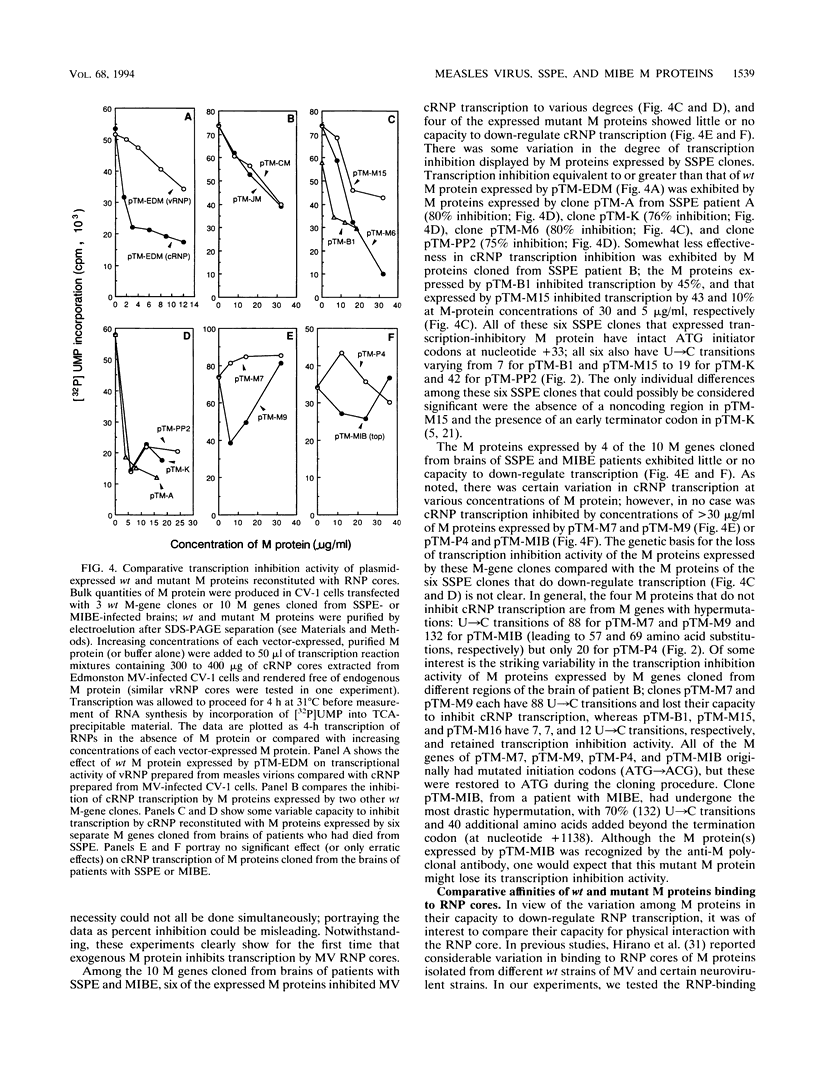
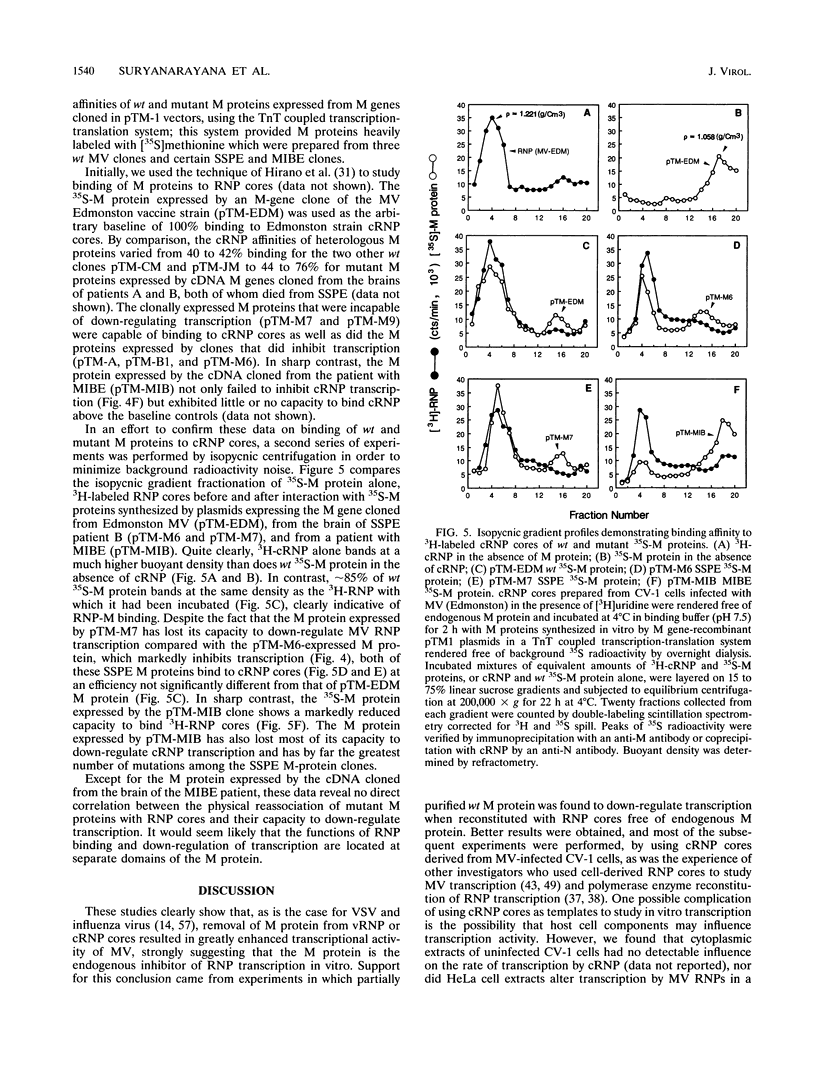
Abstract
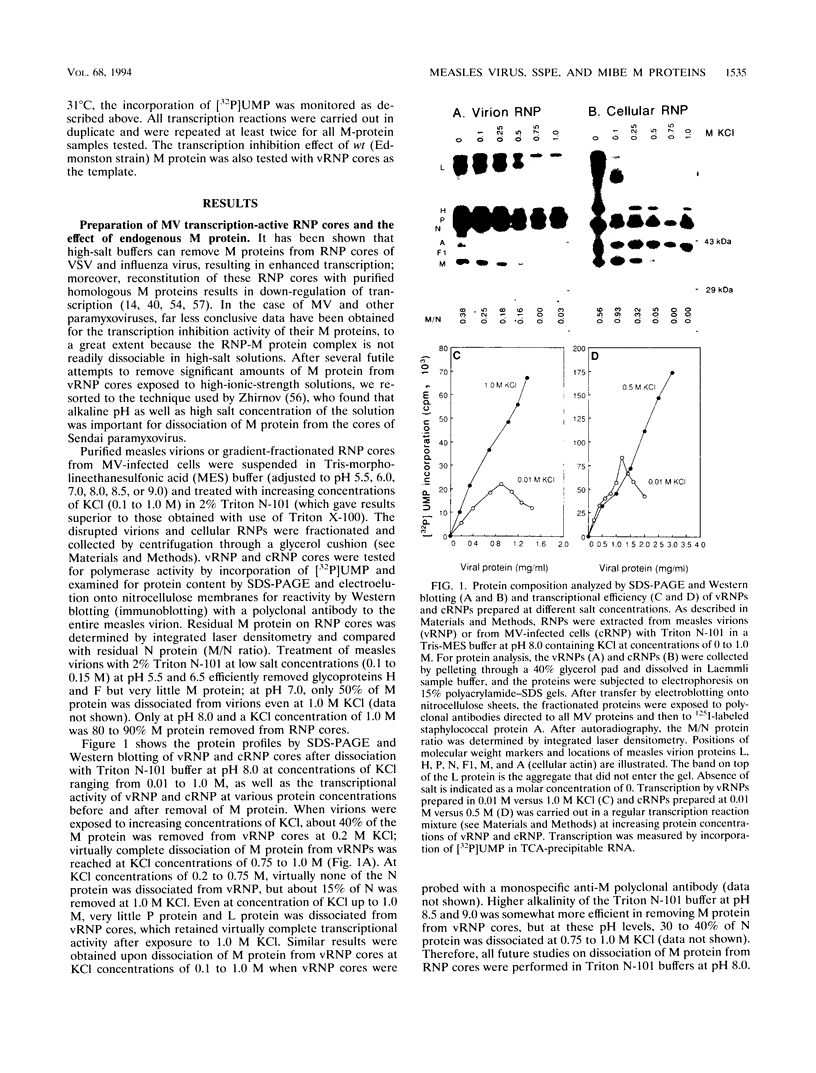
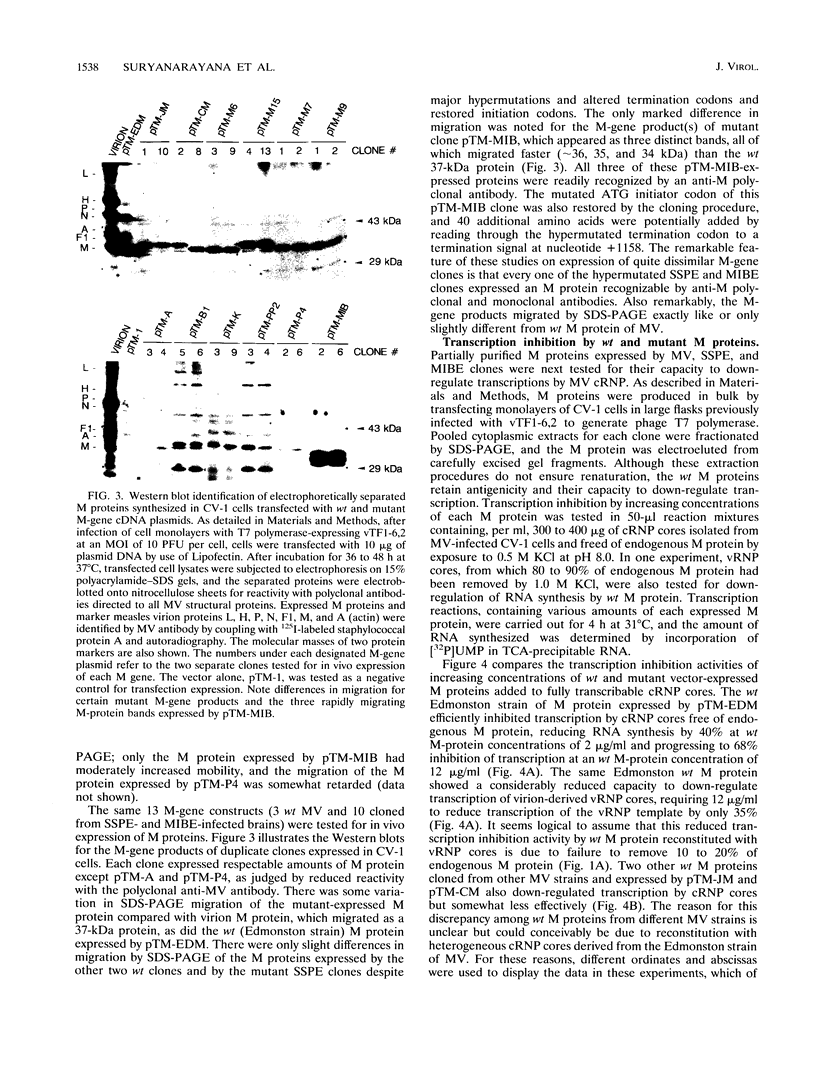
Ribonucleoprotein (RNP) cores extracted from virions of wild-type (Edmonston strain) measles virus (MV) or obtained from MV-infected cells (cRNP) were shown to be capable of transcribing RNA in vitro but at relatively low efficiency. The tightly bound matrix (M) protein could be effectively removed from virion RNP (vRNP) and from cRNP by exposure to buffers of high ionic strength (0.5 to 1.0 M KCl) but only at pH 8.0 or higher. The vRNP and cRNP cores complexed with M protein exhibited markedly reduced transcriptional activity at increasing concentrations, whereas vRNP and cRNP cores free of M protein exhibited linear and substantially higher transcriptional activity; these data suggest that M protein is the endogenous inhibitor of MV RNP transcription. M-gene cDNA clones derived from three strains of wild-type (wt) MV and 10 clones from mRNAs isolated from the brain tissue of patients who had died from subacute sclerosing panencephalitis (SSPE) and from measles inclusion body encephalitis (MIBE) were recloned in the pTM-1 expression vector driven by the bacteriophage T7 RNA polymerase expressed by a coinfecting vaccinia virus recombinant. All 10 mutant SSPE and MIBE clones expressed in vitro and in vivo M proteins that reacted with monospecific anti-M polyclonal antibody and migrated on polyacrylamide gels to positions identical to or only slightly different from those of the M proteins expressed by wt MV clones. When reconstituted with cRNP cores, the three expressed wt M proteins and 6 of the 10 mutant-expressed M proteins showed equivalent capacity to down-regulate MV transcription. Three of the M proteins from SSPE clones and one from the MIBE clone showed little or no capacity to down-regulate transcription when reconstituted with cRNP cores. The only plausible explanations for loss of transcription inhibition activity by the four SSPE/MIBE M proteins were exceedingly high degrees of hypermutations leading to U-->C transitions and cloning-corrected mutations in the initiator codon (ATG-->ACG) of the four M genes. However, only the hypermutated M protein expressed by the MIBE cDNA clone exhibited virtually no capacity to bind cRNP cores in a reconstitution assay. These experiments provide some preliminary data to support the hypothesis that MV encephalitis may result from certain selective mutations in the M gene.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayata M., Hirano A., Wong T. C. Structural defect linked to nonrandom mutations in the matrix gene of biken strain subacute sclerosing panencephalitis virus defined by cDNA cloning and expression of chimeric genes. J Virol. 1989 Mar;63(3):1162–1173. doi: 10.1128/jvi.63.3.1162-1173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Brinckmann U., Pardowitz I., Rima B. K., ter Meulen V. Nucleotide sequence of the genes encoding the matrix protein of two wild-type measles virus strains. J Gen Virol. 1991 Sep;72(Pt 9):2279–2282. doi: 10.1099/0022-1317-72-9-2279. [DOI] [PubMed] [Google Scholar]
- Baczko K., Carter M. J., Billeter M., ter Meulen V. Measles virus gene expression in subacute sclerosing panencephalitis. Virus Res. 1984 Oct;1(7):585–595. doi: 10.1016/0168-1702(84)90015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Lampe J., Liebert U. G., Brinckmann U., ter Meulen V., Pardowitz I., Budka H., Cosby S. L., Isserte S., Rima B. K. Clonal expansion of hypermutated measles virus in a SSPE brain. Virology. 1993 Nov;197(1):188–195. doi: 10.1006/viro.1993.1579. [DOI] [PubMed] [Google Scholar]
- Baczko K., Liebert U. G., Billeter M., Cattaneo R., Budka H., ter Meulen V. Expression of defective measles virus genes in brain tissues of patients with subacute sclerosing panencephalitis. J Virol. 1986 Aug;59(2):472–478. doi: 10.1128/jvi.59.2.472-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Liebert U. G., Cattaneo R., Billeter M. A., Roos R. P., ter Meulen V. Restriction of measles virus gene expression in measles inclusion body encephalitis. J Infect Dis. 1988 Jul;158(1):144–150. doi: 10.1093/infdis/158.1.144. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H., Cattaneo R., Billeter M. A. Biased hypermutation of viral RNA genomes could be due to unwinding/modification of double-stranded RNA. Cell. 1989 Feb 10;56(3):331–331. doi: 10.1016/0092-8674(89)90234-1. [DOI] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Richardson C. D., Rozenblatt S., Lazzarini R. A. Matrix genes of measles virus and canine distemper virus: cloning, nucleotide sequences, and deduced amino acid sequences. J Virol. 1986 May;58(2):408–416. doi: 10.1128/jvi.58.2.408-416.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Rozenblatt S., Arnheiter H., Richardson C. D. Measles virus P gene codes for two proteins. J Virol. 1985 Mar;53(3):908–919. doi: 10.1128/jvi.53.3.908-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Trudgett A., McFarlin D. E. Purification of measles virus with preservation of infectivity and antigenicity. J Gen Virol. 1979 Jun;43(3):633–639. doi: 10.1099/0022-1317-43-3-633. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda S. J., Wong T. C. Measles virus synthesizes both leaderless and leader-containing polyadenylated RNAs in vivo. J Virol. 1989 Jul;63(7):2977–2986. doi: 10.1128/jvi.63.7.2977-2986.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Billeter M. A. Mutations and A/I hypermutations in measles virus persistent infections. Curr Top Microbiol Immunol. 1992;176:63–74. doi: 10.1007/978-3-642-77011-1_5. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Kaelin K., Baczko K., Billeter M. A. Measles virus editing provides an additional cysteine-rich protein. Cell. 1989 Mar 10;56(5):759–764. doi: 10.1016/0092-8674(89)90679-x. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Rose J. K. Cell fusion by the envelope glycoproteins of persistent measles viruses which caused lethal human brain disease. J Virol. 1993 Mar;67(3):1493–1502. doi: 10.1128/jvi.67.3.1493-1502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Billeter M. A., Sheppard R. D., Udem S. A. Multiple viral mutations rather than host factors cause defective measles virus gene expression in a subacute sclerosing panencephalitis cell line. J Virol. 1988 Apr;62(4):1388–1397. doi: 10.1128/jvi.62.4.1388-1397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Eschle D., Baczko K., ter Meulen V., Billeter M. A. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988 Oct 21;55(2):255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Spielhofer P., Kaelin K., Baczko K., ter Meulen V., Pardowitz J., Flanagan S., Rima B. K., Udem S. A. Mutated and hypermutated genes of persistent measles viruses which caused lethal human brain diseases. Virology. 1989 Dec;173(2):415–425. doi: 10.1016/0042-6822(89)90554-0. [DOI] [PubMed] [Google Scholar]
- Dowling P. C., Blumberg B. M., Menonna J., Adamus J. E., Cook P., Crowley J. C., Kolakofsky D., Cook S. D. Transcriptional map of the measles virus genome. J Gen Virol. 1986 Sep;67(Pt 9):1987–1992. doi: 10.1099/0022-1317-67-9-1987. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Ringold G. M. Cationic liposome-mediated transfection. Nature. 1989 Jan 26;337(6205):387–388. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffre R. M., Tovell D. R., Kay C. M., Tyrrell D. L. Evidence for an interaction between the membrane protein of a paramyxovirus and actin. J Virol. 1982 Jun;42(3):963–968. doi: 10.1128/jvi.42.3.963-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hardwick J. M., Bussell R. H. Glycoproteins of measles virus under reducing and nonreducing conditions. J Virol. 1978 Feb;25(2):687–692. doi: 10.1128/jvi.25.2.687-692.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasel K. W., Day S., Millward S., Richardson C. D., Bellini W. J., Greer P. A. Characterization of cloned measles virus mRNAs by in vitro transcription, translation, and immunoprecipitation. Intervirology. 1987;28(1):26–39. doi: 10.1159/000149994. [DOI] [PubMed] [Google Scholar]
- Hirano A., Ayata M., Wang A. H., Wong T. C. Functional analysis of matrix proteins expressed from cloned genes of measles virus variants that cause subacute sclerosing panencephalitis reveals a common defect in nucleocapsid binding. J Virol. 1993 Apr;67(4):1848–1853. doi: 10.1128/jvi.67.4.1848-1853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A., Wang A. H., Gombart A. F., Wong T. C. The matrix proteins of neurovirulent subacute sclerosing panencephalitis virus and its acute measles virus progenitor are functionally different. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8745–8749. doi: 10.1073/pnas.89.18.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikami S. M., Moyer S. A. Synthesis of leader RNA and editing of the P mRNA during transcription by purified measles virus. J Virol. 1991 Oct;65(10):5342–5347. doi: 10.1128/jvi.65.10.5342-5347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Leopardi R., Hukkanen V., Vainionpä R., Salmi A. A. Cell proteins bind to sites within the 3' noncoding region and the positive-strand leader sequence of measles virus RNA. J Virol. 1993 Feb;67(2):785–790. doi: 10.1128/jvi.67.2.785-790.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx P. A., Portner A., Kingsbury D. W. Sendai virion transcriptase complex: polyeptide composition and inhibition by virion envelope proteins. J Virol. 1974 Jan;13(1):107–112. doi: 10.1128/jvi.13.1.107-112.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Elroy-Stein O., Mizukami T., Alexander W. A., Fuerst T. R. Product review. New mammalian expression vectors. Nature. 1990 Nov 1;348(6296):91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Horikami S. M. Host cell proteins required for measles virus reproduction. J Gen Virol. 1990 Apr;71(Pt 4):775–783. doi: 10.1099/0022-1317-71-4-775. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Lessard J. L. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5405–5409. doi: 10.1073/pnas.83.15.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wiener J. R., Volk W. A., Wagner R. R. Monoclonal antibodies to the M protein of vesicular stomatitis virus (Indiana serotype) and to a cDNA M gene expression product. J Virol. 1985 Aug;55(2):298–306. doi: 10.1128/jvi.55.2.298-306.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R. W., Moyer S. A. Initiation and replication of vesicular stomatitis virus genome RNA in a cell-free system. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3198–3202. doi: 10.1073/pnas.80.11.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rataul S. M., Hirano A., Wong T. C. Irreversible modification of measles virus RNA in vitro by nuclear RNA-unwinding activity in human neuroblastoma cells. J Virol. 1992 Mar;66(3):1769–1773. doi: 10.1128/jvi.66.3.1769-1773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J., Fujinami R. S. Characterization of in vitro transcription and transcriptional products of measles virus. J Virol. 1987 Nov;61(11):3381–3387. doi: 10.1128/jvi.61.11.3381-3387.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. D., Berkovich A., Rozenblatt S., Bellini W. J. Use of antibodies directed against synthetic peptides for identifying cDNA clones, establishing reading frames, and deducing the gene order of measles virus. J Virol. 1985 Apr;54(1):186–193. doi: 10.1128/jvi.54.1.186-193.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rima B. K., Baczko K., Clarke D. K., Curran M. D., Martin S. J., Billeter M. A., ter Meulen V. Characterization of clones for the sixth (L) gene and a transcriptional map for morbilliviruses. J Gen Virol. 1986 Sep;67(Pt 9):1971–1978. doi: 10.1099/0022-1317-67-9-1971. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Buonocore L., Whitt M. A. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. Biotechniques. 1991 Apr;10(4):520–525. [PubMed] [Google Scholar]
- Schmid A., Cattaneo R., Billeter M. A. A procedure for selective full length cDNA cloning of specific RNA species. Nucleic Acids Res. 1987 May 26;15(10):3987–3996. doi: 10.1093/nar/15.10.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A., Spielhofer P., Cattaneo R., Baczko K., ter Meulen V., Billeter M. A. Subacute sclerosing panencephalitis is typically characterized by alterations in the fusion protein cytoplasmic domain of the persisting measles virus. Virology. 1992 Jun;188(2):910–915. doi: 10.1016/0042-6822(92)90552-z. [DOI] [PubMed] [Google Scholar]
- Seifried A. S., Albrecht P., Milstien J. B. Characterization of an RNA-dependent RNA polymerase activity associated with measles virus. J Virol. 1978 Mar;25(3):781–787. doi: 10.1128/jvi.25.3.781-787.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Meissner H. C. Measles and SSPE viruses: similarities and differences. Prog Med Virol. 1982;28:65–95. [PubMed] [Google Scholar]
- Wong T. C., Ayata M., Hirano A., Yoshikawa Y., Tsuruoka H., Yamanouchi K. Generalized and localized biased hypermutation affecting the matrix gene of a measles virus strain that causes subacute sclerosing panencephalitis. J Virol. 1989 Dec;63(12):5464–5468. doi: 10.1128/jvi.63.12.5464-5468.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. C., Ayata M., Ueda S., Hirano A. Role of biased hypermutation in evolution of subacute sclerosing panencephalitis virus from progenitor acute measles virus. J Virol. 1991 May;65(5):2191–2199. doi: 10.1128/jvi.65.5.2191-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z. P., Baylor N. W., Wagner R. R. Transcription-inhibition and RNA-binding domains of influenza A virus matrix protein mapped with anti-idiotypic antibodies and synthetic peptides. J Virol. 1989 Sep;63(9):3586–3594. doi: 10.1128/jvi.63.9.3586-3594.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa Y., Tsuruoka H., Matsumoto M., Haga T., Shioda T., Shibuta H., Sato T. A., Yamanouchi K. Molecular analysis of structural protein genes of the Yamagata-1 strain of defective subacute sclerosing panencephalitis virus. II. Nucleotide sequence of a cDNA corresponding to the P plus M dicistronic mRNA. Virus Genes. 1990 Jul;4(2):151–161. doi: 10.1007/BF00678406. [DOI] [PubMed] [Google Scholar]
- Zhirnov O. P. Solubilization of matrix protein M1/M from virions occurs at different pH for orthomyxo- and paramyxoviruses. Virology. 1990 May;176(1):274–279. doi: 10.1016/0042-6822(90)90253-n. [DOI] [PubMed] [Google Scholar]
- Zvonarjev A. Y., Ghendon Y. Z. Influence of membrane (M) protein on influenza A virus virion transcriptase activity in vitro and its susceptibility to rimantadine. J Virol. 1980 Feb;33(2):583–586. doi: 10.1128/jvi.33.2.583-586.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]