Abstract
Serotonin (5-HT) 1A and 5-HT1B receptors have been implicated in the incidence and treatment of depression in part through the examination of animals lacking these receptors. Although these receptors have been repeatedly implicated in ingestive behavior there is little information about how 5-HT 1A and 5-HT1B receptor mutant mice react to solutions of varying palatability. In the present experiment male and female 5-HT 1A and 5-HT1B mutant and wild-type mice were presented with increasing concentrations of sucrose using a two-bottle choice procedure. In addition fasting blood glucose levels were assessed. Both male and female 5-HT1B mutant mice drank more sucrose than WT mice but also consumed more water. Female, but not male, 5-HT1A mutant mice similarly showed increased sucrose consumption, but did not demonstrate increased consumption of water. In addition, the pattern of increased sucrose consumption over genotype and sex was related to fasting blood glucose concentrations such that levels in male 5-HT1B mutant mice were reduced relative to wild-type and 5-HT1A mutant males, but similar to those of females. The findings in 5-HT1B mutant mice emphasize the role of the 5-HT1B receptor in regulating ingestive behavior, whereas female sex hormones and 5-HT1A receptors may interact to alter sucrose consumption in 5-HT1A mutant mice. In addition, these findings may have implications for the role of these receptors in the incidence and treatment of depression since the intake of sucrose has been used as an index of anhedonia in animal models of depression and antidepressant efficacy.
Keywords: Serotonin, 5-HT1A, 5-HT1B, sucrose, glucose, anhedonia, drinking
1. Introduction
The neurotransmitter serotonin (5-HT) is diffusely located throughout the brain where it regulates a diverse array of behavioral effects [1]. The ability of 5-HT to regulate behavioral satiety and macronutrient selection provides the basis for the pharmacologic treatment of obesity and eating disorders. Both 5-HT1A and 5-HT1B receptors have been implicated in the regulation of feeding. Findings regarding the 5-HT1B receptor are relatively straightforward, in that pharmacological stimulation of this receptor routinely led to decreased feeding [2–4]. Accordingly, animals lacking the 5-HT1B receptor consumed more food than their wild-type counterparts especially as they aged, although increased intake remained proportional to their body weight [e.g. 5]. Pharmacological stimulation of 5-HT1A receptors using 8-OH-DPAT has been reported to either increase [e.g. 6] or decrease feeding [e.g. 7], presumably depending on the differential activation of presynaptic receptors on 5-HT cell bodies or postsynaptic 5-HT1A receptors in terminal fields, respectively [e.g. 8]. In line with this suggestion, blockade of carbohydrate feeding provoked by 5-HT in the paraventricular nucleus (PVN) was prevented by blockade of either 5-HT1A or 5-HT1B receptors in the PVN [9].
5-HT is also widely recognized for its potential role in the etiology of psychiatric disorders and their pharmacologic treatment (for review see [10–12]). Research with mice with genetic deletion of 5-HT receptors produces phenotypic changes in anxiety and depressive behaviors and alter the behavioral effects of antidepressant drugs [for review see 13]. The roles of 5-HT in affective and anxiety disorders, however, may not remain completely distinct from the role of 5-HT in feeding, especially since ingestive behavior is used in tests that presume to measure affective behavior in animal models. For example, prolonged exposure to a period of chronic mild stress has been reported to reduce the consumption or preference for palatable fluids, such as sucrose, and this behavior has been used as a model for anhedonia or depression in rodents [14]. Although individual 5-HT receptors have been implicated in feeding, satiety [8, 15] and the ingestion of solutions of varying palatability [16] using pharmacological techniques, few studies have examined the role of 5-HT receptor subtypes using genetically modified mice.
The present studies sought to provide information on the role of 5-HT1A and 5-HT1B receptors in the voluntary intake of palatable solutions using mice with specific genetic deletion of these receptors. Specifically, we examined intake and preference of increasing concentrations of sucrose using a two-bottle choice paradigm in 5-HT1A−/− and 5-HT1B−/− mice. Because sucrose intake could be related to genetic alterations in blood glucose levels, fasting blood glucose levels were measured in the 5-HT mutant mice and related to their intake of sucrose. Finally, male and female mice were considered separately to evaluate gender differences in ingestive behavior and glucose levels.
2. Materials and methods
2.1 Animals
A total of 203 male and female 5-HT1A receptor knockout (5-HT1A−/−; 31 males and 30 females), 5-HT1B receptor knockout (5-HT1B−/−; 32 males and 38 females) and wild-type (WT; 40 males and 32 females) mice with a 129/Sv-ter background were used in the present experiments. Mice were generated from founders obtained originally from Dr. René Hen at Columbia University and bred in the animal colony at the University of Pennsylvania. Their generation and breeding have been previously described [14, 15]. All animals, regardless of genotype, were housed three to five per cage in the colony with continuous access to food pellets and water and a 12-hour light-dark cycle (lights on at 07:00). Animals were handled only for cage changing prior to the start of the first experiment. Animals were 3–6 months of age at the beginning of the experiments and tested for a maximum of 4 weeks. This age range was chosen to ensure that all of the tested animals fell within the adult age range and was required to generate the large number of animals used in the present studies. The estrous cycle of female mice was not monitored. Instead, sucrose solutions were presented for 4 days, a period presumed to sample across the 4–6 day ovarian reproductive cycle of mice [17]. The National Institutes of Health Guide for the Care and Use of Laboratory Animal was followed in conducting these studies and the protocol was approved by the University of Pennsylvania IACUC.
2.2. Sucrose-drinking two-bottle choice procedure
In two experiments using cohorts of the same sex, male and female 5-HT1A−/− (10 males and 12 females), 5-HT1B−/− (10 males and 12 females) and wild-type (20 males and 12 females) mice were tested using a two-bottle choice drinking procedure. Data for male knockout mice were collected in two cohorts resulting in a larger sample size for male wild-type mice. Because there were so significant differences between these groups and to simplify the presentation of the data, these data have been combined. Mice were moved from colony cages to individual hanging wire cages and given access to tap water through two 35-ml graduated cylinders fitted with 3 inch long 1 inch bend open drinking tubes (Ancare, Bellmore, NY). These open drinking tubes were similar to those available in their original home cages. The mice were allowed to habituate to the housing and drinking conditions for 4 days during which water consumption was recorded. During this phase animals samples equally between groups from the two bottles containing water, as evidenced by a lack of group effects on their preference ratios (Females: WT 0.56 ± 0.17, 5-HT1A−/− 0.56 ± 0.13, 5-HT1B−/− 0.52 ± 0.11; Males: WT 0.51 ± 0.16, 5-HT1A−/− 0.54 ± 0.28, 5-HT1B−/− 0.54 ± 0.18). During the choice phase of the experiment, one of the two water bottles was replaced with a bottle containing sucrose in increasing concentrations (1%, 2%, 4%, 8% and 16%). During the final 4-day water phase of the experiment, both bottles were presented and contained only water. The left and right positions of the two bottles were reversed every 48 h to control for side preference. At these times, mice were weighed and bottles were cleaned and replaced on the cages. Each concentration of sucrose was available for 4 days and presented in ascending order over the experiment. Throughout the experiment, evaporation estimates were calculated from bottles placed on an empty cage with one containing water and the other containing the appropriate sucrose solution. Any change in the volume of a given solution compared to the previous day was deducted from consumption values of that solution for that day. Daily consumption from each tube (ml) was used in combination with body weight to calculate sucrose intake (ml/kg), water intake (ml/kg) and total consumption (ml/kg). In addition daily intakes were used to calculate the sucrose preference ratio (sucrose intake/total intake). Average values for the four-day access period to each solution were generated and served as the dependent variables. Consumption of chow was not recorded.
2.3. Fasting blood glucose levels
In order to avoid distress evoked by prolonged fasting, a separate group of naïve animals was deprived of food on the morning of testing [18]. In accordance with the National Institutes of Health standard protocol, animals were fasted between 07:00 and 13:00 and blood was drawn at 13:00. Tail blood samples were taken from male and female 5-HT1A−/− (21 males and 18 females), 5-HT1B−/− (22 males and 26 females) and wild-type (20 males and 20 females) mice. Blood glucose levels were determined using a glucose meter (Abbott Diabetes Care, Alameda, CA).
3. Statistics
Intake of sucrose and water were measured over each 24 hour periods. Average intake was determined for the 4-day access period for each concentration of sucrose. Fluid Intake of sucrose, water, sucrose preference and total fluid intake were compared between sex, genotype, and sucrose concentration using three-way ANOVAs. Fasting blood glucose levels were compared between genotype and sex with a two-way ANOVA. Follow-up comparisons were done to examine significant main effects or interactions using Fisher’s PLSD test.
4. Results
4.1. Body Weight
The average starting body weights for male WT, 5-HT1A−/− and 5-HT1B−/− were 29.6, 27.7 and 30.3 g respectively. The average body weight for female WT, 5-HT1A−/− and 5-HT1B−/− at the start of the experiment were 23.5, 21.7 and 22.9 g respectively. There were no significant main effects of genotype on body weight in either male or female mice.
4.2. Sucrose intake
A three-way ANOVA revealed significant main effects of sex [F(1,280) = 12.5, p < .001], genotype [F(2,280) = 13.8, p < .0001], and sucrose concentration [F(4,280) = 457.7, p < .0001], significant two-way interactions between sex and genotype [F(2,280) = 5.3, p < .01], sex and sucrose concentration [F(4,280) = 20.0, p < .0001], and genotype and sucrose concentration [F(8,280) = 5.7, p < .0001], as well as a significant three-way interaction between sex, genotype and sucrose concentration [F(8,280) = 7.3, p < .0001].
Female 5-HT1A−/− mice tended to drink more sucrose solution than both 5-HT1B−/− and WT females, although when certain sucrose concentrations were available 5-HT1B−/− female mice exceeded the intakes of WT (2%) or both WT and 5-HT1A−/− (16%) mice (Figure 1). Two-way ANOVA for sucrose intake between genotype and concentration yielded significant main effects of genotype [F(2,132) = 7.6, p < .005] and sucrose concentration [F(4,132) = 189.2, p < .0001] and a genotype by sucrose concentration interaction [F(8,132) = 6.6, p < .0001]. Follow-up one-way ANOVAs with Fisher’s PLSD comparisons revealed that 5-HT1A−/− female mice drank more 1% (p < .01) sucrose than WT female mice. In addition, both 5-HT1A−/− and 5-HT1B−/− female mice both drank more 2% (p < .05 and p < .01), 8% (p < .005 and p < .01) and 16% (p < .005 and p < .0001) sucrose than WT females, with 5-HT1B−/− intakes also exceeding intakes of 5-HT1A−/− at 16% sucrose (p < .05).
Figure 1.
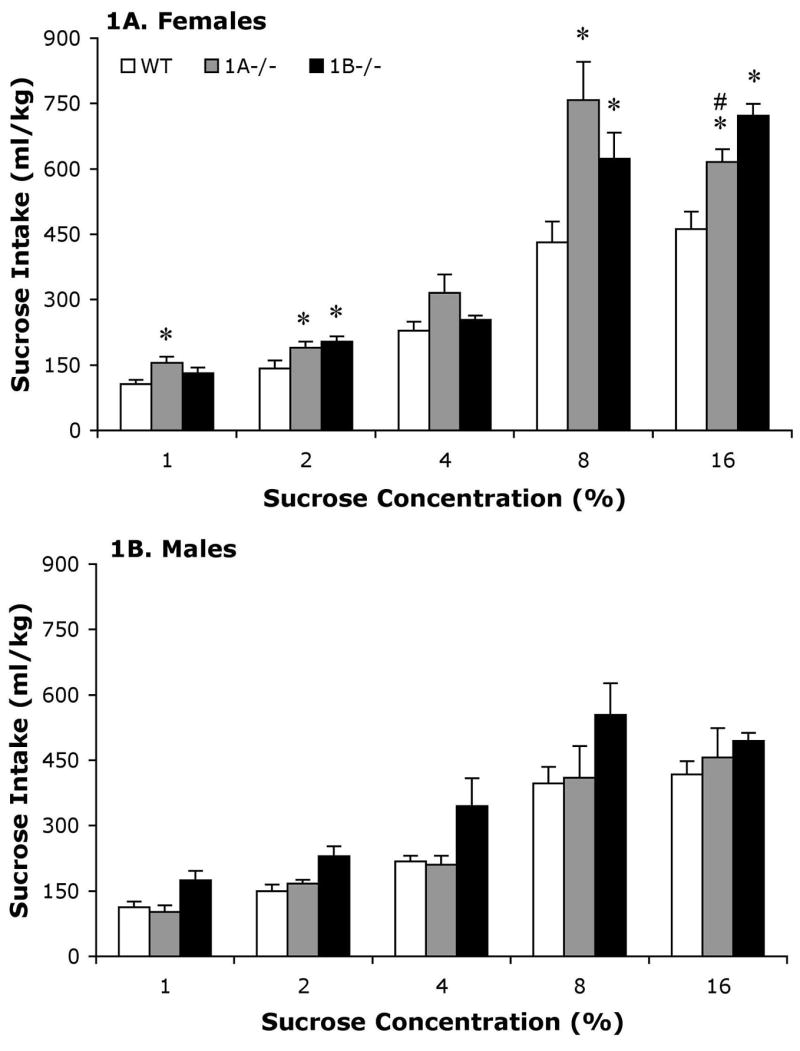
Mean (+SEM) sucrose intake (mg/kg) for female (A) and male (B) wild-type (WT), 5-HT1A, (1A−/−) and 5-HT1B (1B−/−) mice. *; Significantly different from WT of same sex. #; Significantly different from 1B−/− of same sex. In male mice follow-ups to a significant main effects of genotype revealed that male 5-HT1B−/− mice drank more sucrose overall than WT male mice (p < .05).
Unlike the female 5-HT receptor knockout mice that both tended to drink more sucrose than WT mice, only 5-HT1B−/− male mice, and not 5-HT1A−/− male mice, drank more sucrose than WT mice. That is, 5-HT1B−/− male mice drank more sucrose than WT male mice, independent of the concentration of sucrose that was available. This conclusion was supported by a two-way ANOVA for sucrose intake between genotype and concentration that yielded significant main effects of genotype [F(2,148) = 4.0, p < .05] and sucrose concentration [F(4,148) = 91.6, p < .0001], but no significant genotype by sucrose concentration interaction. Follow-up comparisons of this main effect collapsing across sucrose concentration revealed that male 5-HT1B−/− mice drank more sucrose overall than WT male mice (p < .05).
4.3. Water intake
A three-way ANOVA revealed significant main effects of sex [F(1,420) = 9.8, p < .005], genotype [F(2,420) = 19.3, p < .0001], and sucrose concentration [F(6,420) = 1014.6, p < .0001], significant two-way interactions between sex and genotype [F(2,420) = 4.2, p < .05], sex and sucrose concentration [F(6,420) = 2.2, p < .05], and genotype and sucrose concentration [F(12,420) = 11.2, p < .0001], as well as a significant three-way interaction between sex, genotype and sucrose concentration [F(12,420) = 2.6, p < .01].
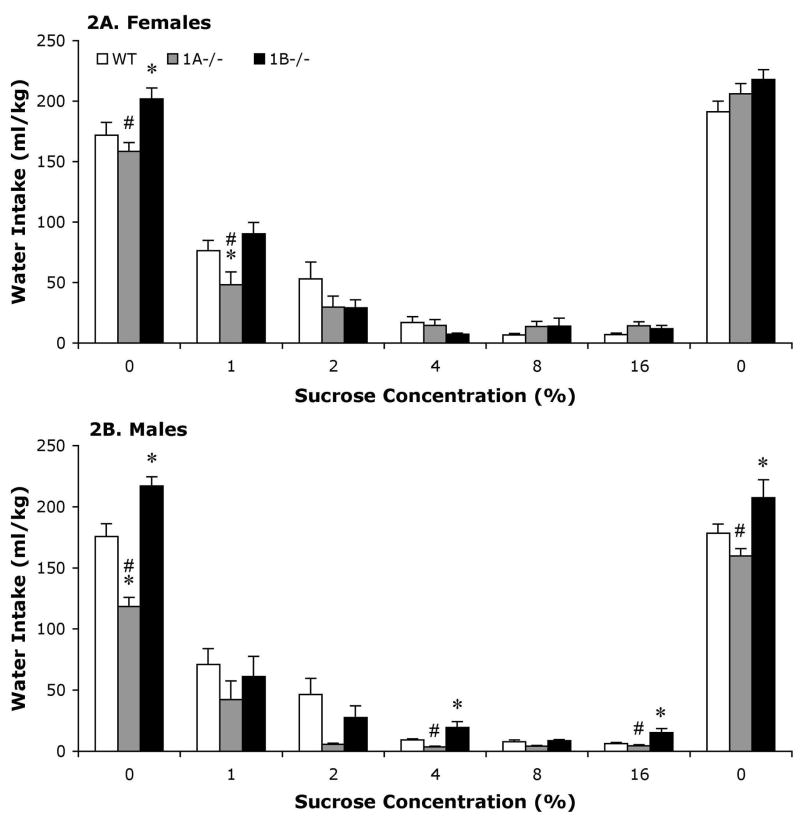
The influence of genotype on water intake varied in female mice depending on the concentration of sucrose that was available. The ANOVA comparing water intake between genotype and sucrose concentration yielded a significant main effect of sucrose concentration [F(6,198) = 395.6, p < .0001] and a significant genotype by sucrose concentration interaction [F(12,198) = 3.6, p < .0001] (Figure 2). During the first presentation of water, female 5-HT1B−/− mice drank more water than both WT (p < .05) and 5-HT1A−/− (p < .005) female mice. Also, when the lowest sucrose concentration (1%) was available, 5-HT1A−/− female mice drank less water than both 5-HT1B−/− (p < .005) and WT (p < .005) female mice.
Figure 2.
Bars represent mean (+SEM) water intakes (mg/kg) for female (A) and male (B) wild-type (WT), 5-HT1A, (1A−/−) and 5-HT1B (1B−/−) mice. *; Significantly different from WT mice of same sex. #; Significantly different from 1B−/− mice of same sex.
As in female mice, the effects of genotype on water intake in males varied depending on the concentration of sucrose that was available at the time. However, in general 5-HT1B−/− male mice drank more water than both, 5-HT1A−/− and WT male mice. The two-way ANOVA for water intake between genotype and sucrose concentration demonstrated significant main effects of genotype [F(2,222) = 7.7, p < .005] and sucrose concentration [F(6,222) = 245.9, p < .0001] and a significant genotype by sucrose concentration interaction [F(12,222) = 4.1, p < .0001]. Follow-up comparisons demonstrated that 5-HT1B−/− male mice drank more water than both WT and 5-HT1A−/− male mice during the first (p < .01 and p < .0001) and last (p < .05 and p < .005) presentations of water without sucrose available and also when 4% (p < .005 and p < .0005) and 16% (p < .0005 and p < .0005) sucrose were available. In addition 5-HT1A−/− male mice drank less water than WT male mice during the first presentation of water without sucrose available.
4.4. Total intake
A three-way ANOVA revealed significant main effects of sex [F(1,420) = 19.4, p < .0001], genotype [F(2,420) = 17.2, p < .0001], and sucrose concentration [F(6,420) = 366.9, p < .0001], significant two-way interactions between sex and genotype [F(2,420) = 8.2, p < .001], sex and sucrose concentration [F(6,420) = 15.8, p < .0001], and genotype and sucrose concentration [F(12,420) = 7.8, p < .0001], as well as a significant three-way interaction between sex, genotype and sucrose concentration [F(12,420) = 6.1, p < .0001].
Across the available sucrose concentrations, female 5-HT1B−/− mice tended to drink more total solution than WT female mice and in some cases 5-HT1A−/− female mice (Table 1). A two-way ANOVA for total intake between genotype and sucrose concentration resulted in significant main effects of genotype [F(2,198) = 8.2, p < .001] and sucrose concentration [F(6,198) = 153.7, p < .0001] and a significant genotype by sucrose concentration interaction [F(12,198) = 6.9, p < .0001]. During the first presentation of only water, female 5-HT1B−/− mice drank significantly more total solution that both WT (p < .05) and 5-HT1A−/− (p < .005) female mice. When 1% (p < .005) or 2% (p < .005) sucrose was available, female 5-HT1B−/− mice drank significantly more total volume than WT mice. At 8% sucrose, both 5-HT1A−/− (p < .005) and 5-HT1B−/− (p < .05) female mice drank significantly more total solution than WT mice. Finally, when 16% sucrose was available, female 5-HT1B−/− mice drank significantly more total solution than both WT (p < .0001) and 5-HT1A−/− (p < .05) female mice, with female 5-HT1a−/− mice drinking significantly more that WT mice (p < .005).
Table 1.
Mean and ± SEM of average total fluid intake (ml/kg) for each sex and genotype across the available sucrose concentrations.
| Sucrose Concentration (%) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 8 | 16 | 0 | |
| Female | |||||||
| WT | 172 ± 11 | 183 ± 8 | 195 ± 11 | 245 ± 20 | 438 ± 49 | 469 ± 40 | 191 ± 9 |
| 1A−/− | 158 ± 7b | 203 ± 7 | 219 ± 9 | 330 ± 41 | 771 ± 89a | 630 ± 31ab | 206 ± 8 |
| 1B−/− | 201 ± 9a | 221 ± 11a | 232 ± 7a | 261 ± 9 | 638 ± 61a | 734 ± 27a | 218 ± 8 |
|
| |||||||
| Male | |||||||
| WT | 175 ± 10 | 183 ± 12 | 196 ± 10 | 227 ± 13 | 404 ± 38 | 423 ± 30 | 178 ± 8 |
| 1A−/− | 119 ± 8 | 145 ± 8 | 173 ± 9 | 214 ± 21 | 414 ± 73 | 461 ± 68 | 159 ± 6 |
| 1B−/− | 217 ± 8 | 236± 9 | 257 ± 17 | 364 ± 66 | 563 ± 73 | 510 ± 19 | 207 ± 15 |
significantly different from WT
significantly different from 1B−/−
Male 5-HT1B−/− mice drank consistently more total volume of the combined solutions across the increasing sucrose gradient. This observation was supported by a two-way ANOVA, which yielded significant main effects of genotype [F(2,222) = 6.4, p < .005] and sucrose concentration [F(6,222) = 153.7, p < .0001], but no significant genotype by sucrose concentration interaction.
4.5. Sucrose preference
A three-way ANOVA revealed a significant main effect of genotype [F(2,280) = 7.9, p < .001], and sucrose concentration [F(4,280) = 457.7, p < .0001], a significant two-way interaction between genotype and sucrose concentration [F(8,280) = 5.2, p < .0001], and a significant three-way interaction between sex, genotype and sucrose concentration [F(8,280) = 2.5, p < .01].
Preference for sucrose in female mice rapidly became maximal with increasing concentrations of sucrose (Table 2). The two-way ANOVA resulted in a significant main effect of sucrose concentration [F(4,132) = 71.7, p < .0001] and a significant genotype by sucrose concentration interaction [F(8,132) = 3.5, p < .05]. Follow-up comparisons revealed that female 5-HT1A−/− mice preferred 1% sucrose to a greater extent than WT (p < .05) and 5-HT1B−/− (p < .05) female mice.
Table 2.
Mean and ± SEM of average preference ratio for each sex and genotype across the available sucrose concentrations.
| Sucrose Concentration (%) | |||||
|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 16 | |
| Female | |||||
| WT | 0.58 ± 0.14 | 0.71 ± 0.26 | 0.91 ± 0.10 | 0.98 ± 0.01 | 0.98 ± 0.01 |
| 1A−/− | 0.75 ± 0.20 a b | 0.86 ± 0.16 | 0.94 ± 0.08 | 0.98 ± 0.02 | 0.98 ± 0.01 |
| 1B−/− | 0.58 ± 0.17 | 0.87 ± 0.12 | 0.97 ± 0.01 | 0.98 ± 0.02 | 0.98 ± 0.02 |
|
| |||||
| Male | |||||
| WT | 0.60 ± 0.27 | 0.76 ± 0.25 | 0.96 ± 0.02 | 0.98 ± 0.02 | 0.98 ± 0.01 |
| 1A−/− | 0.70 ± 0.30 | 0.97 ± 0.02 | 0.98 ± 0.01 | 0.99 ± 0.01 | 0.99 ± 0.01 |
| 1B−/− | 0.73 ± 0.22 | 0.88 ± 0.14 | 0.94 ± 0.06 | 0.98 ± 0.01 | 0.97 ± 0.02 |
significantly different from WT
significantly different from 1B−/−
In male mice preference for sucrose also became maximal very rapidly as the concentration of sucrose increased across the experiment. The two-way ANOVA yielded a significant main effect of sucrose preference [F(4,148) = 32.3, p < .0001], but no significant effect of genotype or their interaction.
4.6. Blood glucose
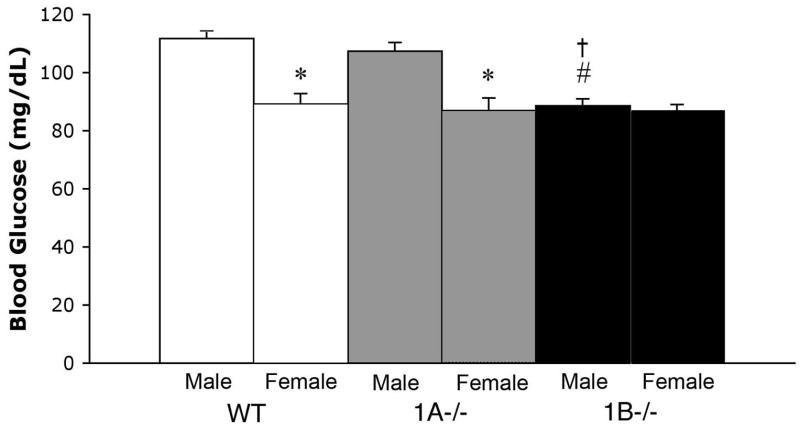
In general, male mice exhibited higher blood glucose levels than female mice (main effect of sex [F(1,121) = 36.2, p < .0001]; Figure 3). However, 5-HT1B−/− males had blood glucose levels that were similar to their female counterparts. A two-way ANOVA for the effects of genotype and sex on blood glucose resulted in significant main effects of genotype [F(2,121) = 10.1, p < .0001] and sex [F(1,121) = 36.2, p < .0001] and significant genotype X sex interaction [F(2,121) = 7.7, p < .001]. Follow-up comparisons demonstrated that WT (p < .0001) and 5-HT1A−/− (p < .0001) male mice had higher blood glucose levels than their female counterparts and there were no significant differences within each sex between these genotypes. In contrast, male 5-HT1B−/− were not significantly different from females of any genotype. Male 5-HT1B−/− mice also demonstrated significantly lower blood glucose levels than both WT (p < .0001) and 5-HT1A−/− (p < .0001) male mice.
Figure 3.
Bars represent mean (+SEM) blood glucose ((mg/dl) for female (A) and male (B) wild-type (WT), 5-HT1A, (1A−/−) and 5-HT1B (1B−/−) mice. *; Significantly different male mice of the same genotype. #; Significantly different from WT mice of same sex. †; Significantly different from 1A−/− mice of same sex.
5. Discussion
This study systematically compared male and female 5-HT1A−/− and 5-HT1B−/− mice for 24-hour intake of varying concentrations of sucrose solutions. Both male and female 5-HT1B−/− mice drank more sucrose than WT mice. Similar effects were observed in female, but not male 5-HT1A−/− mice. In addition, the pattern of effects on sucrose drinking across sex and genotype was related to fasting blood glucose concentrations. Although male mice typically maintained higher fasting glucose levels than females, glucose levels in male 5-HT1B−/− mice were similar to those of females. These data are in agreement with numerous previous findings suggesting a role for 5-HT1B receptors in ingestive behavior and extend those findings to suggest that sex hormones and 5-HT1A receptors interact in females to alter ingestive behavior. In addition, these findings may have implications for the role of these receptors in the incidence and treatment of depression since intake of sucrose has been used as an index of anhedonia in animal models of depression and antidepressant efficacy.
The increased sucrose intake observed in 5-HT1B−/− mice may have been mediated by a general tendency for these mice to consume more fluid than WT mice, which has been previously reported in male, but not female, 5-HT1B−/− mice [5]. However, examination of proportional intakes of water and sucrose make this interpretation seem unlikely. When only water was available, male and female 5-HT1B−/− mice drank an average of 120% and 116% respectively more water than that consumed by WT mice of the same sex. In contrast, averaging across the five sucrose concentrations, male and female 5-HT1B−/− mice drank 145% and 136% respectively of the sucrose taken in by WT mice of the same sex. Taken together, these findings suggest that although general changes in fluid intake may account for some of the observed increased sucrose intake in 5-HT1B−/− mice, this explanation does not account for the entirety of the data. Thus, these findings in the least emphasize the role of the 5-HT1B receptor in the promotion of satiety and may also suggest that the 5-HT1B receptor may participate in the expression of anhedonia. A similar pro-hedonic notion has been proposed with respect to the response of 5-HT1B−/− mice to cocaine. These mice demonstrate elevated basal [19] and cocaine induced [20] dopamine levels in the nucleus accumbens, exhibit enhanced locomotor response to cocaine, and are willing to work harder to obtain cocaine reinforcements [21]. Taken together, these findings suggest that mutations of 5-HT1B the receptor may confer an increased hedonic drive.
Sucrose intake was increased in female, but not male, 5-HT1A−/− mice in the absence of gross changes in fluid consumption. Only female 5-HT1A−/− mice showed significantly increased sucrose preference. This suggests that sex hormones and 5-HT1A receptors interact to regulate the intake of palatable solutions or to induce anhedonia. To date, there are little data available to explain this relationship with respect to sex differences in terms of either feeding or hedonic state. It is however known that female sex hormones interact with serotonin systems to alter food food intake [22]. For example, the anorectic effects of fenfluramine are enhanced during the estrous phase of the female rat reproductive cycle [23]. Further, there is a precedent in the literature for interactions between the 5-HT1A receptor and estrogen in measures relevant to moos such as the production of receptive sexual behavior [24] and also antidepressant [25] and anxiolytic [26] effects. In the present experiments sucrose intakes were averaged across the 4–6 day reproductive cycle of mice [17] and thus estrus cycle was not monitored. Further studies using female mice with controlled hormone cycles to identify the exact mechanisms through which 5-HT1A receptors and sex hormones in females interact to yield these effects are warranted.
The range of sucrose concentration tested was very highly preferred in both sexes of all three genotypes. Only the lowest concentrations (1% and 2%) demonstrated any variability that might have allowed the detection of significant differences in preference that were distinguishable from and independent of differences in total fluid intake. However, the pattern of findings, such as the proportion of sucrose intake in mutant mice relative to WT mice suggest that real differences in preference were present. Substantiating this hypothesis, the only significant effects on preference were detected in female 5-HT1A−/− mice that did not demonstrate greater overall fluid intakes, but tended to drink the most sucrose relative to their body weight than any of the other animals tested. Future studies using lower, less highly preferred solutions may help to verify that differences in preference dictate differences in intake [27].
Consistent with previous reports that females benefit from the anti-diabetic effects of estrogen [28], in the present study female mice tended to have lower blood glucose levels than male mice. In addition, blood glucose levels were predictive of the relationship between sex and sucrose intake. That is, male and female 5-HT1B−/− and female 5-HT1A−/− mice drank more sucrose than WT mice and had similar fasting blood glucose levels suggesting that lower blood glucose levels may be permissive to greater sucrose intakes. 5-HT1A and not 5-HT1B receptor activation reportedly decreases blood glucose levels [29] indicating that perhaps a constitutive lack of the 5-HT1B receptor results in compensatory upregulation of 5-HT1A receptors in male mice. Although it is not entirely clear how deletion of the 5-HT1B receptor would decrease blood glucose levels only in males, profound phenotypic sex differences in 5-HT1B−/− mice have been reported previously [e.g. 30].
In summary, deletion of the 5-HT1A and 5-HT1B receptors resulted in increased sucrose consumption with these effects being specific to female mice in the 5-HT1A−/− genotype. Further, lower fasting blood glucose levels among the genotypes were predictive of greater sucrose intake suggesting that lower glucose levels may allow greater intake of sucrose. These add to a large literature on the role of these receptors in feeding and also highlight some previously unexplored topics such as the interplay between sex hormones and the 5-HT1A and 5-HT1B receptors and their effect on both sucrose intake and blood glucose levels.
Acknowledgments
This research was supported by USPHS grants PO1-MH48125, T32-MH14854 and by research funds provided by the National Alliance for Research in Schizophrenia and Affective Disorder (AJB). Many thanks to Dr. Kenny J. Simansky for his helpful discussions on the association between measures of appetitive and affective behaviors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44(3):151–62. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 2.Halford JC, Blundell JE. The 5-HT1B receptor agonist CP-94,253 reduces food intake and preserves the behavioural satiety sequence. Physiol Behav. 1996;60(3):933–9. doi: 10.1016/0031-9384(96)00073-x. [DOI] [PubMed] [Google Scholar]
- 3.Kennett GA, Curzon G. Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors; hypophagia induced by RU 24969 only requires 5-HT1B receptors. Psychopharmacology (Berl) 1988;96(1):93–100. doi: 10.1007/BF02431539. [DOI] [PubMed] [Google Scholar]
- 4.Kennett GA, Dourish CT, Curzon G. 5-HT1B agonists induce anorexia at a postsynaptic site. Eur J Pharmacol. 1987;141(3):429–35. doi: 10.1016/0014-2999(87)90561-9. [DOI] [PubMed] [Google Scholar]
- 5.Bouwknecht JA, et al. Male and female 5-HT(1B) receptor knockout mice have higher body weights than wildtypes. Physiol Behav. 2001;74(4–5):507–16. doi: 10.1016/s0031-9384(01)00589-3. [DOI] [PubMed] [Google Scholar]
- 6.Aulakh CS, Hill JL, Murphy DL. A comparison of feeding and locomotion responses to serotonin agonists in three rat strains. Pharmacol Biochem Behav. 1988;31(3):567–71. doi: 10.1016/0091-3057(88)90231-6. [DOI] [PubMed] [Google Scholar]
- 7.Aulakh CS, et al. Food intake, neuroendocrine and temperature effects of 8-OHDPAT in the rat. Eur J Pharmacol. 1988;146(2–3):253–9. doi: 10.1016/0014-2999(88)90300-7. [DOI] [PubMed] [Google Scholar]
- 8.De Vry J, Schreiber R. Effects of selected serotonin 5-HT(1) and 5-HT(2) receptor agonists on feeding behavior: possible mechanisms of action. Neurosci Biobehav Rev. 2000;24(3):341–53. doi: 10.1016/s0149-7634(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 9.Mancilla-Diaz JM, et al. Role of 5-HT1A and 5-HT1B receptors in the hypophagic effect of 5-HT on the structure of feeding behavior. Med Sci Monit. 2005;11(3):BR74–9. [PubMed] [Google Scholar]
- 10.Mann J. Neurobiology of suicidal behaviour. Nature Reviews. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 11.Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res. 2003;37(5):357–73. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 12.Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry. 2000;61(Suppl 6):4–6. [PubMed] [Google Scholar]
- 13.Bechtholt AJ, Lucki I. Effects of serotonin-related gene deletion on measures of anxiety, depression and neurotransmission. In: Roth BL, editor. The serotonin receptors: From molecular pharmacology to human therapeutics. Human Press Inc.; Totowa, NJ: 2006. pp. 577–606. [Google Scholar]
- 14.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134(4):319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 15.Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73(1–2):37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 16.Islam AK, et al. Naltrexone, serotonin receptor subtype antagonists, and carbohydrate intake in rats. Pharmacol Biochem Behav. 1994;48(1):193–201. doi: 10.1016/0091-3057(94)90516-9. [DOI] [PubMed] [Google Scholar]
- 17.Silver L. Mouse Genetics: Concepts and Applications. New York: Oxford University Press; 1995. [Google Scholar]
- 18.Breyer MD, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16(1):27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 19.Shippenberg TS, Hen R, He M. Region-specific enhancement of basal extracellular and cocaine-evoked dopamine levels following constitutive deletion of the Serotonin(1B) receptor. J Neurochem. 2000;75(1):258–65. doi: 10.1046/j.1471-4159.2000.0750258.x. [DOI] [PubMed] [Google Scholar]
- 20.Scearce-Levie K, et al. 5-HT receptor knockout mice: pharmacological tools or models of psychiatric disorders. Ann N Y Acad Sci. 1999;868:701–15. doi: 10.1111/j.1749-6632.1999.tb11350.x. [DOI] [PubMed] [Google Scholar]
- 21.Rocha BA, et al. Intravenous cocaine self-administration in mice lacking 5-HT1B receptors. Pharmacol Biochem Behav. 1997;57(3):407–12. doi: 10.1016/s0091-3057(96)00444-3. [DOI] [PubMed] [Google Scholar]
- 22.Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav. 2004;82(1):35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Eckel LA, Rivera HM, Atchley DP. The anorectic effect of fenfluramine is influenced by sex and stage of the estrous cycle in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288(6):R1486–91. doi: 10.1152/ajpregu.00779.2004. [DOI] [PubMed] [Google Scholar]
- 24.Ahlenius S. Brain monoaminergic neurotransmission in the mediation of lordosis behavior in the female rat. Neurosci Biobehav Rev. 1993;17(1):43–9. doi: 10.1016/s0149-7634(05)80229-5. [DOI] [PubMed] [Google Scholar]
- 25.Estrada-Camarena E, Lopez-Rubalcava C, Fernandez-Guasti A. Facilitating antidepressant-like actions of estrogens are mediated by 5-HT1A and estrogen receptors in the rat forced swimming test. Psychoneuroendocrinology. 2006;31(8):905–14. doi: 10.1016/j.psyneuen.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Andrade TG, et al. Anxiolytic effect of estradiol in the median raphe nucleus mediated by 5-HT1A receptors. Behav Brain Res. 2005;163(1):18–25. doi: 10.1016/j.bbr.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Lewis SR, et al. Inbred mouse strain survey of sucrose intake. Physiol Behav. 2005;85(5):546–56. doi: 10.1016/j.physbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep. 2004;6(3):180–5. doi: 10.1007/s11883-004-0030-9. [DOI] [PubMed] [Google Scholar]
- 29.Uvnas-Moberg K, et al. Effects of selective serotonin and dopamine agonists on plasma levels of glucose, insulin and glucagon in the rat. Neuroendocrinology. 1996;63(3):269–74. doi: 10.1159/000126970. [DOI] [PubMed] [Google Scholar]
- 30.Jones MD, Lucki I. Sex Differences in the Regulation of Serotonergic Transmission and Behavior in 5-HT Receptor Knockout Mice. Neuropsychopharmacology. 2005;30:1039–1047. doi: 10.1038/sj.npp.1300664. [DOI] [PubMed] [Google Scholar]