Abstract
Welding fumes contain metals and other toxic substances known or strongly suspected to be related with oxidative stress and premature cellular senescence. Apolipoprotein J/Clusterin (ApoJ/CLU) is a glycoprotein that is differentially regulated in various physiological and disease states including ageing and age-related diseases. In vitro data showed that exposure of human diploid fibroblasts to hexavalent chromium (Cr(VI)) resulted in premature senescence and significant upregulation of the ApoJ/CLU protein. In this study we analyzed blood and urine samples from shipyard industry welders being exposed to different levels of Cr(VI) over a period of five months in order to assay in vivo the relation of ApoJ/CLU serum levels with Cr(VI). Our findings confirmed the previously reported in vitro data since reduction of Cr levels, after a worksite intervention, associated with lower levels of ApoJ/CLU serum levels. We concluded that the human ApoJ/CLU gene is responsive to the acute in vivo oxidative stress induced by heavy metals such as hexavalent chromium.
1. INTRODUCTION
Welders are exposed to many air contaminants such as iron oxide, manganese, nickel, cadmium oxide, zinc oxide, chromium, fluoride, ozone, nitrogen oxides, carbon monoxide, and others [1]. Previous studies suggested that the increased generation of highly reactive oxygen species, which result in oxidative tissue damage, is responsible for the toxicity of Cr(VI), Fe, Ni, and other metals [2–6]. Welding processes, like manual metal finishing, are commonly used in stainless steel welding and produce mainly chromium, nickel, manganese, fluorides, nitrogen oxide, and ozone [1].
The genotoxic, mutagenic, and cytotoxic effects of hexavalent chromium (Cr(VI)) exposure are well documented [2–6]. Chromium is absorbed via the gastrointestinal and respiratory tracts and the skin. Even though chromium kinetics is not fully clear, key features mainly include differential absorption of Cr(VI) and Cr(III), rapid reduction of Cr(VI) to Cr(III) in all body fluids and tissues, modest incorporation of chromium into bone, and concentration- dependent urinary clearance [7]. Compared to the Cr(III) ions that cross the membranes slowly by simple diffusion, Cr(VI) readily crosses cell membranes in the form of tetrahedral chromate anions through the general anion transport system and for that reason intracellular Cr is considered as indicative of Cr(VI) exposure [8–10]. Inside the cell, Cr(VI) is reduced to Cr(III), generating intermediate Cr(V) and Cr(IV) ions, oxygen, and organic radicals. The existing evidence points to Cr(V) as the main reactive species in Cr(VI)-induced genotoxicity [11, 12] through direct redox reactions with DNA, formation of DNA adducts, and Zn(II) thiolate complexes [13] which, in addition to Cr(VI) complexes formed mainly with cellular thiols, are likely triggers of a chain of events leading to carcinogenesis [14]. Products including DNA strand breaks, Cr-DNA adducts, and DNA-protein cross-links have been shown to occur in vivo and in vitro [15, 16].
The distribution of chromium to different compartments, the possibility of different transport mechanisms and pathways combined with the potential for reduction of Cr(VI), complicates further the kinetic models as far as it concerns excretion [17, 18]. The decrease of Cr(VI) levels from body fluids seem to follow a biphasic blood clearance and a bi/multiphasic urinary excretion pattern, which suggest the existence of several slow-releasing storage compartments. Several studies have shown half-times ranging between a few days (2–6) up to more than 3 months or even two years for the fast/medium and slow phase elimination, respectively [19–21].
Recently reported data suggested that exposure of human diploid fibroblasts to hexavalent chromium Cr(VI) at concentrations equal or 10 fold lower than the maximum permissive values (MPV) resulted in cell death or premature cellular senescence, respectively [22]. The cellular senescence phenotype was accompanied by elevated protein levels of apolipoprotein J/clusterin (ApoJ/CLU) [22].
Human ApoJ/CLU is a heterodimeric secreted glycoprotein that was initially purified from serum and identified as an apolipoprotein [23]. Not only ApoJ/CLU functions as an apolipoprotein, but it is also implicated in additional intra-or extracellular processes. For instance, it has been proposed that the secreted ApoJ/CLU protein functions as an extracellular chaperone [24]. CLU is differentially regulated in many severe physiological disturbance states including ageing, several neurological diseases, and in vivo cancer progression [23].
Interestingly and surprisingly, in a cross-sectional field survey, it has been also found that welders and sandblasters (known to be exposed to high levels of heavy metals and other chemicals) exhibited lower ApoJ/CLU serum levels as compared to other low chemically exposed occupational groups like white collars and electricians [22]. Given these observations in this study we analyzed blood and urine samples from shipyard industry welders being exposed to different levels of Cr(VI) over a period of five months in order to assay in vivo the relation of ApoJ/CLU serum levels with Cr(VI).
2. MATERIALS AND METHODS
2.1. Sample collection
Blood and urine samples were collected from male welders (n = 75) and sandblasters (n = 5) of a shipyard industry according to standard procedures. Subjects aged between 22 to 58 years old (mean 40.14) had worked for 2 to 35 years in the shipyard (mean 18.5) and agreed to participate in this study after signing an informed consent. The male welders examined in this study were selected among welders who welded on both MS and SS. Welding has taken place inside workshops or SS tanks. Welders used electrodes containing various metals in different concentrations like Mn (0.8–6.5%), Ni (0.02–8.8%), Cr (0.03–22.5%), Mo, Si, Fe, Zn, Cu, and other substances. All welders had access to local suction at their workplace and used it more than 75% of the welding time. None wore airstream helmet, but all occasionally wore filter mask for personal respiratory protection and consistently used protective clothing and gloves. Each participant completed a comprehensive questionnaire on individual welding history and on welding methods and intensity applied during the previous two weeks, month, and the previous year. They were also asked about their welding employment histories and the duration of SS welding in their careers. As determined after detailed medical examination, none of the subjects suffered any serious chronic disease.
All welders with Cr blood levels above 2 μg/L (n = 9) were selected to enter an intervention phase. This worksite intervention aimed to lower Cr(VI) exposure through a minimization of stainless steel welding. Five months later, sample collection was repeated in this selected group of workers. The five months period was selected based on half time of chromium life and life cycle of erythrocytes.
In parallel to blood and urine collection, a number of additional parameters were recorded such as age, anthropometrical characteristics, smoking status, duration of employment, medical history, and detailed occupational history.
Measurement of Cr and ApoJ/CLU levels in blood and urine samples —
Cr levels in blood and urine samples were measured by using a Perkin-Elmer 600 atomic absorption spectrometer. Samples were appropriately diluted (1/2 for urine and 1/5 for blood) with Triton X-100 in a nitric acid solution. They were then analyzed by the standard addition calibration procedure using graphite furnace [25] at a detection limit of 0.1 μg/L. to correct the differences in fluid intake, the urinary values were also related to the respective creatinine values. Quantitative measurement of ApoJ/CLU serum levels by ELISA was performed as described previously [22]. Additional biological parameters assayed included γ-glutamyltranspeptidase (γ-GT; an indicator of liver function), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), urea nitrogen, uric acid, fasting glucose, erythrocyte sedimentation rate at one hour, white blood cell count and type, platelet count, and hemoglobin. Prostate specific antigen (PSA) level was determined in subjects aged above 45 years old. The biochemical parameters were measured by using a Hitachi 917 analyzer and the hematological parameters at an autoanalyzer Pedra 120.
2.2. Statistical analysis
Results were expressed as mean (SD) or geometric mean (minimum-maximum). Differences between groups were examined by Student's t-test or Mann-Whitney U-test depending on the normality of the distribution. Pearson correlation analysis was used to determine possible correlations between variables. A log transformation was used for laboratory variables not fitting to a normal distribution. Wilcoxon signed rank test was employed to assess the difference on paired observations in intervention group. For comparisons, the two-tailed test was used with a type I error of α = 0.05. Data analyses were conducted by means of the SPSS for Windows 14.1.0 statistical package.
3. RESULTS
One out of three welders had a normal body mass index (BMI) (20–25 kg/m2), while 18.2% were obese (BMI >30 kg/m2). Only 19.1% of the subjects were nonsmokers and statistical analysis revealed a significant positive correlation between cigarette smoking and elevated triglyceride and hematocrit levels. Body mass index (BMI) besides age was also positively correlated to triglyceride level, uric acid, and γ-GT (Table 1). Welders had been welding for 11–25 days the previous month and for 180–240 days the previous year.
Table 1.
Individual characteristics and laboratory results in shipyard workers.
| SS welders | Sandblasters | Welders | |
|---|---|---|---|
| n = 47 | n = 5 | n = 28 | |
| Mean sd | Mean sd | Mean sd | |
| Age (years) | 40.4 (9.0) | 46.8 (6.6) | 38.6 (10.8) |
| Duration of employment (years) | 17.4 (11.5) | 26.3 (7.8) | 18.8 (12.6) |
| BMI (kg/m2) | 29.1 (3.7) | 28.0 (1.7) | 26.5 (2.6) |
| Glucose (mg/dL) | 89.2 (12.4) | 98.4 (11.0) | 85.0 (12.1) |
| Urea (mg/dL) | 36.0 (8.8) | 40.6 (10.3) | 31.1 (6.7) |
| Creatinine | 1.0 (0.1) | 0.9 (0.1) | 0.9 (0.1) |
| Uric acid (mg/dL) | 5.5 (1.3) | 5.0 (0.2) | 5.4 (0.9) |
|
| |||
| Liver function indicators | |||
|
| |||
| ALT (GPT) (U/L) | 36.3 (18.4) | 29.6 (12.1) | 29.3 (13.2) |
| AST (GOT) (U/L) | 22.7 (7.7) | 18.4 (3.1) | 21.0 (6.1) |
| GGT (U/L) | 31.4 (15.5) | 24.6 (6.4) | 25.7 (17.4) |
|
| |||
| Lipid metabolism | |||
|
| |||
| Cholesterol (mg/dL) | 216.7 (43.8) | 200 (35.3) | 207.2 (35.8) |
| LDL (mg/dL) | 138.4 (44.4) | 131.2 (29.6) | 134.7 (31.8) |
| HDL (mg/dL) | 47.7 (8.5) | 44.2 (4.6) | 46.2 (7.9) |
| Triglycerides (mg/dL) | 143.0 (122.7) | 122.6 (53.7) | 133.3 (54.1) |
|
| |||
| Hematology | |||
|
| |||
| Hematocrit (%) | 45.0 (2.6) | 45.6 (3.3) | 45.4 (2.7) |
| ESR (mm) | 6.8 (5.6) | 7.80 (7.1) | 3.6 (2.0) |
| Leukocytes (WBC) (103) | 7.51 (2.08) | 7.57 (0.55) | 8.10 (1.99) |
| Neutocytes (103) | 4.23 (1.64) | 4.29 (0.26) | 4.62 (1.40) |
| Lymphocytes (103) | 2.63 (0.77) | 2.67 (0.25) | 2.77 (0.51) |
| Monocytes (103) | 0.43 (0.19) | 0.40 (0.07) | 0.44 (0.13) |
| Cr blood (μg/L) | 1.14 (1.16) | 0.26 (0.13) | 0.64 (0.68) |
| Cr urine (μg/L) | 1.84 (7.25) | 0.20 (0.14) | 0.68 (0.92) |
| Cr urine (μg/g creatinine) | 1.20 (4.08) | 0.09 (0.04) | 0.45 (0.98) |
| Clusterin/ApoJ (OD492) | 1165.1 (338.1) | 1140.9 (178.4) | 1176.3 (274.9) |
BMI = body mass index; ALT (GPT) = alanine aminotrasferase; AST (GOT) = aspartate aminotransferase; GGT = γ-glutamyltranspeptidase; LDL = low-density lipoprotein; HDL = high-density lipoprotein; ESR = erythrocyte sedimentation rate.
Cr blood levels in the first sampling ranged between 0.1–6.1 μg/L (mean 0.91; geometric mean 0.64) and urine Cr levels ranged between 0.1–50.2 μg/L (mean 1.33; geometric mean, 0.43) or 0.03–27.27 μg/g creatinine (mean 0.87; geometric mean 0,25). In Table 1, relative data of subgroups are presented. ApoJ/CLU serum concentration ranged between 653 and 3075 (mean 1168; geometric mean 1129) (OD492). None of the hematological parameters, biochemical indicators, age, BMI, or smoking status showed any statistical significant relation with ApoJ/CLU levels. Urine Cr levels exhibited a weak association with ApoJ/CLU levels (r = 0.160, P = .157) raised when only SS welders were included in the analysis but it remained insignificant (r = 0.245, P = .097). Correlation of urine Cr corrected for creatinine and ApoJ/CLU levels was also not significant (r = 0.185, P = .101) but also raised in SS welders (r = 0.217, P = .143). Having observed that trend, we analyzed data from the first sampling concerning measurements of those welders known (from their detailed occupational history) to be involved recently (less than 10 weeks) in stainless steel welding. Indeed, when only this group (n = 17) was included in the analysis, the correlation coefficient (r) of ApoJ/CLU with urine Cr rose from 0.160 to 0.333 (P = .192) and with urine Cr corrected for creatinine rose from 0.245 to 0.453 (P = .068) but did not reach statistical significance (P = .206).
As we described previously, we implemented an intervention to lower Cr(VI) blood levels in order to study the regulation of ApoJ/CLU levels in human serum. All welders (n = 9) with blood Cr levels higher than 2 μg/L were assigned to two intervention groups with different grade of intensity, followed a five-month intervention program which consisted of a significant differentiation (lowering) in the volume and amount of stainless steel-mediated welding. After this period, we recollected blood and urine and assayed the Cr and ApoJ/CLU serum levels. In both groups, we found a significant reduction in the Cr levels in all but one blood sample (Table 2). Urine levels exhibited various trends partly explained by the intensity of intervention, in addition to evidence that urine Cr reflects more recent exposure and it has greater variability [26].
Table 2.
Chromium blood and urine levels of welders entered worksite intervention (n = 9).
| Welder | Age | Smoking status1 | Cr blood (μg/L) | Cr urine (μg/g creatinine) | Cr urine (μg/L) | |||
|---|---|---|---|---|---|---|---|---|
| Intervention | Intervention | Intervention | ||||||
| pre | post | pre | post | pre | post | |||
| 1st | 58 | N | 6.1 | 4.0 | 27.27 | 24.27 | 50.20 | 5.20 |
| 2nd | 46 | H | 2.0 | 1.2 | 0.72 | 4.04 | 0.80 | 3.90 |
| 3rd | 38 | N | 5.0 | 0.6 | 0.64 | 0.95 | 1.50 | 0.50 |
| 4th | 40 | M | 2.9 | 0.3 | 1.03 | 0.43 | 1.10 | 0.20 |
| 5th | 36 | M | 3.7 | 0.2 | 0.31 | 0.09 | 0.90 | 0.10 |
| 6th | 37 | L | 2.5 | 2.7 | 0.65 | 2.40 | 1.20 | 1.60 |
| 7th | 42 | M | 2.1 | 1.9 | 1.13 | 1.83 | 1.20 | 2.20 |
| 8th | 35 | L | 3.0 | 1.6 | 1.94 | 2.64 | 3.10 | 3.20 |
| 9th | 35 | M | 2.7 | 1.2 | 0.20 | 4.98 | 0.30 | 3.20 |
|
| ||||||||
| All mean | 40.78 | 3.33 | 1.522 | 3.77 | 4.62 | 6.70 | 2.23 | |
| (SD) | (7.40) | (1.38) | (1.22) | (8.83) | (7.54) | (16.33) | (1.79) | |
1N: nonsmokers; L: light (1–15 pack years); M: medium (16–30 pack years); H: heavy (>30 pack years)
2 P < .05, Wilcoxon signed ranks test.
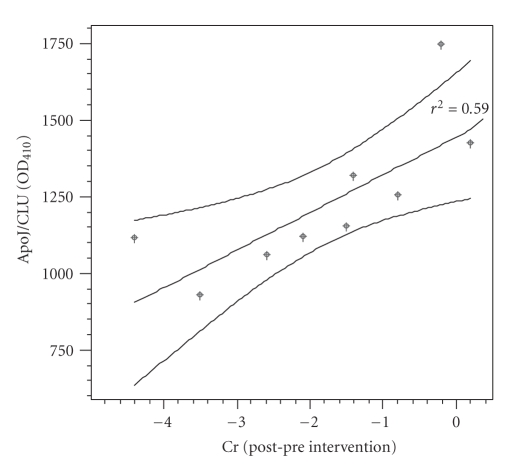
Interestingly, we found that the reduction of Cr levels in blood due to this intervention was related to lower ApoJ/CLU serum levels (0.77, P = .009) (Figure 1). Higher intensity of intervention (lower exposure to hexavalent chromium) was also related to lower ApoJ levels in a statistical significant level (1042 versus 1362, P = .032).
Figure 1.
Relation of ApoJ/CLU levels and the reduction of chromium blood levels (micrograms/L) (lines represent 95% mean prediction interval).
The results of multivariate analysis modeling ApoJ/CLU levels have shown that blood Cr is the main determinant of ApoJ/CLU levels. Urine Cr holds a mild significant inverse association (negative β) reflecting perhaps differences in the kinetics between compartments (Table 3).
Table 3.
Linear regression results modeling for ApoJ/CLU serum levels.
| Variable | Units of β | β coefficient (95% CI) | P value | Model r 2 |
|---|---|---|---|---|
| MODEL 1 | 0.656 | |||
|
| ||||
| Cr blood | μg/L | 265.57 (71.38 to 1233.93) | .015 | |
| Cr urine | μg/g creatinine | − 32.53 (− 70.04 to − 7.02) | .024 | |
|
| ||||
| MODEL 2 | 0.590 | |||
|
| ||||
| pre/post intervention ΔCr blood | μg/L | − 122.69 (− 213.69 to − 31.69) | .015 | |
4. DISCUSSION
The unifying factor in determining toxicity and carcinogenicity for most, if not all, heavy metals including iron, copper, chromium, vanadium, cobalt, mercury, cadmium, and nickel is the generation of reactive oxygen and nitrogen species [27]. Metal-mediated formation of free radicals causes various modifications to DNA bases, enhanced lipid peroxidation and altered calcium and sulfhydryl homeostasis. Lipid peroxides, formed by the attack of radicals on polyunsaturated fatty acid residues of phospholipids, can further react with redox metals finally producing mutagenic and carcinogenic substances [27].
We have previously shown that welders and sandblasters, who are exposed to high levels of heavy metals, exhibited lower ApoJ/CLU serum levels as compared to other occupational groups [22]. This finding was unanticipated since exposure of normal human diploid fibroblasts to low noncytotoxic levels of Cr(VI) induced premature cellular senescence and resulted in the upregulation of the ApoJ/CLU protein [22]. ApoJ/CLU has a nearly ubiquitous expression pattern in human tissues and has been implicated in various physiological processes and in many severe physiological disturbance states including ageing, cancer progression, vascular damage, diabetes, kidney, and neuron degeneration [28]. Although unrelated in their etiology and clinical manifestation, these diseases represent states of increased oxidative stress [29, 30]. By combining these findings, we proposed recently that ApoJ/CLU upregulation during ageing or at age-related diseases does not correlate to chronological age, but it rather relates to increased oxidative damage which can be “sensed” by the regulatory elements of the CLU gene promoter [28].
Although chronic exposure may induce secondary molecular changes, which extend beyond the effects of oxidative stress (e.g., inflammation or initiation of tumor formation) [22], our current findings strengthen further the notion that the ApoJ/CLU is a sensitive biomarker of the organismal oxidative stress. More specifically, we report that the CLU serum levels correlate positively to the workers exposure to heavy metals and to Cr blood and urine concentration. Cr in blood has been shown to reflect occupational exposure to hexavalent chromium in stainless steel welding [31] which gives support to the argument that the significant reduction of Cr levels after the intervention is mainly due to the reduction of hexavalent chromium which is in turn assumed to be the responsible agent for the relation with ApoJ/CLU found in our study. However, it should be considered that regarding the exposure to other metals there might be some uncertainty in the assessment of the related biological effect. Lack of detailed knowledge of the kinetics of Cr in the blood and in the elimination compartments, especially urine, makes difficult to evaluate the correct time and site for sampling and the number of samples that should be taken. It is suggested that a longitudinal study in occupationally exposed participants besides Cr and ApoJ/CLU should also include the measurement of the levels of well-known markers of oxidative damage such as the products of lipid peroxidation [malondialdehyde(MDA)], DNA damage (modified bases such as 8-oxo-dG), and/or protein carbonylation in order to add firm basis to the proposed Cr-mediated oxidative stress in the cells of occupational groups with similar exposures as other studies have shown with various markers [32–37].
In any case, this preliminary study gives evidence that the human ApoJ/CLU gene is responsive to the acute oxidative stress induced by heavy metals as hexavalent chromium, and this finding may prove valuable during the monitoring and re-evaluation of the long-term workers health effects in certain occupational environments.
Abbreviations —
Apolipoprotein J/Clusterin: ApoJ/CLU, (hexavalent) chromium: Cr(VI).
ACKNOWLEDGMENT
The authors wish to thank all welders, sandblasters, and their foremen for their participation, and Mr Ioakim Kantartzis, Dipl. MEng., for his valuable technical advice.
References
- 1.Alexopoulos EC. [Chemical hazards] in [Health effects of welding], ELINYAE Ed. 19–25, (in Greek), Athens, Greece, 2007, http://www.elinyae.gr/el/item_details.jsp?cat_id=33&item_id=7126.
- 2.Kasprzak KS. The role of oxidative damage in metal carcinogenicity. Chemical Research in Toxicology. 1991;4(6):604–615. doi: 10.1021/tx00024a002. [DOI] [PubMed] [Google Scholar]
- 3.Beckman KB, Ames BN. The free radical theory of aging matures. Physiological Reviews. 1998;78(2):547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 4.Hartwig A. Recent advances in metal carcinogenicity. Pure and Applied Chemistry. 2000;72(6):1007–1014. [Google Scholar]
- 5.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biology and Medicine. 1995;18(2):321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 6.Antonini JM, Leonard SS, Roberts JR, et al. Effect of stainless steel manual metal arc welding fume on free radical production, DNA damage, and apoptosis induction. Molecular and Cellular Biochemistry. 2005;279(1-2):17–23. doi: 10.1007/s11010-005-8211-6. [DOI] [PubMed] [Google Scholar]
- 7.O'Flaherty EJ, Kerger BD, Hays SM, Paustenbach DJ. A physiologically based model for the ingestion of chromium(III) and chromium(VI) by humans. Toxicological Sciences. 2001;60(2):196–213. doi: 10.1093/toxsci/60.2.196. [DOI] [PubMed] [Google Scholar]
- 8.Alexander J, Aaseth J. Uptake of chromate in human red blood cells and isolated rat liver cells: the role of the anion carrier. The Analyst. 1995;120(3):931–933. doi: 10.1039/an9952000931. [DOI] [PubMed] [Google Scholar]
- 9.Kortenkamp A, Beyersmann D, O'Brien P. Uptake of Cr(III) complexes by erythrocytes. Toxicological and Environmental Chemistry. 1987;14:23–32. [Google Scholar]
- 10.Cohen MD, Kargacin B, Klein CB, Costa M. Mechanisms of chromium carcinogenicity and toxicity. Critical Reviews in Toxicology. 1993;23(3):255–281. doi: 10.3109/10408449309105012. [DOI] [PubMed] [Google Scholar]
- 11.Levina A, Codd R, Dillon CT, Lay PA. Progress in Inorganic Chemistry. Vol. 51. New York, NY, USA: John Wiley & Sons; 2002. Chromium in biology: toxicology and nutritional aspects; pp. 145–250. [Google Scholar]
- 12.Sugden KD, Stearns DM. The role of chromium(V) in the mechanism of chromate-induced oxidative DNA damage and cancer. Journal of Environmental Pathology, Toxicology and Oncology. 2000;19(3):215–230. [PubMed] [Google Scholar]
- 13.Levina A, Lay PA. Mechanistic studies of relevance to the biological activities of chromium. Coordination Chemistry Reviews. 2005;249(3-4):281–298. [Google Scholar]
- 14.Barchowsky A, O'Hara KA. Metal-induced cell signaling and gene activation in lung diseases. Free Radical Biology and Medicine. 2003;34(9):1130–1135. doi: 10.1016/s0891-5849(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 15.De Flora S, Wetterhahn KE. Mechanisms of chromium metabolism and genotoxicity. Life Chemistry Reports. 1989;7(3):169–244. [Google Scholar]
- 16.Standeven AM, Wetterhahn KE. ls there a role for reactive oxygen species in the mechanism of chromium(VI) carcinogenesis? Chemical Research in Toxicology. 1991;4(6):616–625. doi: 10.1021/tx00024a003. [DOI] [PubMed] [Google Scholar]
- 17.Salem H, Katz SA. Speciation, bioavailability, and systemic distribution of chromium from Whetlerite dust. The Science of the Total Environment. 1989;86(1-2):59–64. doi: 10.1016/0048-9697(89)90193-9. [DOI] [PubMed] [Google Scholar]
- 18.Lim TH, Sargent T, III, Kusubov N. Kinetics of trace element chromium(III) in the human body. The American Journal of Physiology. 1983;244(4):R445–R454. doi: 10.1152/ajpregu.1983.244.4.R445. [DOI] [PubMed] [Google Scholar]
- 19.Bragt PC, van Dura EA. Toxicokinetics of hexavalent chromium in the rat after intratracheal administration of chromates of different solubilities. The Annals of Occupational Hygiene. 1983;27(3):315–322. doi: 10.1093/annhyg/27.3.315. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi S, Sano K, Shimojo N. On the biological half-time of hexavalent chromium in rats. Industrial Health. 1983;21(1):25–34. doi: 10.2486/indhealth.21.25. [DOI] [PubMed] [Google Scholar]
- 21.Schaller KH, Csanady G, Filser J, Jüngert B, Drexler H. Elimination kinetics of metals after an accidental exposure to welding fumes. International Archives of Occupational and Environmental Health. 2007;80(7):635–641. doi: 10.1007/s00420-007-0176-1. [DOI] [PubMed] [Google Scholar]
- 22.Katsiki M, Trougakos IP, Chondrogianni N, Alexopoulos EC, Makropoulos V, Gonos ES. Alterations of senescence biomarkers in human cells by exposure to CrVI in vivo and in vitro. Experimental Gerontology. 2004;39(7):1079–1087. doi: 10.1016/j.exger.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Trougakos IP, Gonos ES. Clusterin/Apolipoprotein J in human aging and cancer. The International Journal of Biochemistry & Cell Biology. 2002;34(11):1430–1448. doi: 10.1016/s1357-2725(02)00041-9. [DOI] [PubMed] [Google Scholar]
- 24.Poon S, Easterbrook-Smith SB, Rybchyn MS, Carver JA, Wilson MR. Clusterin is an ATP-independent chaperone with very broad substrate specificity that stabilizes stressed proteins in a folding-competent state. Biochemistry. 2000;39(51):15953–15960. doi: 10.1021/bi002189x. [DOI] [PubMed] [Google Scholar]
- 25.Angerer J, Schaller KH. Analysis of Hazardous Substances in Biological Materials. New York, NY, USA: Wiley-VCH; 1999. [Google Scholar]
- 26.Stridsklev IC, Schaller KH, Langård S. Monitoring of chromium and nickel in biological fluids of stainless steel welders using the flux-cored-wire (FCW) welding method. International Archives of Occupational and Environmental Health. 2004;77(8):587–591. doi: 10.1007/s00420-004-0560-z. [DOI] [PubMed] [Google Scholar]
- 27.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Current Medicinal Chemistry. 2005;12(10):1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 28.Trougakos IP, Gonos ES. Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radical Research. 2006;40(12):1324–1334. doi: 10.1080/10715760600902310. [DOI] [PubMed] [Google Scholar]
- 29.Floyd RA, West MS, Eneff KL, et al. Conditions influencing yield and analysis of 8-hydroxy-2′ -deoxyguanosine in oxidatively damaged DNA. Analytical Biochemistry. 1990;188(1):155–158. doi: 10.1016/0003-2697(90)90544-j. [DOI] [PubMed] [Google Scholar]
- 30.Grune T, Jung T, Merker K, Davies KJA. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. The International Journal of Biochemistry & Cell Biology. 2004;36(12):2519–2530. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Edmé JL, Shirali P, Mereau M, et al. Assessment of biological chromium among stainless steel and mild steel welders in relation to welding processes. International Archives of Occupational and Environmental Health. 1997;70(4):237–242. doi: 10.1007/s004200050213. [DOI] [PubMed] [Google Scholar]
- 32.Travacio M, Polo JM, Llesuy S. Erratum to “Chromium (VI) induces oxidative stress in the mouse brain”. Toxicology. 2001;162(2):139–148. doi: 10.1016/s0300-483x(00)00423-6. [DOI] [PubMed] [Google Scholar]
- 33.Hodges NJ, Ádám B, Lee AJ, Cross HJ, Chipman JK. Induction of DNA-strand breaks in human peripheral blood lymphocytes and A549 lung cells by sodium dichromate: association with 8-oxo-2-deoxyguanosine formation and inter-individual variability. Mutagenesis. 2001;16(6):467–474. doi: 10.1093/mutage/16.6.467. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y-D, An S-C, Oyama T, Kawamoto T, Kim H. Oxidative stress, hogg1 expression and NF-κB activity in cells exposed to low level chromium. Journal of Occupational Health. 2003;45(5):271–277. doi: 10.1539/joh.45.271. [DOI] [PubMed] [Google Scholar]
- 35.Lee AJ, Hodges NJ, Chipman JK. Interindividual variability in response to sodium dichromate-induced oxidative DNA damage: role of the Ser326Cys polymorphism in the DNA-repair protein of 8-oxo-7,8-dihydro-2′ -deoxyguanosine DNA glycosylase 1. Cancer Epidemiology Biomarkers & Prevention. 2005;14(2):497–505. doi: 10.1158/1055-9965.EPI-04-0295. [DOI] [PubMed] [Google Scholar]
- 36.Goulart M, Batoréu MC, Rodrigues AS, Laires A, Rueff J. Lipoperoxidation products and thiol antioxidants in chromium exposed workers. Mutagenesis. 2005;20(5):311–315. doi: 10.1093/mutage/gei043. [DOI] [PubMed] [Google Scholar]
- 37.Wang X-F, Xing M-L, Shen Y, Zhu X, Xu L-H. Oral administration of Cr(VI) induced oxidative stress, DNA damage and apoptotic cell death in mice. Toxicology. 2006;228(1):16–23. doi: 10.1016/j.tox.2006.08.005. [DOI] [PubMed] [Google Scholar]