Abstract
Numerous studies indicate that C3H/HeJ (C3H) mice are mildly responsive to mechanical loading compared to C57BL/6J (C57) mice. Guided by data indicating high baseline periosteal osteoblast activity in 16 wk C3H mice, we speculated that simply allowing the C3H mice to age until basal periosteal bone formation was equivalent to that of 16 wk C57 mice would restore mechanoresponsiveness in C3H mice. We tested this hypothesis by subjecting the right tibiae of 32 wk old C3H mice and 16 wk old C57 mice to low magnitude rest-inserted loading (peak strain: 1235με) and then exposing the right tibiae of 32 wk C3H mice to low (1085με) or moderate (1875 με) magnitude cyclic loading. The osteoblastic response to loading on the endocortical and periosteal surfaces was evaluated via dynamic histomorphometry. At 32 wk of age, C3H mice responded to low magnitude rest-inserted loading with significantly elevated periosteal mineralizing surface, mineral apposition rate and bone formation compared to unloaded contralateral bones. Surprisingly, the periosteal bone formation induced by low magnitude rest-inserted loading in C3H mice exceeded that induced in 16 wk C57 mice. At 32 wk of age, C3H mice also demonstrated an elevated response to increased magnitudes of cyclic loading. We conclude that a high level of basal osteoblast function in 16 wk C3H mice appears to overwhelm the ability of the tissue to respond to an otherwise anabolic mechanical loading stimulus. However, when basal surface osteoblast activity is equivalent to that of 16 wk C57 mice, C3H mice demonstrate a clear ability to respond to either rest-inserted or cyclic loading.
Keywords: mechanotransduction, bone formation, osteoblast function, mechanical loading, C3H/HeJ and C57BL/6J
Introduction
Beginning with the earliest stages of skeletal development through the attainment of peak bone mass and maintenance of homeostasis during adulthood, genetic and environmental factors interact to modulate skeletal morphology [1–3]. Given their relative ease of genetic manipulation, mice provide an effective model system to explore how specific genetic and environmental factors might influence these biologic processes. This is particularly due to the availability of transgenic and knockout mice that have defined numerous proteins and cytokines regulating skeletal phenotype, as well as availability of in-bred and cross-bred strains with distinct skeletal morphologies [4–11].
Within the realm of environmental influences, mechanical loading can serve as a powerful anabolic stimulus for bone following completion of skeletal development [12]. However, in human exercise trials augmentation of bone mass is modest and highly variable [13–16]. Observations that different genetic strains of mice vary greatly in their ability to respond to mechanical loading suggest that genetic variations in the ability of the skeleton to perceive and respond to mechanical loading may account for the equivocal results of exercise trials [17–23]. Consequently, it has been reasonably argued that a better understanding of the genetic contribution to bone’s response to mechanical loading would enable tailored design of exercise interventions such that the beneficial effects of skeletal loading is realized across a diverse population [18].
The availability of various in-bred mouse strains facilitates a study of genetic interactions with mechanical loading. C3H/HeJ (C3H) mice in particular have been found to be less responsive to mechanical loading than other strains of mice (e.g., C57BL/6J (C57)). In a variety of studies, C3H mice have been found to be unresponsive to low magnitude loading, have consistently required greater magnitudes of mechanical stimulation to induce any anabolic response, and when induced, observed responses are muted compared to other strains of mice [17–19, 24, 25]. These studies have spawned a number of trait mapping efforts to identify specific genetic loci responsible for deficient mechanotransduction in C3H mice [26–31], as well as attempts to isolate specific alterations in downstream bone cell function underlying the muted tissue level responses [32–34].
Recently, our group reported in a preliminary study that inserting a 10 s rest period between each load cycle of a cyclic loading regimen significantly elevated bone’s response to low magnitude mechanical loading in C57 mice [35]. The surprising anabolic effect of inserting a brief zero load interval between each load cycle was subsequently confirmed by our group and others [36–39]. At the tissue level, we have found that low magnitude rest-inserted loading is even capable of stimulating periosteal bone formation in senescent animals [40]. Given that rest-inserted loading is so stimulatory for surface osteoblast activity, we hypothesized that rest-inserted loading may provide a means of overcoming a genetic predisposition toward decreased mechanoresponsiveness in C3H mice. To test this hypothesis, we performed a series of in vivo experiments in which we exogenously loaded the tibiae of C3H and C57 mice.
First, we assessed the response of 16 wk C3H mice (an age comparable to previous studies) to rest-inserted loading of a magnitude that is sufficient to induce prolific periosteal bone formation in 16 wk C57 mice. While the regimen did not alter periosteal bone formation rates compared to contralateral non-loaded tibiae, we noted high levels of basal periosteal osteoblast activity in the C3H mice. As it is difficult to imagine how an osteogenic mechanical loading regimen would further augment an already extremely high level of periosteal osteoblast activity (e.g., 55% periosteal mineralizing surface (Ps.MS, [41]) in 16 wk C3H mice [17]), we speculated that if C3H mice were aged until basal periosteal osteoblast activity was equivalently low to that found in 16 wk C57 mice, then the skeletal response to anabolic mechanical loading would be restored. To test this hypothesis, we eliminated the potentially confounding influence of high basal periosteal activity in the experiments by using C3H mice at an age (32 wk) where their basal periosteal bone formation was equivalent to that of 16 wk C57 mice. We first assessed whether low magnitude rest-inserted loading would elevate periosteal bone formation in C3H and C57 mice. We then examined whether 32 wk C3H mice would respond to two different magnitudes of repetitive cyclic loading to assess whether the observed response was due entirely to rest-inserted loading.
Materials and Methods
Age-Matched Study
In this study, 16 wk C3H mice (n = 5) underwent 50 cycles of loading with a 10 s rest interval inserted between each load cycle. Based upon a previous study reporting cross-sectional areas of 16 wk C3H mice [42], we estimated a priori a loading regimen that would induce peak periosteal normal strains of 2200με. This magnitude of waveform was 40% greater than a rest-inserted waveform that induced significant periosteal bone formation rate (Ps.BFR) in 16 wk C57 mice [43]. Post-hoc animal specific estimates of induced strains were determined using beam theory (as described in the section on calibration).
Because we noted a high level of baseline osteoblastic activity in 16 wk C3H mice during this study, we evaluated basal osteoblast activity as C3H mice aged in order to equilibrate it to the baseline activity of 16 wk C57 mice. A C3H mouse was sacrificed every 2 wk following 16 wk of age and dynamic histomorphometry was used to determine Ps.BFR/BS at the tibia mid-diaphysis. We found that Ps.BFR/BS in C3H mice at 32 wk of age was equivalent to within 5% of that observed in 16 wk C57 mice in our previous studies [43]. C3H mice at 32 wk of age were therefore used in the following studies to examine if the C3H strain was capable of responding to low and moderate magnitude mechanical stimuli without the confounding influence of high basal surface osteoblast activity.
Rest-Inserted Study
The Rest-Inserted Study contrasted the response of 32 wk old C3H mice and 16 wk old C57 mice to rest-inserted loading protocols that induced equivalent peak periosteal normal strains. Groups of 32 wk old C3H mice (n = 8) and 16 wk C57 mice (n = 8) were exposed to 50 cycles/d of low magnitude loading with a 10 s unloaded rest interval inserted between each load cycle. Applied end loads were established a priori from calibration experiments such that peak periosteal normal strains of 1200 με would be equivalently induced across groups.
Cyclic Study
We next explored whether two strain magnitudes of repetitive cyclic loading (1 Hz, no rest-intervals) would stimulate bone formation in 32 wk old C3H mice. The loading protocols applied 50 cycles/d using end loads that, based on preliminary calibrations, were anticipated to induce peak periosteal normal strains of either 1200 με or 1900 με (termed Low and Moderate magnitude, respectively).
In Vivo Mechanical Loading
We mechanically loaded the right tibiae of female mice with a non-invasive device that applies cantilever bending to the tibia [44]. For each bout of loading, the mouse was anesthetized (2% isoflurane) and, proximal to the tibial crest, the right tibia was gripped between a brass block on the lateral side and a brass gripping cup on the medial side. An aluminum loading tine attached to a computer controlled linear actuator applied force to the lateral distal tibia, placing the tibia in cantilever bending. Digital signals controlled the strain rate (0.01/s), applied force and a 1 s load cycle (1-Hz trapezoidal waveform with dwell times at peak load decreased for increased magnitude loading such that strain rate was equivalent across protocols). Normal cage activity was allowed between loading sessions. Loading was applied 3 d/wk beginning on day 1, with contralateral tibiae serving as non-externally loaded controls. Calcein (15 mg/kg IP) was administered on days 10 and 19 and all mice were sacrificed on day 22. Experimental procedures were approved by the University of Washington Institutional Animal Care and Use Committee (IACUC).
Calibration of Induced Normal Strains
The strain environment induced by our murine tibia loading device is non-uniform due to the irregular geometry of the tibia and the applied cantilever bending [44]. In this study, a further complication arose in that the cross-sectional tibia morphology of C3H and C57 mice differ substantially. As a result, it was not possible to simultaneously induce equivalent endocortical and periosteal normal strains in C3H and C57 mice with the same loading protocol. Given the primary importance of periosteal osteoblast function as a means of increasing bone’s mechanical properties, we focused on inducing equivalent periosteal normal strains between C3H and C57 mice. A combination of in situ strain gage application and finite element modeling was used to quantify peak periosteal and endocortical normal strains at the tibia mid-diaphysis. One 16 wk C57 mouse and one 32 wk C3H mouse were utilized for calibration of induced strains. Immediately following sacrifice, two uniaxial strain gages (FLK-1–11, Texas Measurements, Inc.) were attached to the medial and lateral surfaces of the right tibia diaphysis. The calibration mouse was positioned within the loading device and the right tibia was loaded for five sequential load cycles over a range of loading magnitudes (0.15 to 0.5 N, random order). Five trials were performed at each load magnitude (with mice removed from the loading device between each trial). Strain gage signals were amplified using a 2120A amplifier (Vishay Measurement Groups, Inc.), with induced strains recorded using DAPView (Microstar Laboratories, Inc.).
Each calibration tibia with attached fibula was dissected and subjected to high resolution (18 μm per voxel) microCT scanning (μCT 20, Scanco Medical AG, Bassersdorf Switzerland). Serial scans of the entire tibia-fibula were used to create a finite element (FE) model using custom multi-module automated software (PV Wave, Visual Numerics Inc.), which was then imported into Patran (MSC Software Corporation) to create an isotropic finite element model of the mouse tibia. Using previously published bone material properties for C57 and C3H mice [45, 46], the boundary conditions produced in the mouse tibia loading device were simulated in the FE model and tibia strains for the tibia-fibula model were calculated. Over the range of loads considered, the induced strains exhibited a linear relation to applied loads. At the strain gages sites, the mean (± SE) FE predictions were within 7.3% of strain gage data at the strain gage sites. The load-strain calibration curve was used to determine load levels required to induce protocol-specific strains in the in vivo experiment.
Post-experimental Determination of Animal Specific Peak Strains
Animal specific peak normal strains were calculated for all experimentally loaded tibiae using beam theory. Using strain distributions from the validated FE model, we applied beam theory to calculate force and moment boundary conditions for tibia mid-diaphysis loading [43]. Following sacrifice, the boundary conditions were applied to the morphology of the mid-diaphysis section of the mirrored contralateral left tibia (as used in dynamic histomorphometry, described below) to determine the strain distribution induced at the initiation of the loading experiments for each mouse utilized in the studies.
Dynamic Histomorphometry
Post sacrifice, the experimental (right) and contralateral (left) bones were dissected and sectioned at mid-diaphysis. Thick sections (200 μm) were removed at identical sites 2 mm proximal to the tibia-fibula junction, mounted on plastic slides, ground to 90 μm and digitally imaged with an epi-fluorescent microscope at 200× (Eclipse E600, Nikon). Images were analyzed to obtain measures of single label surface (s.Ls), double label surface (d.Ls) and interlabel thickness (Ir.L.Th). Standard histomorphometric measures of endocortical and periosteal mineralizing surface (Ec.MS/BS, Ps.MS/BS, with BS = bone surface), endocortical and periosteal mineral apposition rate (Ec.MAR, Ps.MAR) and endocortical and periosteal surface referent bone formation rate (Ec.BFR/BS, Ps.BFR/BS) were then calculated for both the periosteal and endocortical surfaces [41].
Statistical Analysis
Statistical analyses were performed separately for each of the studies with the design based on the complexity of the particular study. All data are reported as mean ± SE, with p ≤ 0.05 considered to be statistically significant for all comparisons.
In the Age-Matched Study, a paired t-test was utilized to determine if rest-inserted loading significantly enhanced MAR, MS/BS and BFR/BS in experimental vs. contralateral bones at the endocortical and periosteal surfaces.
For the Rest-Inserted Study, a 2 × 1 factorial MANOVA was used to examine if response variables (i.e., MAR, MS/BS, BFR/BS) at the endocortical and periosteal surfaces in contralateral controls or in experimentally loaded bones attained significance in the different mouse strains (i.e., factors C57, C3H). If the MANOVA test (Pillai’s Trace) attained significance, then between subject significance for any given response measure was considered only if the regression between the residuals of the response measure in experimental vs. contralateral bones were not significant and positive. This caveat ensured that our examination of between subject effects was restricted to those measures where loading induced activity was not confounded by baseline activity at the tibia mid-diaphysis. Finally, paired t-tests were performed to examine if loading significantly enhanced bone response measures at the endocortical and periosteal surfaces compared to that of contralateral bones. The statistical analysis for the Cyclic Study was identical to that of the Rest-Inserted Study, with the exception that the main effect factor considered was the magnitude of applied cyclic loading rather than the strain of mouse.
Results
Peak Normal Strains Induced by the Loading Regimens
Mean (± SE) peak endocortical and periosteal normal strain magnitudes were consistent within each experimental group (Table 1). The average coefficient of variation of the induced peak strain across all groups was 7.7%. For the Rest-Inserted Study, the peak periosteal strain induced in the 32 wk C3H and 16 wk C57 mice was nearly identical (1.9% difference, p = 0.26). However, despite equivalent end loads the mean peak strains induced in the Cyclic Study Low magnitude group were significantly less than those induced in the Rest-Inserted Study C3H group (−12.1%; p = 0.004), making the planned comparison between groups not statistically valid.
Table I.
Mouse strain, age, end loads and animal specific peak normal strain magnitude (mean ± SE) for each loading study.
| Study | Mouse | Age (wk) | n | Force (N) | Strain (με), periosteal | Strain (με), endocortical |
|---|---|---|---|---|---|---|
| Age Match | C3H | 16 | 5 | 0.465 | 2210 ± 67 | 1180 ± 40 |
| Rest | C3H | 32 | 8 | 0.27 | 1235 ± 30 | 690 ± 18 |
| Rest | C57 | 16 | 8 | 0.3 | 1210 ± 20 | 885 ± 23 |
| Cyclic | C3H | 32 | 8 | 0.27 | 1085 ± 31* | 625 ± 16 |
| Cyclic | C3H | 32 | 8 | 0.465 | 1875 ± 65 | 1070 ± 41 |
p < 0.01 vs. Rest, C3H
Age-Matched Study
At the endocortical surface in 16 wk C3H mice, Ec.MS/BS, Ec.MAR and Ec.BFR/BS in loaded tibiae were not significantly altered compared to contralateral tibiae (Fig. 1). At the periosteal surface, the high level of Ps.MS/BS observed in the non-loaded contralateral tibiae (52.2% ± 2.8) was not altered by rest-inserted loading (49.2% ± 2.4, p = 0.52, Fig. 1). Similarly, neither Ps.MAR (+11.0% vs. contralaterals, p = 0.11) or Ps.BFR/BS (+5.0% vs. contralaterals, p = 0.64) were significantly altered by the loading regimen.
Figure 1.
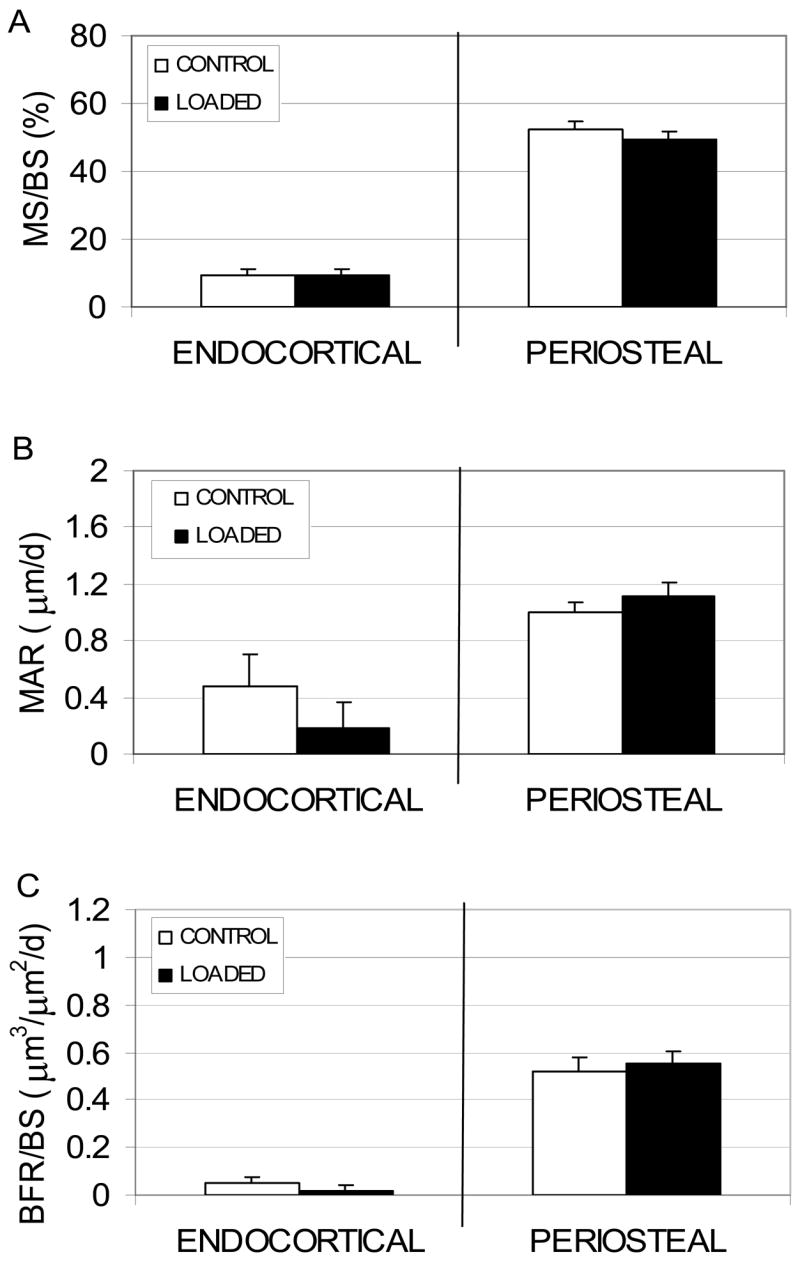
Endocortical and periosteal (mean + SE) MS/BS (A), MAR (B) and BFR/BS (C) induced by rest-inserted mechanical loading in 16 wk C3H mice (2210 με peak periosteal normal strain). Consistent with previous studies, none of the response measures were significantly elevated by a loading protocol previously found to be highly osteogenic in C57 mice.
Rest-Inserted Study
Endocortical and periosteal measures of osteoblast activity in the contralateral non-loaded tibiae were equivalent between the groups (MS/BS, Fig. 2). Low magnitude rest-inserted loading did not significantly alter endocortical osteoblast activity in either C57 or C3H mice. At the periosteal surface, rest-inserted loading induced significantly higher Ps.MS/BS in both the C3H (125.6% vs. contralateral, p < 0.001) and C57 (112.3% vs. contralateral, p = 0.003). Likewise, Ps.MAR was significantly elevated by the loading regimen in both C3H (103.9% vs. contralateral, p = 0.005) and C57 (83.7% vs. contralateral, p = 0.034) mice. As a result, Ps.BFR/BS was significantly elevated for C3H (310.5% vs. contralateral, p = 0.001) and C57 (187.0% vs. contralateral, p = 0.013) mice. Additionally, both Ps.MS/BS and Ps.BFR/BS in loaded bones were significantly elevated in C3H compared to C57 mice (81.4%, p < 0.001; and 113.9%, p = 0.015, respectively).
Figure 2.
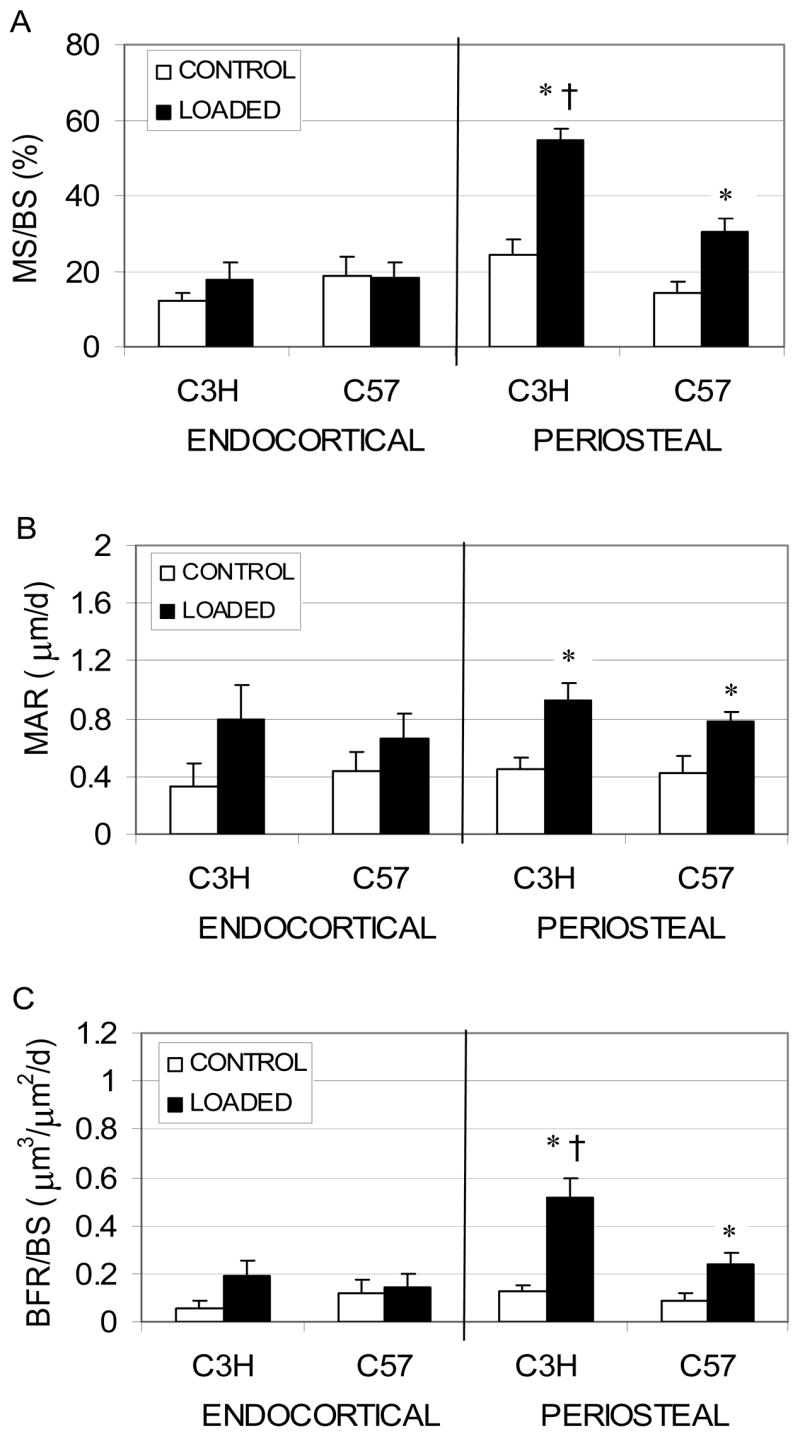
Endocortical and periosteal (mean + SE) MS/BS (A), MAR (B) and BFR/BS (C) induced by rest-inserted loading in 16 wk C57 and 32 wk C3H mice. Peak periosteal normal strains were equivalent between groups. Response measures attaining significance are noted (*: p < 0.05, Loaded tibiae vs. non-loaded contralateral tibiae; †: p < 0.05, C3H vs. C57)
Cyclic Study
No differences were observed in measures of endocortical and periosteal MS/BS, MAR, and BFR/BS in the contralateral non-loaded tibiae between the two groups. At the endocortical surface, Ec.MS/BS following Low magnitude loading was significantly increased compared to contralateral tibiae (111.6%, p = 0.009, Fig. 3). In contrast, Ec.MS/BS (258.6%, p = 0.002), Ec.MAR (422.0%, p < 0.001), and Ec.BFR/BS (1483.2%, p = 0.001) were all significantly increased compared to contralateral tibiae following Moderate magnitude cyclic loading. All endocortical bone formation measures (Ec.MS/BS, Ec.MAR, and Ec.BFR/BS) in the Moderate group significantly exceeded those of the Low group (all p ≤ 0.004).
Figure 3.
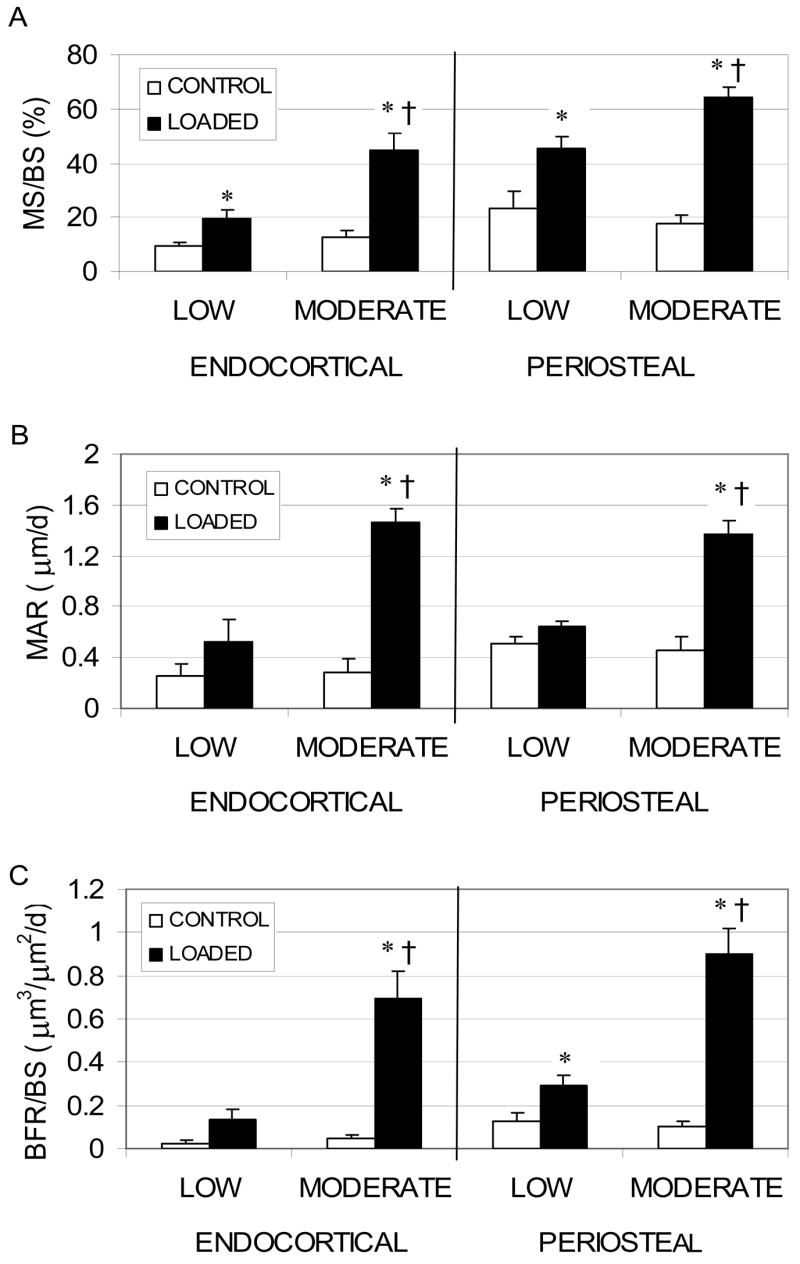
Endocortical and periosteal (mean ± SE) MS/BS (A), MAR (B) and BFR/BS (C) induced by cyclic mechanical loading of 32 wk C3H mice at peak periosteal strains of 1085 με (Low) and 1875 με (Moderate). Response measures attaining significance are noted (*: p < 0.05, Loaded tibiae vs. non-loaded contralateral tibiae; †: p < 0.05, C3H vs. C57)
At the periosteal surface, Ps.MS/BS (92.4%, p < 0.001) and Ps.BFR/BS (134.4%, p < 0.001) were elevated in the Low magnitude loading group compared to their contralateral tibiae. In the Moderate group, Ps.MS/BS (267.4%, p < 0.001), Ps.MAR (199.4%, p < 0.001), and Ps.BFR/BS (798.3%, p < 0.001) were all significantly elevated compared to contralateral tibiae. The Ps.MS/BS, Ps.MAR, and Ps.BFR/BS induced by Moderate magnitude loading significantly exceeded that induced by the Low group (42.7%, p = 0.008; 111.8%, p < 0.001; 205.3%, p = 0.001, respectively).
Discussion
We assessed the ability of low and moderate magnitude rest-inserted and cyclic loading to stimulate new bone formation in C3H mice. As has been previously reported in the literature for cyclic loading [17–19, 25], our initial study also indicated that 16 wk C3H mice did not respond to a rest-inserted loading protocol that had previously been found to be highly osteogenic in C57 mice [43]. However, at 32 wk of age, when C3H mice demonstrated baseline Ps.BFR/BS within 5% of that of 16 wk old C57 mice (a common age for use in mechanotransduction studies), a rest-inserted loading regimen of much lower amplitude (45% less than the initial study) significantly elevated Ps.BFR/BS in C3H mice. Further, the Ps.BFR/BS induced in 32 wk C3H mice was significantly enhanced compared to that in 16 wk C57 mice. We then used two different magnitudes of cyclic loading to demonstrate that 32 wk C3H mice respond not only to rest-inserted loading, but differentially to increasing magnitudes of cyclic loading. These data therefore directly challenge the current consensus that C3H mice demonstrate a reduced ability to respond to mechanical loading.
Prior to loading mice tibiae, we used a combined finite element and beam theory approach to identify end loads that would induce equivalent peak normal strains across the strains of mice. One clear limitation with this approach is evident. Given that the tibiae of the C3H and C57 mice clearly differ in cross-sectional morphology (the C3H cortex is thicker, the C57 endocortical and periosteal areas are both greater), it is not possible to simultaneously match endocortical and periosteal peak normal strains. We chose to match periosteal normal strains across groups. As a result, endocortical strains in the C57 mice exceeded those of the C3H mice by 22% in the Rest-Inserted Study. However, if a bias resulted from this imbalance, it would favor the C57 mice as the mean peak normal strain between the endocortical and periosteal surfaces in C57 exceeded that of the C3H mice. Instead, we found that the response observed in loaded tibiae from the C3H mice actually exceeded those observed in the C57 mice in the Rest-Inserted Study.
The two studies examining bone formation in 32 wk C3H mice sought to use C3H mice at a developmental stage where surface baseline bone formation was comparably quiescent to that observed in 16 wk C57 mice. A single study that explored the response of 36 wk C57 and C3H mice to mechanical loading used measures of total area, periosteal circumference and total density to evaluate response to loading [19]. While total density did not change in either C57 or C3H mice, increases in the total area and periosteal circumference were greater in C57 mice. However, it is difficult to directly contrast that study with ours for several reasons. First, four point bending was used to load the tibia, but this device is known to confound cellular responses at the periosteal surface [47, 48]. Second, the induced peak strains greatly exceed those induced in our study (> 200%). Finally, the authors noted substantial woven bone formation that would inflate static morphology assessments (all surface responses were lamellar in our studies). To our knowledge, all other previous mechanical loading studies using C3H mice have been performed when the mice were 20 wk or younger [17, 18, 20, 24, 25, 49].
One of the first studies in which a long bone of a C3H mouse was mechanically loaded did note that the observed lack of responsiveness may have arisen as a result of high levels of ambient periosteal osteoblast activity in 16 wk C3H mice [17]. Based on this observation and that of our Age-Matched Study, we hypothesized that: 1) the high basal level of periosteal osteoblast activity in 16 wk C3H mice confounded bone’s response to an otherwise osteogenic loading protocol; or 2) C3H mice are substantially deficient in their ability to sense and respond to mechanical loading (including rest-inserted loading), as has been proposed. The two subsequent studies were designed to clarify these possibilities by eliminating the potential confounding influence of basal surface osteoblast activity.
Data from the Rest-Inserted Study clearly supported the first supposition. In higher order mammals and large birds, the periosteal surface of long bones is primarily quiescent once growth plates fuse [50, 51]. While mice do not demonstrate closed long bone growth plates, the change in bone mineral density (BMD) across their lifespan generally mimics that of humans. Mice reach peak BMD as young adults (4 to 8 mo), maintain BMD through middle age (12 to 13 mo) and demonstrate declining BMD in old age (18 to 24 mo) [52–55]. The age required to achieve periosteal surface quiescence as young adults varies substantially across strains of mice, given continued growth through life, and differs depending on the site within the same skeleton [56]. C57 mice demonstrate a predominantly quiescent tibial periosteum by 16 wk of age, and 36 wk old C57 mice have been found to be less responsive to mechanical loading than 16 wk C57 mice [19]. C3H mice did not reach an equivalently low level of Ps.BFR/BS until 32 wk of age. Surprisingly, we found that 32 wk old C3H mice were highly responsive to rest-inserted loading, even though this regimen was nearly 50% lower in magnitude than was found to be ineffective in 16 wk old C3H mice. Further, the Ps.BFR/BS induced in 32 wk C3H mice by this rest-inserted regimen exceeded that induced in 16 wk C57 mice by 113.9% (the data in the C57 mice were similar to previous data from our group [43]). This study also demonstrates that optimal timing for the application of mechanical loading to enhance bone formation can vary with the genetic makeup and developmental stage of the individual animal.
We also assessed whether eliminating the confounding influence of basal Ps.BFR/BS would also enable C3H mice to respond to repetitive cyclic loading. Previous studies found that cyclic loading inducing peak strain magnitudes less than 2000 με did not alter periosteal bone formation in 16 to 20 wk old C3H mice compared contralateral non-loaded bones [18, 24]. The minimal response observed from our Low cyclic group (average peak strain: 1085με) was consistent with studies suggesting that the minimal effective strain required to induce significant Ps.BFR/BS via 1 Hz cyclic mechanical loading is less than or near 1000 με [57–59]. However, we then found that the Moderate cyclic loading group with peak normal strains less than 2000 με demonstrated significantly enhanced endocortical and periosteal Ps.BFR/BS. Although the comparison was initially intended, we did not directly contrast the effect of rest-inserted loading vs. cyclic loading in 32 wk C3H mice since post experimental peak strain determination indicated that induced strains were higher for rest-inserted loading. This confounding variable arose due to issues with randomization sometimes arising in studies with the group size used here (as applied end loads were equivalent).
We found that once the C3H mice reached an age where the confounding influence of basal periosteal osteoblast activity was eliminated, C3H mice differentially responded to mechanical loading protocols that were anticipated to possess different levels of osteogenic potential. Our results therefore clearly indicate that a high level of basal periosteal osteoblast activity confounds the ability of bone to respond to an otherwise anabolic mechanical loading stimulus. The literature indicates that osteoblasts from young C3H mice do not proliferate as rapidly as C57 cells, and C3H cells have a lower rate of apoptosis than C57 osteoblasts [33, 34]. Differing developmental time courses and cell activity may account for a high level of basal osteoblast activity in C3H mice compared to C57 mice, while the presence or absence of systemic factors such as IGF-1 may also influence this physiology [32]. While we have observed variations in basal osteoblast activity between C3H and C57 mice, the lack of mechanoresponsiveness in C3H mice may also be complicated by signaling pathways responsible for negatively regulating mechanically induced bone formation in younger C3H mice, or hormone-induced inhibitors of osteoblast excitation that may diminish in potency with age. It may therefore prove interesting to explore whether such pathways or systemic factors are present in other strains of mice, but are inactivated at much younger ages.
In conclusion, we have found that 32 wk C3H mice are responsive to mechanical loading and, surprisingly, they respond more prolifically than 16 wk C57 mice to low magnitude rest-inserted loading. Further, we found that 32 wk C3H mice are also responsive to increases in cyclic loading magnitude. C3H mice are therefore responsive to mechanical loading when the confounding influence of a highly active periosteum is eliminated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eisman JA, Sambrook PN, Kelly PJ, Pocock NA. Exercise and its interaction with genetic influences in the determination of bone mineral density. Am J Med. 1991;91(5B):5S–9S. doi: 10.1016/0002-9343(91)90239-t. [DOI] [PubMed] [Google Scholar]
- 2.Krall EA, Dawson-Hughes B. Heritable and life-style determinants of bone mineral density. J Bone Miner Res. 1993;8(1):1–9. doi: 10.1002/jbmr.5650080102. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TV, Howard GM, Kelly PJ, Eisman JA. Bone mass, lean mass, and fat mass: same genes or same environments? Am J Epidemiol. 1998;147(1):3–16. doi: 10.1093/oxfordjournals.aje.a009362. [DOI] [PubMed] [Google Scholar]
- 4.Cao X, Chen D. The BMP signaling and in vivo bone formation. Gene. 2005;357(1):1–8. doi: 10.1016/j.gene.2005.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itoh S, Udagawa N, Takahashi N, Yoshitake F, Narita H, Ebisu S, Ishihara K. A critical role for interleukin-6 family-mediated Stat3 activation in osteoblast differentiation and bone formation. Bone. 2006;39(3):505–12. doi: 10.1016/j.bone.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 6.Sims NA, Jenkins BJ, Quinn JM, Nakamura A, Glatt M, Gillespie MT, Ernst M, Martin TJ. Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways. J Clin Invest. 2004;113(3):379–89. doi: 10.1172/JCI19872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi Y, Watanabe S, Ishii G, Takeda S, Nakayama K, Fukumoto S, Kaneta Y, Inoue D, Matsumoto T, Harigaya K, Fujita T. Interleukin-11 as a stimulatory factor for bone formation prevents bone loss with advancing age in mice. J Biol Chem. 2002;277(50):49011–8. doi: 10.1074/jbc.M207804200. [DOI] [PubMed] [Google Scholar]
- 8.Fretz JA, Zella LA, Kim S, Shevde NK, Pike JW. 1,25-Dihydroxyvitamin D3 induces expression of the Wnt signaling co-regulator LRP5 via regulatory elements located significantly downstream of the gene’s transcriptional start site. J Steroid Biochem Mol Biol. 2007;103(3–5):440–5. doi: 10.1016/j.jsbmb.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakanishi R, Akiyama H, Kimura H, Otsuki B, Shimizu M, Tsuboyama T, Nakamura T. Osteoblast-Targeted Expression of Sfrp4 in Mice Results in Low Bone Mass. J Bone Miner Res. 2007 doi: 10.1359/jbmr.071007. [DOI] [PubMed] [Google Scholar]
- 10.Raum K, Hofmann T, Leguerney I, Saied A, Peyrin F, Vico L, Laugier P. Variations of microstructure, mineral density and tissue elasticity in B6/C3H mice. Bone. 2007 doi: 10.1016/j.bone.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Judex S, Garman R, Squire M, Donahue LR, Rubin C. Genetically based influences on the site-specific regulation of trabecular and cortical bone morphology. J Bone Miner Res. 2004;19(4):600–6. doi: 10.1359/JBMR.040101. [DOI] [PubMed] [Google Scholar]
- 12.Frost HM. Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275(2):1081–101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 13.Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res. 2003;18(2):352–9. doi: 10.1359/jbmr.2003.18.2.352. [DOI] [PubMed] [Google Scholar]
- 14.Daly RM. The effect of exercise on bone mass and structural geometry during growth. Med Sport Sci. 2007;51:33–49. doi: 10.1159/000103003. [DOI] [PubMed] [Google Scholar]
- 15.Modlesky CM, Lewis RD. Does exercise during growth have a long-term effect on bone health? Exerc Sport Sci Rev. 2002;30(4):171–6. doi: 10.1097/00003677-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Borer KT. Physical activity in the prevention and amelioration of osteoporosis in women: interaction of mechanical, hormonal and dietary factors. Sports Med. 005;35(9):779–830. doi: 10.2165/00007256-200535090-00004. [DOI] [PubMed] [Google Scholar]
- 17.Akhter MP, Cullen DM, Pedersen EA, Kimmel DB, Recker RR. Bone response to in vivo mechanical loading in two breeds of mice. Calcif Tissue Int. 1998;63(5):442–9. doi: 10.1007/s002239900554. [DOI] [PubMed] [Google Scholar]
- 18.Judex S, Donahue LR, Rubin C. Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. Faseb J. 2002;16(10):1280–2. doi: 10.1096/fj.01-0913fje. [DOI] [PubMed] [Google Scholar]
- 19.Kesavan C, Mohan S, Oberholtzer S, Wergedal JE, Baylink DJ. Mechanical loading-induced gene expression and BMD changes are different in two inbred mouse strains. J Appl Physiol. 2005;99(5):1951–7. doi: 10.1152/japplphysiol.00401.2005. [DOI] [PubMed] [Google Scholar]
- 20.Kodama Y, Umemura Y, Nagasawa S, Beamer WG, Donahue LR, Rosen CR, Baylink DJ, Farley JR. Exercise and mechanical loading increase periosteal bone formation and whole bone strength in C57BL/6J mice but not in C3H/Hej mice. Calcif Tissue Int. 2000;66(4):298–306. doi: 10.1007/s002230010060. [DOI] [PubMed] [Google Scholar]
- 21.Bonjour JP, Chevalley T, Rizzoli R, Ferrari S. Gene-environment interactions in the skeletal response to nutrition and exercise during growth. Med Sport Sci. 2007;51:64–80. doi: 10.1159/000103005. [DOI] [PubMed] [Google Scholar]
- 22.Takeshita T, Yamagata Z, Iijima S, Nakamura T, Ouchi Y, Orimo H, Asaka A. Genetic and environmental factors of bone mineral density indicated in Japanese twins. Gerontology. 1992;38(Suppl 1):43–9. doi: 10.1159/000213362. [DOI] [PubMed] [Google Scholar]
- 23.Robling AG, Burr DB, Turner CH. Skeletal loading in animals. J Musculoskelet Neuronal Interact. 2001;1(3):249–62. [PubMed] [Google Scholar]
- 24.Robling AG, Turner CH. Mechanotransduction in bone: genetic effects on mechanosensitivity in mice. Bone. 2002;31(5):562–9. doi: 10.1016/s8756-3282(02)00871-2. [DOI] [PubMed] [Google Scholar]
- 25.Akhter MP, Cullen DM, Recker RR. Bone adaptation response to sham and bending stimuli in mice. J Clin Densitom. 2002;5(2):207–16. doi: 10.1385/jcd:5:2:207. [DOI] [PubMed] [Google Scholar]
- 26.Robling AG, Warden SJ, Shultz KL, Beamer WG, Turner CH. Genetic effects on bone mechanotransduction in congenic mice harboring bone size and strength quantitative trait loci. J Bone Miner Res. 2007;22(7):984–91. doi: 10.1359/jbmr.070327. [DOI] [PubMed] [Google Scholar]
- 27.Kesavan C, Mohan S, Srivastava AK, Kapoor S, Wergedal JE, Yu H, Baylink DJ. Identification of genetic loci that regulate bone adaptive response to mechanical loading in C57BL/6J and C3H/HeJ mice intercross. Bone. 2006 doi: 10.1016/j.bone.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Robling AG, Li J, Shultz KL, Beamer WG, Turner CH. Evidence for a skeletal mechanosensitivity gene on mouse chromosome 4. Faseb J. 2003;17(2):324–6. doi: 10.1096/fj.02-0393fje. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava AK, Kapur S, Mohan S, Yu H, Kapur S, Wergedal J, Baylink DJ. Identification of novel genetic loci for bone size and mechanosensitivity in an ENU mutant exhibiting decreased bone size. J Bone Miner Res. 2005;20(6):1041–50. doi: 10.1359/JBMR.041239. [DOI] [PubMed] [Google Scholar]
- 30.Rosen CJ, Ackert-Bicknell C, Beamer WG, Nelson T, Adamo M, Cohen P, Bouxsein ML, Horowitz MC. Allelic differences in a quantitative trait locus affecting insulin-like growth factor-I impact skeletal acquisition and body composition. Pediatr Nephrol. 2005;20(3):255–60. doi: 10.1007/s00467-004-1612-z. [DOI] [PubMed] [Google Scholar]
- 31.Kesavan C, Baylink DJ, Kapoor S, Mohan S. Novel loci regulating bone anabolic response to loading: expression QTL analysis in C57BL/6JXC3H/HeJ mice cross. Bone. 2007;41(2):223–30. doi: 10.1016/j.bone.2007.04.185. [DOI] [PubMed] [Google Scholar]
- 32.Lau KH, Kapur S, Kesavan C, Baylink DJ. Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J Biol Chem. 2006;281(14):9576–88. doi: 10.1074/jbc.M509205200. [DOI] [PubMed] [Google Scholar]
- 33.Sheng MH, Lau KH, Beamer WG, Baylink DJ, Wergedal JE. In vivo and in vitro evidence that the high osteoblastic activity in C3H/HeJ mice compared to C57BL/6J mice is intrinsic to bone cells. Bone. 2004;35(3):711–9. doi: 10.1016/j.bone.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Sheng MH, Lau KH, Mohan S, Baylink DJ, Wergedal JE. High Osteoblastic Activity In C3H/HeJ Mice Compared to C57BL/6J Mice Is Associated with Low Apoptosis in C3H/HeJ Osteoblasts. Calcif Tissue Int. 2006;78(5):293–301. doi: 10.1007/s00223-005-0303-5. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan S, Gross TS. Intermittent rest enhances osteoblastic activation induced by mechanical loading. Trans Orthop Res Soc. 2000;25:628. [Google Scholar]
- 36.Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low–magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res. 2002;17(9):1613–20. doi: 10.1359/jbmr.2002.17.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robling AG, Burr DB, Turner CH. Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol. 2001;204(Pt 19):3389–99. doi: 10.1242/jeb.204.19.3389. [DOI] [PubMed] [Google Scholar]
- 38.Umemura Y, Sogo N, Honda A. Effects of intervals between jumps or bouts on osteogenic response to loading. J Appl Physiol. 2002;93(4):1345–8. doi: 10.1152/japplphysiol.00358.2002. [DOI] [PubMed] [Google Scholar]
- 39.LaMothe JM, Zernicke RF. Rest insertion combined with high-frequency loading enhances osteogenesis. J Appl Physiol. 2004;96(5):1788–93. doi: 10.1152/japplphysiol.01145.2003. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan S, Agans SC, King KA, Moy NY, Poliachik SL, Gross TS. Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. Bone. 2003;33(6):946–55. doi: 10.1016/j.bone.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 42.Akhter MP, Iwaniec UT, Covey MA, Cullen DM, Kimmel DB, Recker RR. Genetic variations in bone density, histomorphometry, and strength in mice. Calcif Tissue Int. 2000;67(4):337–44. doi: 10.1007/s002230001144. [DOI] [PubMed] [Google Scholar]
- 43.Srinivasan S, Ausk BJ, Poliachik SL, Warner SE, Richardson TS, Gross TS. Rest-Inserted Loading Rapidly Amplifies the Response of Bone to Small Increases in Strain and Load Cycles. J Appl Physiol. 2007 doi: 10.1152/japplphysiol.00507.2006. [DOI] [PubMed] [Google Scholar]
- 44.Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res. 2002;17(3):493–501. doi: 10.1359/jbmr.2002.17.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akhter MP, Fan Z, Rho JY. Bone intrinsic material properties in three inbred mouse strains. Calcif Tissue Int. 2004;75(5):416–20. doi: 10.1007/s00223-004-0241-7. [DOI] [PubMed] [Google Scholar]
- 46.Brodt MD, Ellis CB, Silva MJ. Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res. 1999;14(12):2159–66. doi: 10.1359/jbmr.1999.14.12.2159. [DOI] [PubMed] [Google Scholar]
- 47.Forwood MR, Bennett MB, Blowers AR, Nadorfi RL. Modification of the in vivo four-point loading model for studying mechanically induced bone adaptation. Bone. 1998;23(3):307–10. doi: 10.1016/s8756-3282(98)00090-8. [DOI] [PubMed] [Google Scholar]
- 48.Torrance AG, Mosley JR, Suswillo RF, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periostal pressure. Calcif Tissue Int. 1994;54(3):241–7. doi: 10.1007/BF00301686. [DOI] [PubMed] [Google Scholar]
- 49.Pedersen EA, Akhter MP, Cullen DM, Kimmel DB, Recker RR. Bone response to in vivo mechanical loading in C3H/HeJ mice. Calcif Tissue Int. 1999;65(1):41–6. doi: 10.1007/s002239900655. [DOI] [PubMed] [Google Scholar]
- 50.Skedros JG, Hunt KJ, Hughes PE, Winet H. Ontogenetic and regional morphologic variations in the turkey ulna diaphysis: implications for functional adaptation of cortical bone. Anat Rec A Discov Mol Cell Evol Biol. 2003;273(1):609–29. doi: 10.1002/ar.a.10073. [DOI] [PubMed] [Google Scholar]
- 51.Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35(5):1003–12. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 52.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18(5):397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 53.Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res. 2002;17(6):1044–50. doi: 10.1359/jbmr.2002.17.6.1044. [DOI] [PubMed] [Google Scholar]
- 54.Bar-Shira-Maymon B, Coleman R, Cohen A, Steinhagen-Thiessen E, Silbermann M. Age-related bone loss in lumbar vertebrae of CW-1 female mice: a histomorphometric study. Calcif Tissue Int. 1989;44(1):36–45. doi: 10.1007/BF02556238. [DOI] [PubMed] [Google Scholar]
- 55.Geusens P, Dequeker J, Verstraeten A, Nijs J. Age-, sex-, and menopause-related changes of vertebral and peripheral bone: population study using dual and single photon absorptiometry and radiogrammetry. J Nucl Med. 1986;27(10):1540–9. [PubMed] [Google Scholar]
- 56.Sheng MH, Baylink DJ, Beamer WG, Donahue LR, Rosen CJ, Lau KH, Wergedal JE. Histomorphometric studies show that bone formation and bone mineral apposition rates are greater in C3H/HeJ (high-density) than C57BL/6J (low-density) mice during growth. Bone. 1999;25(4):421–9. doi: 10.1016/s8756-3282(99)00184-2. [DOI] [PubMed] [Google Scholar]
- 57.Frost HM. A determinant of bone architecture. The minimum effective strain. Clin Orthop Relat Res. 1983;(175):286–92. [PubMed] [Google Scholar]
- 58.Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37(4):411–7. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- 59.Turner CH, Forwood MR, Rho JY, Yoshikawa T. Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res. 1994;9(1):87–97. doi: 10.1002/jbmr.5650090113. [DOI] [PubMed] [Google Scholar]


