Abstract
A novel lysine-based trifunctional chelate 3 was designed, synthesized, and characterized, which bears both a chelating moiety (CHX-A”) for sequestering radiometals (86Y or 111In) and the near infrared dye Cy5.5 for dual modality PET (or SPECT) and fluorescence imaging, respectively. Successful conjugation of 3 to the monoclonal antibody trastuzumab (Herceptin) was achieved by efficient thiol-maleimide chemistry, thereby yielding immunoconjugate 2. Analysis of 2 by flow cytometry and competitive binding assay demonstrates that immunoconjugate 2 binds to SKOV3 tumor cells comparably to native trastuzumab and thus may be used as a tumor-targeted monoclonal antibody probe for multimodality imaging.
INTRODUCTION
Multimodality imaging is becoming more common as a primary clinical tool for imaging human diseases, especially cancers.1 Dual labeled imaging probes allow the same target to be evaluated with two different modalities, such as positron emission tomography (PET) and computed tomography (CT),2 and magnetic resonance imaging (MRI) and near-infrared spectroscopy (NIR) optical imaging (OI).3 This allows the strengths of each modality to be combined in a single imaging session thereby improving diagnostic accuracy. The multimodality imaging approach is widely considered to provide a more precise, multiparametric description of a disease process such as its location, extent, metabolic activity, blood flow, and function of target tissue, resulting in better characterization of disease processes. However, the development of imaging agents for multimodality imaging is more challenging than single modality agents, requiring more complex design, multi-step synthesis, and careful selection of nuclear and/or optical tracers to avoid physical-chemical interference between molecular components. Few examples of multimodality agents have been reported in the literature and even fewer have been carried forward for investigation and evaluation in vivo.4-7 For example, Meade et. al. reported a class of dual imaging agents, in which multiple copies of both Gd(III)-DTPA and a fluorescent dye were covalently attached to a macromolecular framework of either poly-lysine or dextran.4 Meijer et. al. labeled a targeting cyclic peptide cNGR with both Gd(III)-DTPA and Oregon Green 488 for potential use in imaging angiogenesis.5 Bornhop and co-workers coupled a Gd(III) chelated peripheral-type benzodiazepine receptor ligand to cyclen-based fluorophores resulting in an agent that was both strongly fluorescent and readily detected by MRI.6 In our laboratory, amino-terminated PAMAM G6 dendrimers have been employed to carry both Gd(III) and near infrared dye Cy5.5 moieties.7 The resulting dual modality dendrimer-based imaging agent demonstrated efficient visualization of sentinel lymph nodes in mice by both MR and fluorescence imaging.7
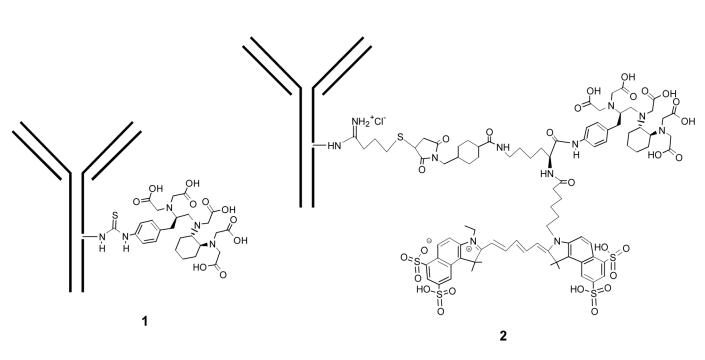
Tumor-specific monoclonal antibodies (mAbs) have been widely used as delivery vectors to transport radiometal ions for cancer imaging and therapy.8,9 The targeted nature of radiolabeled mAb imaging and therapies offers the promise of greater efficacy, less toxicity, and potentially greater treatment success. The humanized mAb trastuzumab (Herceptin) targets the cell surface antigen HER2, which is over-expressed in a variety of epithelial tumors. Radiolabeling of trastuzumab using isothiocyanate derivatives of the bifunctional chelating agents 1B4M (2-(4-aminobenzyl)-6-methyl-diethylenetriaminepentaacetic acid) and CHX-A” (N-[(R)-2-Amino-3-(p-aminophenyl)propyl]-trans-(S,S)-cyclohexane-1,2-diamine-N,N,N’,N”,N”-pentaacetic acid) has been thoroughly investigated for tumor targeting and cancer therapy in our laboratory (for example, see trastuzumab conjugate 1 in Figure 1).9-11 These immunoconjugates have efficient tumor targeting, excellent toleration of the modification, and normal whole body clearance patterns. Therefore, we hypothesized that conjugation of a dual imaging agent to trastuzumab would exploit the proven vector capabilities of monoclonal antibodies to perform multimodality imaging of tumors.
Figure 1.
A schematic presentation of CHX-A” conjugated trastuzumab 1 and trifunctional agent 3 conjugated to trastuzumab to form 2
Herein, we report a modular synthetic approach to assemble radioactive metal chelating agents and optical dyes into a “single” trifunctional (tumor targeting, radionuclide and optical imaging) agent for conjugation to antibodies, peptides, or other potential delivery vectors. In this approach, the near-infrared dye Cy 5.5 and a radiometal chelator, e.g., CHX-A” DTPA (86Y(III) for PET, 111In for SPECT) were introduced to the α-NH2 and α-COOH of l-lysine, respectively, while the ε-NH2 was used to introduce a maleimide moiety thereby permitting conjugation of the novel agent 3 to targeting vectors of interest, in this case, trastuzumab. In addition to the synthesis and characterization of 3, we demonstrate the successful conjugation of this trifunctional agent 3 to trastuzumab. The resulting radiolabeled immunoconjugate 2 (Figure 1) showed excellent tumor cell binding capabilities comparable with trastuzumab and thus may be used as a tumor-targeting probe for dual modality PET (or SPECT) and fluorescence imaging.
RESULTS AND DISCUSSION
In current clinical practice multimodality imaging usually utilizes different imaging agents for each modality. For example, in PET/CT, 18F-deoxyglucose is used for the PET component and iodinated contrast media is used for the CT. If the same imaging agent possessed the signaling components for more than one imaging modality it would be advantageous as it would require a single injection and exact co-localization of pathology based on two or more different parameters. By providing the imaging agents on the same vector, differences in the distribution of the agents would be minimized if not eliminated. This is one of the advantages of multimodality imaging agents such as the trifunctional agent, 3, presented in this study.
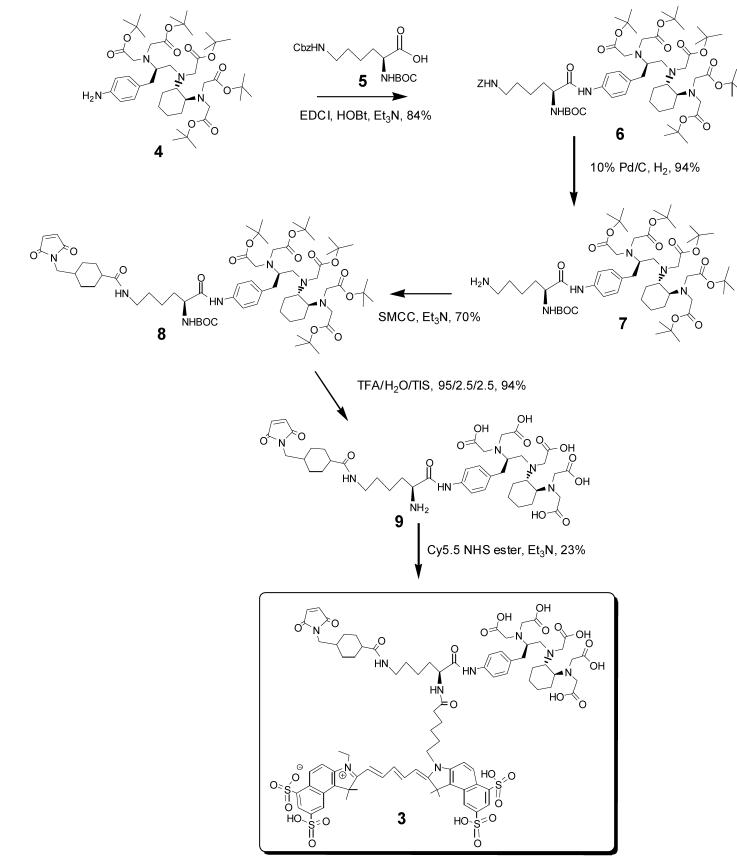
For the synthesis of 3, we chose to take advantage of the selectively protected lysine derivative, N-α-BOC-N-ε-benzyloxycarbonyl-L-lysine, which possesses a carboxylic group and two NH2 groups with distinguishable reactivity.12 Other diaminocarboxylate compounds were considered as starting material for the core of 3, however, this particular lysine derivative was readily available. The synthetic route for preparing 3 is shown in Figure 2. In brief, p-amino functionalized CHX-A” 4 was reacted with lysine derivative 5 using standard peptide coupling conditions to provide adduct 6 (84%). The ε-NH2 benzyl carbamate (Z) protecting group on 6 was quantitatively cleaved by catalytic hydrogenation as monitored by TLC. The liberated ε-NH2 group was reacted with succinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate to yield maleimide 8 (70%). Incorporation of a maleimide function in this step provides a highly reactive group towards thiol groups either extant or introduced into proteins or peptides. The efficient maleimide-thiol coupling chemistry can be performed for the convenient-synthesis of dye-chelate-mAb immunoconjugates. Cleavage of the tert-butyl ester on the CHX-A” moiety and the tert-butyl carbamate (BOC) on the α-NH2 of the lysine were cleanly achieved in one step by treatment of 8 with TFA/H2O/TIS (95:2.5:2.5). Finally, NIR dye Cy5.5 mono NHS ester was reacted with the α-NH2 group of 9 in DMSO. Purification by reverse-phase HPLC using a C18 column gave 3 in ∼ 23% yield. It is noteworthy that the free α-NH2 group in 9 provides almost limitless possibilities of dye options that can be introduced and that, in principle, any dye bearing active ester or isothiocyanate moieties might be chosen. By our design, the NIR dye was intended to be coupled to the lysine core in the final step of the synthesis primarily because quantum yields of Cy dyes are significantly decreased after treatment with strong acids (e.g. TFA). Another reason for incorporating Cy dyes last is related to the high cost(s) of NIR dyes. Compound 3 was fully characterized by 1H NMR, ES-MS and HPLC. In the 1H NMR spectra, the aromatic protons of 3 are consistent with those of the published NMR data of Cy 5.5 dye except two additional doublet peaks from the CHX-A” fragment. Negative ion ES-MS of 3 gave a m/z of 1797.6 and 897.8 for [M-H]- and [M-2H]2- respectively, which confirmed its identity. The HPLC spectra of 3 showed a single, symmetric peak, supportive of its high purity (see supporting information).
Figure 2.
Synthesis of trifunctional chelate Cy5.5-Lys(SMCC)-CHX-A”, 3
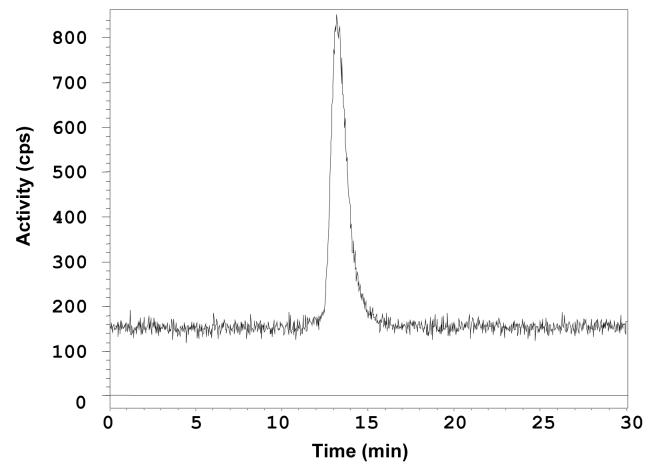
Metal ion complexation of 111In was demonstrated by reversed-phased HPLC of the radiolabeled 3 (111In-3) under neutral pH conditions. Time-resolved elution of 3 (15.0 min) and 111In-3 (16.8 min) was observed following a no-carrier-added complexation reaction, evident from a nearly 2 minute separation between the radiometric and UV peaks, respectively. An analogous carrier-added (with natIn) radiolabeling resulted in convergence of the UV peak (16.9 min) and the radiometric peak for 111In-3 (17.1 min). To conjugate 3 to trastuzumab, the mAb was first dialyzed into the thiolation buffer (50 mM NaHCO3, 150 mM NaCl, and 10 mM EDTA, pH 8.6) and then reacted with 15 equivalents of Traut’s agent using standard procedure.13 Excess reagents were removed by passing the reaction solution through a PD-10 column. The resulting -SH groups on trastuzumab were quantitated by Ellman’s reagent.14,15 In our hands, ∼3.5 -SH groups per trastuzumab were introduced as calculated based on the molar absorptivity at 412 nm. The thiolated trastuzumab was then reacted with 5 equivalents of 3 in the dark at RT for 1 hour to produce compound 2. The unreacted thiols were capped with iodoacetamide to minimize cross-linking of antibody product and to permit a longer shelf life for the immunoconjugate. Finally, the reaction mixture was dialyzed into phosphate-buffered saline (PBS; pH 7.2) at 4°C with 4 buffer changes over 48 hours. The final protein concentration was 0.901 mg/mL. The number of Cy5.5 dye moieties per trastuzumab were calculated to be ∼ 2.4 based on UV absorption at 675 nm, which corresponds to the same number of CHX-A” chelates on trastuzumab according to the 1:1 ratio of Cy5.5 and CHX-A” within 3. Thus, not only does incorporation of Cy5.5 provide a NIR imaging modality in addition to the potential of PET or SPECT imaging, but also provides a direct readout of the degree of protein modification in much the same way that a 14C labeled chelate would provide.
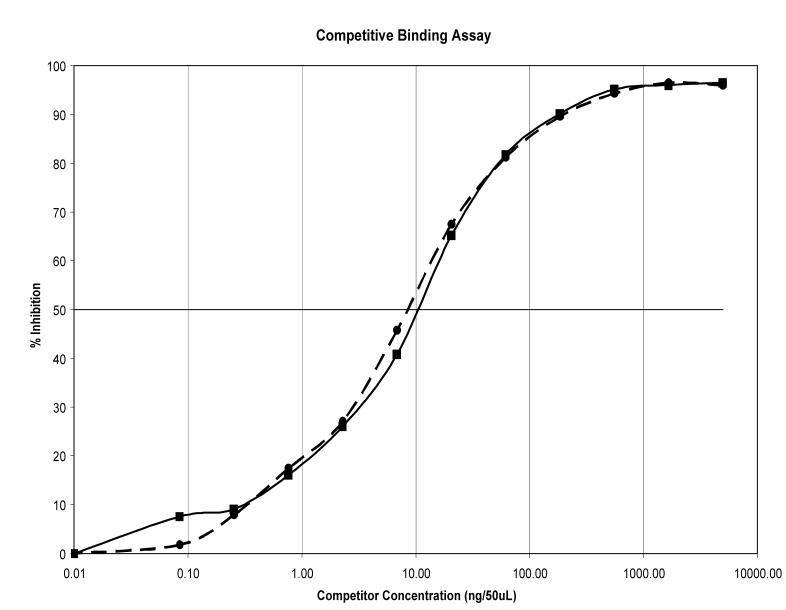
The immunoreactivity of 2 was found to be retained using three in vitro analytical methods. Analysis by flow cytometry (Figure 3) demonstrates the immunoconjugate’s ability to bind HER2; 99.2 % of the cells were positive with a mean fluorescence intensity of 106. The competition radioimmunoassay (RIA) demonstrates that modification of trastuzumab with the trifunctional chelate did not affect immunoreactivity as illustrated in Figure 4. The amount of trastuzumab modified with the trifunctional agent 3 required for 50% inhibition was comparable to the amount of native trastuzumab (10 ng vs. 9 ng).
Figure 3.
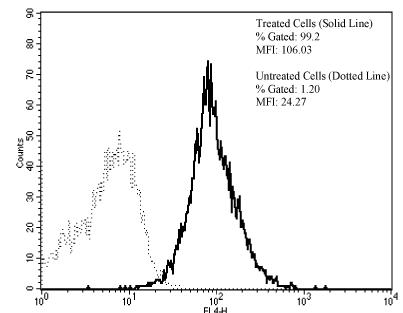
Conjugate 2 was evaluated by flow cytometric analysis. Cells incubated with 2 (solid line) were 99.2 % positive with a MFI of 106.0 while those in buffer (dotted line) only were 1.2 % positive with a MFI of 24.3.
Figure 4.
A competition radioimmunoassay was conducted to demonstrate that modification with 3 did not alter immunoreactivity of trastuzumab. The ability of 2 (solid line) to react with HER2 expressing SKOV-3 cells and to displace 111In-trastuzumab was compared to unmodified trastuzumab (dashed line)
Immunoconjugate 2 was radiolabeled efficiently (>79%) with the SPECT radionuclide 111In within 30 min at room temperature. Figure 5 shows the size exclusion HPLC profile of the labeled product.
Figure 5.
SE-HPLC chromatograph of 111In-2
The immunoreactivity of radiolabeled 2 (111In-2) was then assessed in a direct binding RIA and compared to radiolabeled trastuzumab modified with just the CHX-A”-DTPA chelate. The percent binding of the 111In-2 and 111In-trastuzumab was 67.7 and 57.9 percent, respectively. In the presence of excess unlabeled 2 the values were 17.4% for the 111In-2 and 8.4% for 111Indium-trastuzumab. These values were comparable to those obtained, 13.8% and 7.8%, when excess unmodified trastuzumab was added in the presence of 111In-2 and 111Indium-trastuzumab, respectively.
Radiolabeling of the trifunctional chelating agent and its conjugates was facile and this should be readily applicable to other radionuclides such as 86Y for PET imaging. The ability to label the same vector with both SPECT/PET and optical imaging agents has the advantage of obtaining both a quantitative gross deep tissue assessment and micro-cellular distribution using the same molecule. Products of the trifunctional chelate would also potentially be amenable for use in endoscopy and intraoperative procedures to aid in mapping the borders of tumors during surgery. Lastly, we also note that the chemistry described herein is highly flexible. Variations of both chelating chemistry to adjust for other radionuclides of interest combined with the vast array of available dyes suitable for in vivo imaging open a broad spectrum of imaging application for such agents when conjugated to suitable targeting vectors such as monoclonal antibodies, peptides, and possibly to small molecular weight cell surface receptor agents.
In addition, through this modular strategy of synthesizing the trifunctional agent 3 and its conjugates, potent cytotoxic chemotherapeutic agents, such as geldanamycin or doxorubicin, in principle, can be also chemically modified and then attached with either a chelating moiety CHX-A” or a fluorescent dye Cy5.5, generating a single agent for both therapeutic and imaging and tracking purposes. Therefore, our synthetic approach opens an extensive range of possibilities for combination of different modality imaging agents and/or therapeutic compounds for cancer imaging and therapy.
Ongoing studies are evaluating the combination of 86Y PET imaging with Cy5.5 NIR optical imaging in both subcutaneous and intraperitoneal xenograft tumor targeting model systems. Results will be forthcoming in the appropriate venue.
MATERIALS AND PROCEDURES
N-[(R)-2-Amino-3-(p-aminophenyl)propyl]-trans-(S,S)-cyclohexane-1,2-diamine-N,N,N’,N”,N”-penta-tert-butylacetate (4) was prepared by the previously described procedure.16 Boc-Lys(Z)-OH (5) was purchased from Novabiochem (San Diego, CA). Succinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate (SMCC) was purchased from Pierce Biotechnology (Rockford, IL). Cy5.5 mono NHS ester was obtained from GE Healthcare (Piscataway, NJ). N-hydroxysuccinimide (NHS), 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride (EDCI), peptide sequence grade trifluoroacetic acid were acquired from Aldrich/Sigma Chemical Company (St. Louis, MO) and used as received. All experiments with moisture- and/or air-sensitive compounds were carried out under a dried N2 or Ar atmosphere. For column chromatography, Merck 60 Silica Gel was used (70-230 mesh). Thin-layer chromatography (TLC) was performed on silica gel 60 F-254 plates from EM Reagents. All water used was purified using a Hydro Ultrapure Water Purification system (Rockville, MD).
Proton and 13C NMR data were obtained using a Varian Gemini 300 MHz instrument and chemical shifts are reported in ppm on the δ scale relat ive to TMS, TSP or residual solvent. Proton chemical shifts are annotated as follows: ppm (multiplicity, coupling constant (Hz), integration). Low and high resolution mass spectra (HRMS) were obtained on a Waters’ LCT Premier time-of-flight mass spectrometer using electrospray ionization (ESI/TOF/MS) in positive ion mode operated at a resolution of 10000. The electrospray capillary voltage was 3kV and the sample cone voltage was 60V. Desolvation temperature was 225°C and the desolvation gas was nitrogen at 300 L/hr. Accurate masses were obtained using the lock spray mode with Leu-Enkephalin as the external reference compound.
Trifunctional chelate 3 was purified by reversed-phase HPLC (RP-HPLC) using a Gilson system equipped with Model 811B solvent mixer and Knauer variable wavelength monitor controlled by Gilson Unipoint System Software. RP-HPLC was performed on a Vydac 5μm C18 reversed-phase 10 mm × 25 cm column equilibrated with 15 mM NH4OAc (pH 7). A gradient of CH3CN that increased from 0% at 0 min to 50% at 30 min was employed.
Boc-Lys(Z)-CHX-A” penta-tert-butyl ester (6)
Boc-Lys(Z)-OH 5 (0.50g, 1.32mmol), EDCI (0.51g, 2.64mmol), HOBt (0.36g, 2.64mmol), and DIPEA (0.23mL,1.32mmol) were added to a stirred solution of 4 (1.00g, 1.20mmol) in DMF (15mL). The mixture was stirred for 24h at room temperature (RT) and then concentrated under reduced pressure, diluted with CH2Cl2 (100mL), and washed successively with water (2 × 100 mL), 5% NaHCO3 (2 × 100 mL), and water (1 × 100 mL). The organic layer was concentrated and the residue was chromatographed on silica gel eluted with EtOAc-EtOH (9:1) to afford 6 (1.20g, 84%). 1H NMR (DMSO-d6, 300Hz) δ 9.77 (s, 1H), 7.45 (d, J = 8.7 Hz, 2H), 7.33 (m, 5H), 7.23 (t, J = 6.9 Hz, 1H), 7.16 (d, J = 8.4 Hz, 2H), 6.92 (d, J = 8.1 Hz, 1H), 4.99 (s, 2H), 4.03 (m, 1H), 3.43 (s, 2H), 3.32 (s, 8H), 2.96 (m, 4H), 2.59 (m, 2H), 1.91 (m, 2H), 1.70-1.20 (m, 10H), 1.37 (s, 54H), 1.03 (m, 4H); 13C NMR: 172.1, 171.9, 171.8, 171.6, 171.5, 170.1, 156.7, 156.2, 137.9, 136.6, 135.4, 130.0, 128.6, 128.2, 119.5, 80.4, 80.3, 80.25, 80.2, 66.7, 64.1, 63.2, 62.7, 54.0, 53.6, 53.1, 52.2, 40.3, 36.2, 31.4, 29.5, 29.3, 28.5, 28.4, 28.1, 27.1, 26.1, 25.9, 22.6; HRMS: calcd for C64H103N6O15 [M + H]+: 1195.7481, found 1195.7506; Anal. Calcd. for C64H102N6O15·0.5H2O: C, 63.82; H, 8.62; N, 6.98. Found: C, 63.67; H, 8.57; N, 6.88.
Boc-Lys-CHX-A” penta-tert-butyl ester (7)
A solution of 6 (0.39 g, 0.33 mmol) in MeOH (15mL) was treated with 10% Pd/C (50 mg) and stirred under a hydrogen atmosphere overnight (normal pressure, supply of hydrogen from a balloon). The mixture was filtered through Celite 535 (Fluka) and concentrated. The residue was dried under high vacuum to give amine 7 (0.33 g, 94%), which was used without further purification. 1H NMR (DMSO-d6) δ 9.81(s, 1H), 7.45(d, J = 8.4 Hz, 2H), 7.16(d, J = 8.1 Hz, 2H), 6.95(d, J = 7.0Hz, 1H), 4.12(m, 1H), 3.44(s, 2H), 3.33(s, 8H), 2.96(m, 2H), 2.80-2.40(m, 6H), 1.89(m, 2H), 1.59(m, 4H), 1.39(s, 54H), 1.50-1.20(m, 6H), 1.03(m, 4H); HRMS: calcd for C56H97N6O13 [M + H]+: 1061.7114, found 1061.7140; Anal. Calcd. for C56H96N6O13·H2O: C, 62.31; H, 9.15; N, 7.79. Found: C, 62.09; H, 9.00; N, 8.09.
Boc-Lys(SMCC)-CHX-A” penta-tert-butyl ester (8)
To a solution of amine 7 (0.33 g, 0.31 mmol) in DMF (10 mL) was added succinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate (SMCC) (0.10 g, 0.30 mmol) and Et3N (0.04 mL, 0.31 mmol). The mixture was stirred under N2 atmosphere at room temperature for 18h. Afterwards, the solution was concentrated and the residue was diluted with CH2Cl2 (100 mL) and washed successively with water (1 × 100 mL), 5% NaHCO3 (1 × 100 mL), and water (1 × 100 mL). The organic layer was concentrated and chromatographed on silica gel eluted with EtOAc/EtOH (10:0-9:1) to afford 8 as a white solid (0.27g, 70%). 1H NMR (DMSO-d6, 300MHz) δ 9.78 (s, 1H), 7.64 (t, J = 6.0 Hz, 1H), 7.44 (d, J = 7.8 Hz, 2H), 7.16 (d, J = 8.4 Hz, 2H), 7.02 (s, 2H), 6.88 (d, J = 7.0 Hz, 1H), 4.01 (m, 1H), 3.43 (s, 2H), 3.32 (s, 8H), 3.23 (d, J = 6.3 Hz, 2H), 2.97 (m, 4H), 2.59 (m, 4H), 2.01(m, 2H), 1.80-1.00 (3 × m, 22H), 1.37 (s, 54H); HRMS: calcd for C68H110N7O16 [M + H]+: 1280.8014, found 1280.8040; Anal. Calcd. for C68H109N7O14·1.1H2O: C, 62.80; H, 8.62; N, 7.54. Found: C, 62.46; H, 8.15; N, 7.44.
NH2-Lys(SMCC)-CHX-A” (9)
Lysine derivative 8 (0.25 g, 0.20 mmol) was stirred with 10 mL of TFA/TIS/H2O (9.5/0.25/0.25) for 4h. The reaction mixture was concentrated in vacuum to afford amine 9 (0.17 g, 94%). 1H NMR (DMSO-d6, 300MHz) δ 10.60(s, 1H), 7.76(t, J = 6.0 Hz, 1H), 7.63(d, J = 7.8 Hz, 2H), 7.37(d, J = 8.4 Hz, 2H), 7.11(s, 2H), 4.05(m, 1H), 3.80-2.60 (m, 20H), 1.80-0.90(m, 25H), HRMS: calcd for C43H62N7O14 [M + H]+: 900.4355, found 900.4331.
Cy5.5-Lys(SMCC)-CHX-A” (3)
To a solution of amine 9 (0.84 mg, 0.94 μmol) in DMSO (2 mL) was added Cy5.5 Mono NHS ester (1.0 mg, 0.88 μmol) and Et3N (20 μL). The reaction mixture was stirred under N2 atmosphere at room temperature for 18h and then diluted with diethyl ether (20mL). The precipitated product was purified by reverse-phase (RP) analytical HPLC on a C18 column using 0%-50% CH3CN in 15mM NH4OAc in a 30 min run followed by lyophilization of the solvent from the relevant fraction to yield trifunctional chelate 3 (0.36mg, 22.7%). 1H NMR (D2O, 300MHz) δ 8.85(d, J = 9.0 Hz, 2H), 8.80(s, 2H), 8.36(m, 2H), 8.25(d, J = 9.0 Hz, 2H), 7.83(d, J = 9.3 Hz, 1H), 7.81(d, J = 9.6 Hz, 1H), 7.43(d, J = 8.1 Hz, 2H), 7.29 (d, J = 8.1 Hz, 2H), 6.67(s, 2H), 4.20 (br triplet, 4H), 3.80-2.60(m, 22H), 2.34(t, J = 6.0Hz 2H), 2.15-1.00(m, 46H); ES-MS m/z: calcd for C84H104N9O27S4 [M-H]-, [M-2H]2-: 1796.6, 897.8 found 1797.6, 897.8.
Radiosynthesis and characterization of Cy5.5-Lys(SMCC)-111In-CHX-A” (111In-3)
A 200-μCi portion of 111In (Perkin Elmer, Wellesley, MA) in 0.05 N HCl was added to Cy5.5-Lys(SMCC)-CHX-A” dissolved in 0.15M NH4OAc pH 7. The reaction mixture was incubated at 37 °C for 30 min. An aliquot of the resulting solution was analyzed by RP-HPLC using a Vydac Protein & Peptide C18 column equilibrated with 0.015 M NH4OAc (pH 7). A 0.0 to 50% gradient of increasing CH3CN over 30 min was employed, followed by an additional 10-min plateau at 50% CH3CN. A UV detector and radiometric detector were coupled to measure absorbance at 254 nm and radioactivity, respectively.
Conjugation of 3 to Trastuzumab (General Procedure)
Trastuzumab was dialyzed into thiolation buffer (50 mM NaHCO3, 150 mM NaCl, and 10 mM EDTA, pH 8.6) and reacted with Traut’s reagent13 (Sigma Chemical Co., St. Louis, MO) at a 1:15 molar ratio for 45 min at room temperature. These conditions were empirically determined to yield ∼3.5 -SH groups per trastuzumab molecule. Excess Traut’s reagent was removed by passage of the reaction solution through a PD-10 column eluted with the thiolation buffer. The -SH concentration was measured using Ellman’s reagent.15 Just prior to protein conjugation, 3 was dissolved in thiolation buffer and then added drop wise to the mAb solution to achieve a molar reaction ratio of 5:1 (3:trastuzumab) and gently vortexed. The solution was gently agitated in the dark at 25°C for 1h. Excess free SH groups were capped with iodoacetamide solution (2.0 mM). Finally, the reaction mixture was dialyzed into PBS buffer at 4°C with 4 buffer changes over 48 h.
111In Labeling of the Immunoconjugates
The trifunctionalized immunoconjugate, 2, and CHX-A”-Herceptin, 1, were labeled with 111In.17 The pH of a solution of 111InCl3 in 0.05 M HCl (0.85 mCi, 2.5 μL) in a vial was adjusted to 4.5-5.0 with aqueous NH4OAc (150 μL, 0.15 M, pH 7.0). Solutions of the respective immunoconjugates in PBS (50 μg, 8 - 50 μL) were brought up to 100 μL with NH4OAc (0.15 M, pH 7.0) and then added to the 111In solution, mixed, and incubated at room temperature for 30 min. The reaction was quenched by the addition of a solution of DTPA (10 μL, 0.001 M, pH 6.5). Protein bound 111In was separated from unbound 111In using a PD-10 column pre-equilibrated and eluted with PBS. The labeling efficiency ranged from 79 - 96%. Figure 7 shows the size exclusion HPLC profile of the 111In-2.
Characterization of Trastuzumab-trifunctional Chelate Conjugate
The final protein concentration of the immunoconjugate was quantified by the Lowry method18 with a bovine serum albumin (BSA) standard. In order to assess the final Cy5.5 dye concentration of the trastuzumab-trifunctional chelate conjugate, approximately 1.5 mL of the final product was added to a cuvette and the absorbance measured at 675 nm in a Beckman-Coulter DU 520 spectrophotometer (Beckman-Coulter, Inc., Fullerton, CA, USA).
Cell Culture
The ovarian adenocarcinoma cell line SKOV3, obtained from ATCC (Manassas, VA), was used for experiments assessing imunoreactivity. SKOV-3 has been shown to express at least 5 × 105 HER2 molecules per cell.19 The cells were grown in McCoy’s 5A medium (Quality Biological Inc., Gaithersburg, MD) containing 10% Fetalplex (Gemini Bioproducts, Woodland, CA) and 1% non-essential amino acids (BioWhittaker, Walkersville, MD) in a humidified incubator at 37°C in 5% CO2.
Immunoreactivity Studies
The immunoreactivity of 2 to SKOV3 cells was determined by flow cytometry, competition radioimmunoassay, and radioimmunoassay.
Flow cytometry
Reactivity of 2 was confirmed with HER2 expressing SKOV3 cells using flow cytometric methods as previously described. The cells were incubated with 1.25 ug of trastuzumab for 1 h at 4° C in PBS containing 1% BSA, pH 7.2 (BSA-PBS). Following three washes, the cells were then re-suspended in 1 mL of PBS. The cells (10,000 events collected) were analyzed using a FACSCalibur flow cytometer with CellQuest software (Becton-Dickinson, Franklin Lakes, NJ).
Competition Radioimmunoassay
In preparation for the competition RIA, SKOV3 cells (∼ 3 × 105 cells/well) were plated on a 96-well plate and incubated for 6 d. The wells were washed with cold PBS and 200 μL of a cold 80% MeOH solution was added to each well. Following an overnight incubation at 4° C, the wells were washed three times with 200 μL of PBS and 200 μL of 1% BSA-PBS was added. After 30 min at RT, the wells were aspirated and serial dilutions of 2 (5000 ng - 0.08 ng) were then added to the wells in triplicate (50 μL) followed by the addition of ∼33 nCi (50 μL in BSA-PBS) of 111In-trastuzumab. The RIA was incubated, overnight, at RT. On the following day, the wells were washed three times with 200 μL of BSA-PBS. The radioactivity was removed from the wells by adding 100 μL of 0.2 M NaOH, gently shaking the plate for 10 min and adsorbing the solution to filters (Molecular Device Corp., Sunnyvale, CA). The filters were placed in 12×75 mm polypropylene tubes and the radioactivity counted in a γ-scintillation counter (WizardOne, PerkinElmer, Shelton, CT). The assay included unmodified trastuzumab as well as HuM195, an anti-CD33 humanized mAb, (a generous gift from D. Scheinberg, Memorial Sloan Kettering Cancer Center),20 served as a negative control. The assay also included wells to which no competitor was added. The percent inhibition was calculated and plotted.
Radioimmunoassay (RIA)
The immunoreactivity of the 111In-2 was assessed using methanol-fixed SKOV3 cells. Briefly, the cells were trypsinized, pelleted, and re-suspended in 5 mL of PBS. Cold methanol (20 mL) was then added dropwise while vortexing to yield a final solution of 80% methanol. After sitting overnight at 4° C, the cells were washed with PBS, pelleted, re-suspended in BSA-PBS and aliquoted (2×106 in 50 μL) into 12×75 mm polypropylene tubes. Serial dilutions (∼84 to ∼ 2 nCi) of 111In-2 or 111In-trastuzumab (for comparison) were added to the cells in duplicate and gently vortexed. The cells were washed with BSA-PBS following an overnight incubation at RT, pelleted and the radioactivity measured in a γ-scintillation counter (WizardOne,). The percent binding was calculated for each dilution and the values presented represent an average of the serial dilutions. In order to confirm the specific reactivity of the 111In-2, additional cells were incubated with ∼ 84 nCi of the 111In-2 along with an excess (10 μg) of either unlabeled trastuzumab or unlabeled 2.
Supplementary Material
Acknowledgment
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We would also like to acknowledge the illuminating assistance from Kristian Birkeland in regards to our optical imaging research.
Supporting Information
Analytical data and spectra of intermediate compounds 3, 6-9 are presented as supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.
Abbreviations
- PET
positron emission computed tomography
- CT
computed tomography
- MRI
magnetic resonance imaging
- NIR
near-infrared spectroscopy
- OI
optical imaging
- SPECT
single photon emission computed tomography
- DTPA
diethylenetriaminepentaacetic acid
- PAMAM
polyaminonamido
- G6
generation 6
- Cy
cyanine dyes
- mAbs
monocolonal antibodies
- 1B4M
2-(4-isothiocyanatobenzyl)-6-methyl-diethylenetriamine pentaacetic acid
- CHX-A”
N-[(R)-2-Amino-3-(p-aminophenyl)propyl]-trans-(S,S)-cyclohexane-1,2-diamine-N,N,N’,N”,N”-pentaacetic acid
- BOC
t-butoxycarbonyl
- Z
benzyloxy carbonyl
- TFA
trifluoroacetic acid
- TIS
triisopropylsilane
- PBS
phosphate-buffered saline
- RIA
radioimmunoassay
- SMCC
succinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate
- NHS
N-hydroxysuccinimide
- EDCI
1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride
- HOBt
1-hydroxybenzotriazole.
References
- 1.Moseley M, Donnan G. Multimodality imaging. Stroke. 2004;35:2632–2634. [Google Scholar]
- 2.Blodgett TM, Meltzer CC. Townsend DW. PET/CT: form and function. Radiology. 2007;242:360–85. doi: 10.1148/radiol.2422051113. [DOI] [PubMed] [Google Scholar]
- 3.Jaffer FA, Weissleder R. Molecular imaging in the clinical arena. JAMA. 2005;293:855–862. doi: 10.1001/jama.293.7.855. [DOI] [PubMed] [Google Scholar]
- 4.Hüber MM, Staubli AB, Kustedjo K, Gray MHB, Shih J, Fraser SE, Jacobs RE, Meade TJ. Fluorescently detectable magnetic resonance imaging agents. Bioconjugate Chem. 1998;9:242–249. doi: 10.1021/bc970153k. [DOI] [PubMed] [Google Scholar]
- 5.Dirksen A, Langereis S, Waal B. F. M. d., Genderen M. H. P. v., Meijier EW, Lussanet Q. G. d., Hackeng TM. Design and synthesis of a bimodal target-specific contrast agent for angiogenesis. Org. Lett. 2004;6:4857–5860. doi: 10.1021/ol048084u. [DOI] [PubMed] [Google Scholar]
- 6.Manning HC, Goebel T, Marx JN, Bornhop DJ. Facile, Efficient conjugation of a trifunctional lanthanide chelate to a peripheral benzodiazepine receptor ligand. Org. Lett. 2002;4:1075–1078. doi: 10.1021/ol017155b. [DOI] [PubMed] [Google Scholar]
- 7.Talanov VS, Regino CAS, Kobayashi H, Bernardo M, Choyke PL, Brechbiel MW. Dendrimer-based nanoprobe for dual modality magnetic resonance and fluorescence imaging. Nano Lett. 2006;6:1459–1463. doi: 10.1021/nl060765q. [DOI] [PubMed] [Google Scholar]
- 8.Carter P. Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer. 2001;1:118–129. doi: 10.1038/35101072. [DOI] [PubMed] [Google Scholar]
- 9.Milenic DE, Brady ED, Brechbiel MW. Antibody-targeted radiation cancer therapy. Nature Rev. Drug Disc. 2004;3:488–499. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 10.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma DS, Abdulla A, Brechbiel MW. Targeting of HER2 antigen for the treatment of disseminated peritoneal disease. Clin. Cancer Res. 2004;10:7834–7841. doi: 10.1158/1078-0432.CCR-04-1226. [DOI] [PubMed] [Google Scholar]
- 11.Blend MJ, Stastny JJ, Swanson SM, Brechbiel MW. Labeling anti-HER2/neu monoclonal antibodies with 111In and 90Y using a bifunctional DTPA chelating agent. Cancer Biother. Radiopharm. 2003;18:355–363. doi: 10.1089/108497803322285107. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Kinsel GR, Zhang J, Li M, Rudkevich DM. Calixarene amino acids; building blocks for calixarene peptides and peptide-dendrimers. Tetrahedron. 2003;59:5837–5848. [Google Scholar]
- 13.Traut RR, Bolen A, Sun TT, Hershey JWB, Sundberg J, Pierce LR. Methyl 4-mercaptobutyrimidate as a cleavable crosslinking reagent and its application to the Escherichia coli 30S ribosome. Biochemistry. 1973;12:3266–3273. doi: 10.1021/bi00741a019. [DOI] [PubMed] [Google Scholar]
- 14.Ellman GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 15.Riddles PW, Blakeley RL, Zerner B. Ellman’s reagent: 5, 5′-dithiobis(2-nitrobenzoic acid) - a reexamination. Anal. Biochem. 1978;94:75–81. doi: 10.1016/0003-2697(79)90792-9. [DOI] [PubMed] [Google Scholar]
- 16.Clifford T, Boswell AC, Biddlecombe GB, Lewis JS, Brechbiel MW. microPET/CT Imaging using 86Y-CHX-A”-Octreotide: Validation of a novel CHX-A” derivative suitable for peptide conjugation. J. Med. Chem. 2006;49:4297–4304. doi: 10.1021/jm060317v. [DOI] [PubMed] [Google Scholar]
- 17.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma D, Abdulla A, Brechbiel MW. Targeting of HER2 Antigen for Radioimmunotherapy of Disseminated Peritoneal Disease using 213Bi-Labeled Herceptin. Clin. Cancer Res. 2004;10:7712–7720. doi: 10.1158/1078-0432.CCR-04-1226. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall AJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Xu F, Yu Y, Le XF, Boyer C, Mills GB, Bast RC., Jr. The outcome of Heregulin-induced activation of ovarian cancer cells depends on the relative levels of HER-2 and HER-3 expression. Clin. Cancer Res. 1999;5:3653–3660. [PubMed] [Google Scholar]
- 20.Nikula TK, McDevitt MR, Finn RD, Wu C, Kozak RW, Garmestani K, Brechbiel MW, Curcio MJ, Pippin CG, Tiffany-Jones L, Geerlings MW, Sr., Apostolidos C, Molinet R, Geerlings MW, Jr., Gansow OA, Scheinberg DA. Alpha-Emitting Bismuth Cyclohexylbenzyl DTPA Constructs of Recombinant Humanized Anti-CD33 Antibodies: Pharmacokinetics, Bioactivity, Toxicity and Chemistry. J. Nucl. Med. 1999;40:166–176. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.