage PJ, Psaty BM, Orchard TJ, Robbins JA, Tracy RP. Diabetes in older adults: comparison of 1997 American Diabetes Association classification of diabetes mellitus with 1985 WHO classification. Lancet. 1998;352:1012–1015. doi: 10.1016/S0140-6736(98)04055-0. [DOI] [PubMed] [Google Scholar]
- 42.Testa MA, Simonson DC. Health economic benefits and quality of life during improved glycemic control in patients with type 2 diabetes mellitus: a randomized, controlled, double-blind trial. JAMA. 1998;280:1490–1496. doi: 10.1001/jama.280.17.1490. [DOI] [PubMed] [Google Scholar]
- 43.Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;331:821–827. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]


