Abstract
Background
Pulmonary involvement in leptospirosis remains poorly recognized in regions where it is endemic, despite reports of recent outbreaks and epidemic disease.
Methods
A prospective, population-based study was carried out to identify febrile patients exposed to Leptospira in urban and rural contexts in Iquitos, Peru. Evidence of exposure to Leptospira was obtained by serologic testing, and diagnosis of leptospirosis was confirmed in pulmonary cases by culture or quantitative real-time PCR assay.
Results
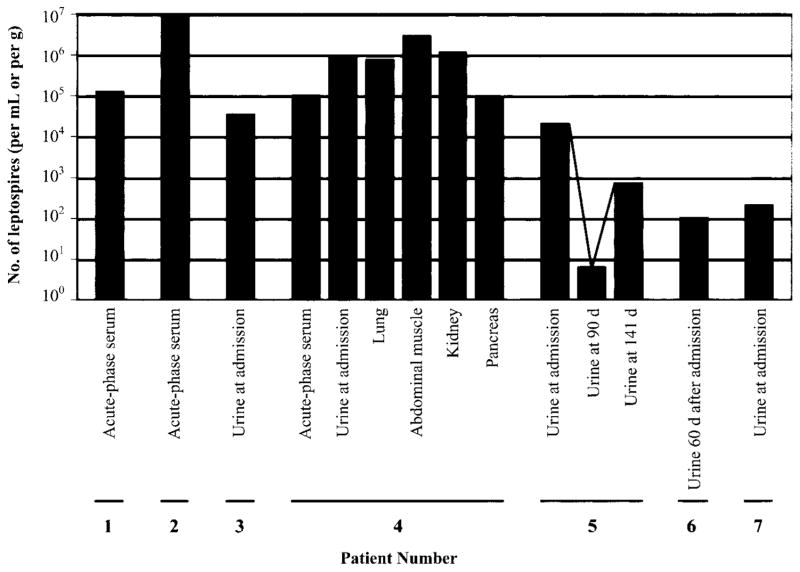
Of 633 consecutively enrolled febrile patients, 321 (50.7%) had antileptospiral IgM antibodies or high titers of antileptospiral antibodies. Seven patients with histories of only urban exposure to leptospires had severe pulmonary manifestations; of these, 5 patients died; 4 of the deaths were caused by pulmonary hemorrhage, and 1 was caused by acute respiratory distress syndrome and multiorgan failure. Real-time, quantitative PCR assay showed high levels of leptospiremia (≥104 leptospires/mL) in most fatal cases; 1 patient, from whom tissue specimens were obtained at autopsy, had ≥105 leptospires/g of lung, kidney, and muscle tissue.
Discussion
This study demonstrates the underdiagnosis of leptospirosis in a region of high endemicity and the underrecognition of grave pulmonary complications. Pulmonary involvement in leptospirosis was present in urban but not rural areas. Presumptive treatment for leptospirosis should be initiated immediately in the appropriate epidemiological and clinical context.
Over the past decade, pulmonary hemorrhage has been increasingly recognized throughout the world as a grave manifestation of leptospirosis [1–8]. Physicians in regions where the disease is endemic often fail to recognize the association of pulmonary involvement with leptospirosis. Available diagnostic methods, such as microscopic agglutination testing (MAT) and culture, are insufficiently sensitive, specific, or timely to be of much practical use. Clinicians typically consider leptospirosis in the differential diagnosis of an acute undifferentiated fever, but only when patients present with classic textbook manifestations, such as the triad of fever, jaundice, and renal failure known as Weil disease. Moreover, when patients present with less common forms of leptospirosis [9], as occurred in the epidemic of leptospirosis resulting in pulmonary hemorrhage that occurred in Nicaragua in 1995, the diagnosis is frequently either not considered or only discovered at autopsy [10].
This report demonstrates the underdiagnosis of leptospirosis and the underrecognition of severe pulmonary involvement with this disease in a region of high endemicity. Pulmonary leptospirosis was present in subjects living in urban, not rural, areas. A quantitative real-time PCR assay provided rapid diagnosis of leptospirosis in severe cases. These cases of pulmonary involvement in leptospirosis, the first to be reported from Peru, again point out the high importance of this syndrome for public health. Appropriate surveillance and control measures need to be established in this and similar regions.
METHODS
Study sites
The city of Iquitos, which has a population of ~400,000 and a surrounding rural population of ~474,000, is a major tourist and shipping center situated 120 m above sea level (73°W, 3°S) at the juncture of the Ucayali and Marañon rivers, which forms the Amazon River proper. The urban arm of the study was conducted in the Hospital de Apoyo Iquitos, a 200-bed regional Peruvian Ministry of Health hospital serving the main city of Iquitos, with an outpatient fever clinic that operates in the context of the local malaria control program. We also studied patients in 3 neighboring rural villages, including Zungarococha (8 km from the city), Varillal (14 km), and Moralillo (16 km). All 3 villages were accessible via the paved Iquitos-Nauta road [11].
Enrollment criteria
Outpatients who were ≥6 years of age with a ≤2-week history of fever were enrolled at the out-patient fever clinic at the Hospital de Apoyo Iquitos. Patients were excluded if results from malaria blood-smear results were positive. A systematic, prospective effort by the study team evaluated all inpatients at the Hospital de Apoyo Iquitos for those with fever and jaundice and requested permission from admitting physicians before such patients were enrolled in the study.
Case definition
All patients had laboratory evidence of recent leptospiral exposure, which was detected using a combination of culture of blood and urine specimens, serologic testing with IgM ELISA, MAT, and/or molecular evidence of the presence of leptospiral DNA in clinical specimens (see below). MAT evidence for seropositivity was defined as ≥1 of the following: seroconversion from negative to positive; 4-fold rise in titer between acute-phase and convalescent-phase samples; or a single titer of ≥800 [12].
Protection of human subjects
This study was approved by the Ethical Committees of the Universidad Peruana Caye-tano Heredia and the Asociación Benéfica PRISMA, Lima; the Hospital de Apoyo Iquitos, Iquitos; and the Directorate of Health (DISA), Loreto, Peru; and the Institutional Review Board of the University of California San Diego, La Jolla. All patients were clinically managed by local Peruvian physicians according to regional standards. As soon as patients were identified by the study team as having possible severe leptospirosis, treating physicians were advised to administer appropriate antibiotics (parenteral ceftriaxone or penicillin) and supportive treatment.
Serologic testing
Venous blood samples were drawn into vacutainer tubes (Becton-Dickinson) without anticoagulant and transported to the study laboratory within 4 h at ambient temperature. Serum was separated, frozen in 1 mL aliquots at −20°C, and transported on dry ice to the National Leptospirosis Reference Laboratory at the Instituto Nacional de Salud in Lima, where the presence of antileptospiral antibodies was determined. An ELISA incorporating 6 pathogenic serovars (strains)—Icterohaemorrhagiae (RGA), Australis (Ballico), Bratislava (Jez Bratislava), Ballum (MUS127), Canicola (Hond Utretch IV), Cynopteri (3522 C), and Grippotyphosa (Moskva V)—was used to detect antileptospiral IgM antibodies [13]. MAT was performed using 26 leptospiral antigens, using the Centers for Disease Control and Prevention panel [14] plus Leptospira biflexa serovar Patoc (Patoc I) and a newly identified, antigenically distinct strain of Leptospira obtained from a febrile subject in Iquitos (provisionally designated Leptospira sp. strain Var10, with further taxonomic classification pending). MAT titers are reported as the reciprocal of the number of dilutions still agglutinating 50% of live bacterial antigen.
Leptospira culture of clinical specimens
Blood from each patient was inoculated at the bedside into semisolid PLM-5 media (Serologicals Corporation) supplemented with 200 cg/mL of 5-fluorouracil and 2 μg/mL neomycin. Urine samples were adjusted to pH 7.0 with 1% sodium hydroxide at the time of collection, then inoculated into culture media. Cultures were transported to the study laboratory at ambient temperature within 4 h after being initiated, incubated at 28°C–30°C, examined weekly by dark-field microscopy, and discarded at 12 weeks if results were negative. Isolates were identified using MAT for serogrouping; identification at the serovar level was not performed. Commercially available rabbit polyclonal antisera (National Veterinary Services Laboratory) were used to serotype isolates. PFGE and 16S ribosomal gene sequencing were performed according to standard procedures [15, 16]; the Basic Local Alignment Search Tool and Clustal W analysis version 1.4 (DNASTAR) were used to determine relationships between 16S gene sequences in leptospiral isolates, to reference Leptospira species sequences in GenBank, and to construct phylogenetic trees for identification purposes. Serotyping was performed at University of California San Diego; 16S gene sequencing and PFGE analysis were also done at University of California San Diego in collaboration and consultation with the Leptospirosis Reference Laboratory at the Centers for Disease Control and Prevention in Atlanta. PFGE profiles were obtained for the leptospiral serovars used in the MAT panel and for other leptospiral isolates (serogroup Icterohaemorrhagiae, Grippotyphosa, Canicola, Var10) from the Iquitos region as a reference database, against which we compared the PFGE patterns of the isolates obtained in this study. The Phoretix 1D suite of software (Nonlinear Dynamics) was used to analyze and quantitatively compare the PFGE patterns and to create phylogenetic trees for classification.
Molecular diagnosis of leptospirosis
Real-time PCR assay was performed to confirm the diagnosis of leptospirosis only for patients with pulmonary manifestations. DNA was extracted from either 200 μL of serum, sediment of urine centrifuged at 10,000 g, or 25 mg of tissue obtained at necropsy, using, in all cases, the Qiagen Tissue DNA extraction kit (Qiagen). The real-time PCR assay is based on TaqMan chemistry that specifically detects pathogenic Leptospira 16S ribosomal genes by means of a published method [17]; the only modification in the assay is the use of the fluorescent probe at a final concentration of 2 μm. A DNA Engine Opticon 2 thermocycler (MJ Research) was used for the assays performed on site in our field laboratory in Iquitos. Standard curves for quantification were performed using Leptospira interrogans serovar Icterohaemorrhagiae and enumerated using a Petroff-Hausser counting chamber on a dark-field microscope. Wells with no template were included in each assay to detect the presence of contaminating DNA.
Statistical analysis
We used a 2-sample test of proportions with a 95% confidence level. One-sided and 2-sided alternative hypotheses were tested against the null (the 2 proportions are similar). We used the statistical software STATA, version 8.0 (STATA).
RESULTS
In the context of a prospective, hospital-based and population-based study of acute fever in the region of Iquitos, 633 patients were enrolled from 1 June 2003 to 1 March 2004. Patients were studied at the urban Hospital de Apoyo Iquitos and at health posts in rural communities surrounding Iquitos (table 1). Rates of leptospiral exposure, as defined by serologic testing (IgM ELISA or MAT), were similar in both the hospital and community populations (table 1). Rates of follow-up were greater in the rural areas, primarily because these patients were more likely to be permanent residents of the catchment area, while patients at hospitals in urban areas often come from distant places and follow-up was difficult (table 1).
Table 1.
Summary of enrollment and follow–up in a study of leptospirosis.
| No. (%) of patients
|
|||||
|---|---|---|---|---|---|
| Location | Enrolled | With clinical follow-up | With available results from leptospiral serologic testing | With Antileptospiral anti-bodies detecteda | With severe pulmonary involvement b |
| Urban | |||||
| Hospital de Apoyo Iquitos | |||||
| Inpatients | 45 | 26 (57.8) | 44 (97.8) | 24 (55) | 5 |
| Outpatients | 344 | 290 (84.3) | 306 (89.0) | 165 (53.9) | 2 |
| Subtotal | 389 | 316 (81.2) | 350 (90.0) | 54 (54)c | 7 |
| Rural | |||||
| Zungarococha | 59 | 57 (96.6) | 59 (100) | 31 (52.5%) | 0 |
| Varillal | 72 | 60 (83.3) | 72 (100) | 44 (61.1%) | 0 |
| Moralillo | 113 | 96 (85.0) | 107 (95.0) | 57 (53.2%) | 0 |
| Subtotal | 244 | 213 (87.3) | 238 (97.5) | 132 (55.4)c | 0 |
| Total | 633 | 529 (83.6) | 588 (92.9) | 321 (54.6) | 7 |
Of patients for whom at least 1 positive result was available from IgM ELISA or Microscopic Agglutination Testing (MAT); positivity was determined according to criteria mentioned in Materials and Methods.
Significantly different. P = .0141 for a one-sided alternative hypothesis (diff>0) and P = .0282 for a two-sided alternative hypothesis.
Not significantly different.
In the rural community, no cases of severe pulmonary leptospirosis occurred among the 244 patients enrolled (table 1). Among the hospital-based patient population, 7 (3.7%) patients with serologic evidence of exposure to Leptospira had severe pulmonary forms of disease: 6 patients had hemoptysis; 1 had acute respiratory distress syndrome without hemoptysis (table 2). No cases of typical Weil disease (i.e, jaundice or renal failure) were observed. The severity of disease differed significantly between urban and rural subjects (table 1). Five patients died, of whom 4 had hemoptysis and 1 had ARDS and experienced multiorgan failure. One outpatient (patient 5) with hemoptysis remained ambulatory and was treated presumptively for pulmonary tuberculosis without evident effect, and resolution of disease occurred simultaneously with self-administration of amoxicillin. One hospitalized patient (patient 6) survived after several days of hemoptysis. A partial autopsy of one patient (patient 4) who had hemoptysis and died of respiratory failure (figure 1, top) had complete hemorrhagic involvement of the right lower lung (figure 1, bottom); other abdominal organs appeared grossly normal. There was no other evidence of visceral hemorrhage, but mucosal bleeding was observed in the lips and gums.
Table 2.
Summary of clinical information on patients with severe pulmonary leptospirosis.
| Age in years, sex | Clinical data
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Presentation | Epidemiologic factors | Treatment | Outcome | Laboratory data |
Chest radiograph findings | Results of leptospiral tests |
|
| 1 | 15, M | 7 days with undifferentiated fever; 4 days with dark urine, icteric sclerae, dyspnea, and bloody sputum; hematuria; oliguria. | Lived in a brick-and-mortar house in San Juan district between Iquitos airport and the city; poor sanitation; no running water; well in backyard; mother died of undi agnosed disease with fever- and jaundice 1 year before | Intravenous ceftriaxone administered every 2 h after admission | Died of respiratory failure while receiving ventilator support on day 2 of hospitalization | Hb, 9.3; Hct, 30%; WBC count, 10,900 cells/mm3; Tbil, 7.2 mg/dL; Dbil, 5.6 mg/dL; SCr, 1.6 mg/dL; calculated ClCr, 67.5 mL/min; 2 smears negative for malaria parasites | Diffuse patchy infiltrates with lower-lobe pre- dominance; right-side pleural-based density | Blood culture, positive for L. interrogans serovar Icterohaemorrhagiae; MAT/ELISA results, negative; acute-phase serum PCR results, positive; urine PCR results, negative |
| 2 | 43, M | Abrupt onset of fever, increased cough, dyspnea, and hemoptysis, preceded by a 2-week afebrile period of productive, nonbloody cough. Fulminant deteriora tion with respiratory failure; no jaundice; refractory hypotension. | Lived 1 block from Moronacocha lake in urban Iquitos; house had running water; rats seen in home; dogs ubiquitous in the area | Regimen of intravenous ceftriaxone started 6 h after admission | Died 17 h after hospital admission | Not done | Diffuse patchy infiltrates with lower-lobe predominance | Blood culture, positive for L. interrogans serovar Canicola; MAT/ELISA results, negative; acute-phase serum PCR results, positive; urine PCR results, negative |
| 3 | 19, F | 3 Weeks of fever, malaise, nonproductive cough, dyspnea; no hemoptysis. Plasmodium vivax parasitemia was diagnosed 2 weeks before presentation, but fever did not respond to chloroquine; refractory shock on day 7. | Lived in San Juan; 2 deep-water wells in backyard, one enclosed, one at ground- level and subject to runoff from ground water | Regimen of ceftriaxone was started 12 h after admission; antitubercu lous drugs | Died | WBC count, 6700 cells/mm3; urinalysis, 30 WBCs/hpf; 3 blood smears negative for malaria parasites | Diffuse “ground-glass” pulmonary infiltrates typical of acute respira tory distress syndrome | ELISA results, negative; MAT results, positive (1/1600 against strain Var10 but not to other serovars); acute-phase serum PCR results, negative; urine PCR results, positive |
| 4 | 19, M | 6 Days with fever, chills, headache, and sore throat; 3 days with jaundice and severe calf pain; abdominal and retrosternal pain; 1 day with blood-tinged vomit. Physical examination showed tachypnea and icteric sclerae and skin, hepatosplenomegaly, and right upper quadrant tenderness. Complications during hospitalization included hyperpyrexia; pericardial friction rub; bleeding from oral mucosa, lips and gums; subconjunctival hemorrhage; hemoptysis; and oliguric renal failure. | Lived in brick-and-mortar house in urban Iquitos; bitten by rat 5 days prior to onset of illness; no other information available | Regimen of intravenous ceftriaxone started 4 h after admission | Died 5 days after hospitalization; autopsy showed frankly hemorrhagic lungs (figure 1, bottom); liver was green; otherwise, viscera normal with no evidence of retroperitoneal hemorrhage | Hb, 9.1; Tbil, 7.4 mg/dL; Dbil, 6.7 mg/dL; SCr, 5.2 mg/dL; arterial blood gas: Po2 = 61 mm Hg; Pco2 =23 mm Hg | Diffuse alveolar infiltrates with lower-lobe pre- dominance (figure 1, top) | Blood and urine culture results, negative; MAT and ELISA results, negative; acute-phase serum, urine, and tissue PCR results, positive |
| 5 | 53, M | 5 Days with fever, headache, myalgia, leg pain, sore throat, and abdominal pain. The patient was seen at the out-patient fever clinic and was sent home with a course of antipyretics. Two weeks later at a follow-up visit, the patient revealed that he had hemoptysis 4 days after being seen at the fever clinic. Two sputum smears negative for M. tuberculosis. | Fruit farmer in San Juan district of Iquitos; rats noticed sporadically at home and at work | Self-medicated with amoxicillin | Symptoms resolved | None available | None available | Blood culture, positive for L. interrogans serovar Icterohaemorrhagiae; acute-phase ELISA results, negative; convalescent-phase ELISA results, positive; MAT titer for acute- phase serum, 6400 against serovar Australis; for convalescent- phase serum, 3200 against serovars Australis and Icterohaemorrhagiae; acute-phase serum PCR results, negative; urine PCR results at admission, at 90 days after hospitalization, and at 121 days, positive |
| 6 | 63, M | 9 Days of fever, abdominal pain, diarrhea, anorexia, and jaundice; self-resolving episodes of hemoptysis 4–5 days before admission. Physical examination: temperature, 383C; tachypnea, normotensive; alert and oriented; icteric sclerae and skin; pulmonary rales; hepatomegaly. | Lived in Iquitos, worked in newspaper printing plant; rats seen there; traveled to oil drilling area in Amazon region of Loreto to do manual field labor | Regimen of intravenous penicillin started 6 h after admission | Disease resolved; patient discharged after 11 days | Hb, 10.8 g/dL, WBC count, 7200 cells/mm3; SCr, 0.65 mg/dL; urinalysis, 10–12 leukocytes/hpf; thick smears negative for malaria parasites | Diffuse, bilateral lower-lobe infiltrates, with right-side predominance | Acute- and convalescent-phase ELISA results, positive; MAT titer on acute-phase serum, 51,200 against serovars Icterohaemorrhagiae and Bratislava (6400 at 30 days after hospitalization and 1600 at 60 days); acute-phase serum PCR results, negative; urine PCR results at 60 days, positive |
| 7 | 37, F | 2 Weeks with generalized myalgia; 4 days with jaundice and acute onset of diffuse, colicky abdominal pain. Physical examination: temperature, 36.53C; normotensive; not tachypneic; icteric mucous membranes and skin; lungs normal; hepatosplenomegaly; abdominal distension and diffuse tenderness on palpation; legs tender bilaterally. Fever, copious hemoptysis, and mucosal hemorrhage (gums, lips) began on day 2 of hospitalization; progressive respiratory deterioration, anuria, and obtundation. | Lived for 1 year in Punchana, a district of Iquitos near the Bellavista Nanay port on the Amazon River | Regimen of intravenous ceftriaxone started 2 h after admission | Died 6 days after presentation | Hypoglycemia (glucose, 60 mg/dL); Hb, 7.5 g/dL; Hct, 24%; platelet count, 302,000 platelets/mL; WBC count, 6300 cells/mL with normal differential; SCr, 5.2 mg/dL | Not done | ELISA results, negative; MAT results, 3200 only against isolate Var10; acute-phase serum PCR results, negative; urine PCR results, positive |
NOTE. ClCr, creatinine clearance; Dbil, direct bilirubin; Hb, hemoglobin level; Hct, hematocrit; SCr, serum creatinine level; Tbil, total bilirubin.
Figure 1.
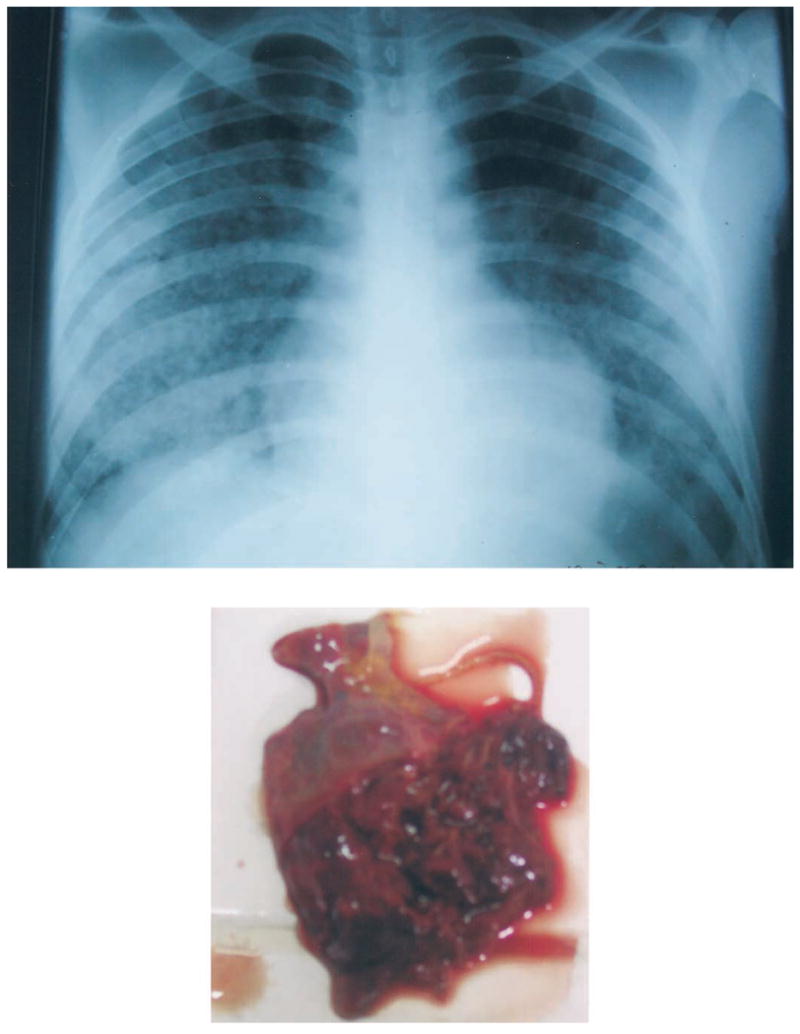
Chest radiograph (top) and gross appearance of right lower lung (bottom) from patient 4, a 19-year-old man who died from leptospiral pulmonary hemorrhage. The chest radiograph shows diffuse, patchy, alveolar infiltrates with a lower-lobe predominance. The right lower lung was extensively hemorrhagic and friable; the portion shown was obtained through the diaphragm through a peri-umbilical incision because only a partial necropsy was authorized.
The diagnosis of leptospirosis was confirmed for all patients with severe cases by a combination of serologic testing, culture, and a real-time PCR assay (table 2 and figure 2). Three patients (patients 1, 2, and 5) had positive blood cultures; 2 isolates were identified as L. interrogans serovar Icterohaemorrhagiae, and 1 was identified as L. interrogans serovar Canicola. One patient (patient 3) had an MAT titer of 1600 against strain Var10, and real-time PCR assay revealed pathogenic leptospires in the patient’s urine. One patient (patient 6) who had an extremely elevated MAT titer (51,200) also had negative blood and urine culture results but positive results of real-time PCR performed on urine 60 days after enrollment in the study. One patient who died (patient 4) underwent a partial necropsy. Pathogenic leptospires were demonstrated and quantified in this patient’s lung, kidney, abdominal wall muscle, serum, and urine by real-time PCR (figure 2). The patient who died of overwhelming sepsis and hemoptysis (patient 7) had negative blood and urine culture results, but real-time PCR had revealed pathogenic Leptospira in the urine.
Figure 2.
Quantification of the leptospiral burden in 7 patients with severe pulmonary manifestations of leptospirosis. Patient numbers correspond to patient descriptions in table 2. Bars show the number of leptospires per milliliter of serum or urine, or (e.g. patient 4) per g of tissue obtained at autopsy, as determined by a real-time polymerase chain reaction assay (see Materials and Methods). The line connecting the data points for patient 6 represents sequential urine samples. D, day; †, fatal case.
Three patients had ≥104 leptospires/mL of serum and 1 patient had 107 leptospires/mL of serum. One patient (patient 4) who had tissue specimens analyzed by real-time PCR had ≥105 leptospires per g of tissue. One patient with pulmonary hemorrhage who survived (patient 5) continued to have leptospiruria for >140 days.
DISCUSSION
In this report, we describe 7 patients with leptospirosis pulmonary involvement in a region of hyperendemicity. A real-time PCR assay was useful in the rapid diagnosis of these cases. The cases of leptospirosis exhibited by these patients, particularly those with fever and hemoptysis as cardinal manifestations, were unexpected by local physicians and health authorities and would have gone undiagnosed without special efforts to find such disease. These findings not only have importance for public health in a tropical region of Peru, but they have widespread applicability.
We found severe pulmonary involvement in leptospirosis only in patients in urban settings. All of these patients lived in and, most probably, according to clinical history, were infected in the city of Iquitos. No cases were found in rural villages around Iquitos, despite evidence that the proportion of patients who had acute febrile illness with IgM antibodies or a high titer of antileptospiral antibodies by MAT was not significantly different in these 2 contrasting regions (table 1). However, no cases of typical Weil disease were found. The reasons for the relation of severe pulmonary leptospirosis to urban acquisition of the disease are unclear. Potential explanations could include ascertainment bias (i.e, sicker patients may go to a hospital rather than an outpatient clinic), different frequencies of exposure to pathogenic leptospires in urban and rural populations [18], differing pathogenicity of serovars present in urban and in rural environments, the focal emergence of pulmonary tropism, or varying levels of infecting Leptospira in environmental water sources of infection (i.e., inoculum effect). In this study, leptospiral seropositivity was apparently not associated with seasonal changes in water levels or the amount of rain. In contrast, in other regions, such as Nicaragua, Brazil, and India, leptospirosis and severe associated disease has been described as epidemic and associated with seasonal flooding or severe weather [4, 6, 19–21], although pulmonary hemorrhagic manifestations were not limited to the urban setting, as was observed, for example, in Nicaragua and La Reunion.
In this study, 5 patients died; 4 deaths were associated with frank pulmonary hemorrhage. Two patients survived without apparent sequelae. In only 1 case did leptospirosis enter the differential diagnosis at the time of illness. In other cases, diagnosis was suspected only by physicians involved in the study. In all 7 patients, a real-time PCR assay provided a potentially immediate, definitive diagnosis of leptospirosis. Despite its cost, this assay has advantages over routine PCR and other PCR-based assays, such as the quantitative micro-plate test for clinical use, as described by Truccolo et al. [22]. The assay used here was rapid, sensitive, and specific, and was performed in closed tubes or microplates, thus minimizing cross-contamination from PCR amplicons. Quantification of Leptospira in clinical samples was also easily performed and potentially could have prognostic value. Postmortem tissue specimens from a patient who died of leptospiral pulmonary hemorrhage had high levels of leptospires in lung, liver, muscle, and kidney tissues. This is the first such quantification, to our knowledge, of leptospires in human tissues. The quantitative results for serum, which showed high levels of leptospiremia in patients who died, mirror those from Truccolo et al. [22], who found, using a quantitative microplate PCR assay, that the presence of at least 104 leptospires/mL of serum was associated with a poor prognosis. As Truccolo et al. [22] propose, multicenter studies are needed to further delineate the association of leptospiremia levels with severity of disease and prognosis, particularly in regions with contrasting patterns of leptospirosis transmission and endemicity.
Acute fever in patients in tropical settings is a diagnostic challenge. This is especially true when complications such as hemoptysis, jaundice, neurological impairment, and renal failure supervene. Other infectious diseases, such as malaria, dengue, yellow fever, viral hepatitis, and tuberculosis, as well as noninfectious disorders, such as small vessel vasculitides, could have conceivably explained the clinical presentations we describe. Without specific investigation for leptospirosis in the context of ongoing surveillance for an ecological and epidemiological study, we would not have made the diagnosis of severe pulmonary leptospirosis, despite its attendant implications for the institution of public health measures and ministerial policy for the diagnosis and control of this disease. Based on the leptospiral strains identified in this and other ongoing studies, control measures aimed against rats (carriers of serovar Icterohaemorrhagiae and strain Var10 [Vinetz, unpublished observation]) and dogs (carriers of serovar Canicola) would have the greatest public health impact.
Interpretation of this study has 2 potential limitations. First, we relied on serologic testing as the primary means of identifying exposure to Leptospira in the patient populations studied. MAT and ELISA simply revealed the presence of high titers of IgM antileptospiral antibodies in the patient populations studied. Some investigators in the leptospirosis field would use the criteria used in this study as evidence of acute leptospirosis [23–25]. Studies that have used serologic testing to diagnose acute leptospirosis in regions of endemicity have used high cutoff values (i.e., MAT titers of ≥200 in areas of nonendemicity or ≥400–800 in areas of endemicity) [23, 26] to enhance the specificity of the test for diagnosis of acute infection. However, MAT titers of ≥800 are not uncommon in asymptomatic ambulatory individuals in rural villages in the Iquitos region (Vinetz, Segura, Gilman, and Cespedes, data not shown). Therefore, in the present study, we interpret the serologic test results only as an indication of probable recent leptospiral exposure [18]. This study likely underestimates the case-fatality rate of acute leptospirosis and the rate of leptospiral pulmonary hemorrhage because of the very high prevalence of high titers of antileptospiral antibodies in acutely febrile patients from this region of endemicity. Because of the high level of leptospiral exposure in our study populations, it is possible that the number of patients with high titers of antileptospiral antibodies caused an overestimation of the number of febrile patients who actually had acute leptospirosis. Here, we estimate that ~4% of patients with high titers of antileptospiral antibodies in urban Iquitos had severe pulmonary involvement, with an overall case-fatality rate of 2%. Second, some patients were lost to follow-up, which potentially caused an underestimation of the number of patients who developed severe pulmonary leptospirosis. The study design included active surveillance of hospitalized patients, and outpatient follow-up was successful for >80% of enrolled patients, with the highest rate of loss to follow-up at the Hospital de Apoyo Iquitos. It is possible that some patients lost to follow-up because they were only temporary inhabitants of Iquitos could have had severe pulmonary leptospirosis. If this were true, the number of severe leptospirosis cases would have been underestimated. Further, with our continuous presence in the rural districts and excellent communication with the village health posts, it is likely that we would have learned of severe cases of illness in the area around Iquitos, even with the few patients that were lost to follow-up. But severe cases of illness in the area around Iquitos did not occur. Therefore, the data support our conclusion that severe leptospirosis in the Iquitos region is primarily an urban problem.
Leptospirosis continues to be misidentified because of its variable and often nonspecific clinical presentation. In Iquitos, Peru, leptospirosis appears to be a highly significant cause of morbidity and mortality, far more so than has hitherto been recognized. Because the results of leptospiral serologic tests are still difficult to interpret in regions where leptospiral exposure is common, more effective diagnostic tests need to be developed to focus on rapid demonstration of the etiologic agent in clinical specimens, such as serum or blood. Such testing modalities could be PCR-based, such as those presented here [17] and elsewhere [22, 27], or they could include antigen detection assays [28]. All inpatients in this study received appropriate antimicrobial therapy at presentation; outpatients did not receive such therapy because they went home before the diagnosis was established or before hemoptysis developed. It remains unclear whether initiation of antimicrobial therapy would have an impact on the prognosis for severe pulmonary leptospirosis, but antimicrobials clearly cannot be withheld from such patients [9]. Improved surveillance is only possible by engaging public health authorities to maintain interest in and support of leptospirosis diagnostic facilities that can provide diagnostic information with a clinically relevant turnaround time, and by educating the public in regions of endemicity to seek early medical attention for fever.
Acknowledgments
We wish to acknowledge the strong encouragement and support of Dr. Fernando Llanos, former Jefe, Instituto Nacional de Salud, Lima, Peru, Dr. Carlos Vidal, Director, and Dr. Hugo Rodríguez, Epidemiologist, of the Dirección de Salud (DISA), Iquitos, Peru, and the outstanding collaborative support of Drs. Kevin Baird, James Olson, Jeff Stancil, and the staff of the US Naval Medical Research Center Detachment, Lima, Peru. We also acknowledge Paul Levett, Ph.D., and Rene Galloway of the Centers for Disease Control and Prevention, Atlanta, for their collaborative efforts in the molecular identification of the leptospiral isolates performed in this study. Special thanks to Flor Pacheco, Fabiola Díaz, Gladys Nahir Chuquipiondo, Juana Calderón, Sandra Cubas in Iquitos, and Lourdes Balda and Dana Gonzalez at the Instituto Nacional de Salud, Lima, for their laboratory and field work contributions to the present study; and to Mirko Zimic, M.Sc., for statistical contributions. We also thank Drs. Joshua Fierer, Carlton Evans, and Katherine Remick for critical review of the manuscript and helpful suggestions. We are grateful to MJ Research for their generous support and helpful technical advice in the Real-Time PCR diagnostic assay.
Financial support. Funding was received from the Fogarty International Center, US National Institutes of Health/US National Institute of Environmental Health Sciences (grant R01TW05860); Tutorial in Tropical Health at JHU/Peru Overseas Sites TG-35 (grant T35AI07646); US National Institutes of Health/Fogarty International Center (grants D43TW00910, D43TW006581, and D43TW007120) and the RG-ER anonymous Tropical Medicine Research Fund.
References
- 1.Dai B. Advances in research on Leptospira and human leptospirosis in China. Chin Med Sci J. 1992;7:239–43. [PubMed] [Google Scholar]
- 2.Park YK, Park SK, Rhee YK, Kang SK. Leptospirosis in Chonbuk Province of Korea in 1987. Korean J Intern Med. 1990;5:34–43. doi: 10.3904/kjim.1990.5.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panaphut T, Domrongkitchaiporn S, Thinkamrop B. Prognostic factors of death in leptospirosis: a prospective cohort study in Khon Kaen, Thailand. Int J Infect Dis. 2002;6:52–9. doi: 10.1016/s1201-9712(02)90137-2. [DOI] [PubMed] [Google Scholar]
- 4.Sehgal SC, Murhekar MV, Sugunan AP. Outbreak of leptospirosis with pulmonary involvement in north Andaman. Indian J Med Res. 1995;102:9–12. [PubMed] [Google Scholar]
- 5.Yersin C, Bovet P, Merien F, et al. Pulmonary haemorrhage as a predominant cause of death in leptospirosis in Seychelles. Trans R Soc Trop Med Hyg. 2000;94:71–6. doi: 10.1016/s0035-9203(00)90445-0. [DOI] [PubMed] [Google Scholar]
- 6.Trevejo RT, Rigau-Perez JG, Ashford DA, et al. Epidemic leptospirosis associated with pulmonary hemorrhage—Nicaragua, 1995. J Infect Dis. 1998;178:1457–63. doi: 10.1086/314424. [DOI] [PubMed] [Google Scholar]
- 7.Silva JJ, Dalston MO, Carvalho JE, Setubal S, Oliveira JM, Pereira MM. Clinicopathological and immunohistochemical features of the severe pulmonary form of leptospirosis. Rev Soc Bras Med Trop. 2002;35:395–9. doi: 10.1590/s0037-86822002000400017. [DOI] [PubMed] [Google Scholar]
- 8.Vinetz JM, Glass GE, Flexner CE, Mueller P, Kaslow DC. Sporadic urban leptospirosis. Ann Intern Med. 1996;125:794–8. doi: 10.7326/0003-4819-125-10-199611150-00002. [DOI] [PubMed] [Google Scholar]
- 9.Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–71. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 10.Zaki SR, Shieh W-J. Leptospirosis associated with outbreak of acute febrile illnesses and pulmonary haemorrhage, Nicaragua, 1995. Lancet. 1996;347:535–6. doi: 10.1016/s0140-6736(96)91167-8. [DOI] [PubMed] [Google Scholar]
- 11.Roshanravan B, Kari E, Gilman RH, et al. Endemic malaria in the Peruvian Amazon region of Iquitos. Am J Trop Med Hyg. 2003;69:45–52. [PubMed] [Google Scholar]
- 12.Russell KL, Montiel Gonzalez MA, Watts DM, et al. An outbreak of leptospirosis among Peruvian military recruits. Am J Trop Med Hyg. 2003;69:53–7. [PubMed] [Google Scholar]
- 13.Céspedes M, Glenny M, Felices V, Balfa L, Suarez V. Prueba de ELISA indirecta para la deteccion de anticuerpos IgM para el diagnóstico de leptospirosis humana. Rev Peru Med Exp Salud Publica. 2002;19:24–7. [Google Scholar]
- 14.Cole JR, Jr, Sulzer CR, Pursell AR. Improved microtechnique for the leptospiral microscopic agglutination test. Appl Microbiol. 1973;25:976–80. doi: 10.1128/am.25.6.976-980.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautom RK. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J Clin Microbiol. 1997;35:2977–80. doi: 10.1128/jcm.35.11.2977-2980.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S Ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smythe LD, Smith IL, Smith GA, et al. A quantitative PCR (TaqMan) assay for pathogenic Leptospira spp. BMC Infect Dis. 2002;2:13. doi: 10.1186/1471-2334-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson MAS, Smith M, Joseph P, et al. Human exposure to Leptospira in three contrasting epidemiological contexts in Peru. Emerg Infect Dis. 2004;10:1016–22. [Google Scholar]
- 19.Ko AI, Galvao Reis M, Dourado CMR, Johnson WD, Riley LW. Urban epidemic of severe leptospirosis in Brazil. Lancet. 1999;354:820–5. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 20.Bharadwaj R, Bal AM, Joshi SA, et al. An urban outbreak of leptospirosis in Mumbai, India. Jpn J Infect Dis. 2002;55:194–6. [PubMed] [Google Scholar]
- 21.Duval G, Michault A, Baranton G, et al. Seroepidemiological study of human leptospirosis at Reunion Island. Rev Epidemiol Sante Publique. 1991;39:135–41. [PubMed] [Google Scholar]
- 22.Truccolo J, Serais O, Merien F, Perolat P. Following the course of human leptospirosis: evidence of a critical threshold for the vital prognosis using a quantitative PCR assay. FEMS Microbiol Letters. 2001;204:317–21. doi: 10.1111/j.1574-6968.2001.tb10904.x. [DOI] [PubMed] [Google Scholar]
- 23.Bajani MD, Ashford DA, Bragg SL, et al. Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis. J Clin Microbiol. 2003;41:803–9. doi: 10.1128/JCM.41.2.803-809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sehgal SC, Vijayachari P, Murhekar MV, Sugunan AP, Sharma S, Singh SS. Leptospiral infection among primitive tribes of Andaman and Nicobar Islands. Epidemiol Infect. 1999;122:423–8. doi: 10.1017/s0950268899002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko AI, Galvao Reis M, Ribeiro Dourado CM, Johnson WD, Jr, Riley LW. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet. 1999;354:820–5. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 26.Sehgal SC, Vijayachari P, Sharma S, Sugunan AP. LEPTO Dipstick: a rapid and simple method for serodiagnosis of acute leptospirosis. Trans R Soc Trop Med Hyg. 1999;93:161–4. doi: 10.1016/s0035-9203(99)90293-6. [DOI] [PubMed] [Google Scholar]
- 27.Merien F, Baranton G, Perolat P. Comparison of polymerase chain reaction with microagglutination test and culture for diagnosis of leptospirosis. J Infect Dis. 1995;172:281–5. doi: 10.1093/infdis/172.1.281. [DOI] [PubMed] [Google Scholar]
- 28.Saengjaruk P, Chaicumpa W, Watt G, et al. Diagnosis of human leptospirosis by monoclonal antibody-based antigen detection in urine. J Clin Microbiol. 2002;40:480–9. doi: 10.1128/JCM.40.2.480-489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]