Abstract
Background
Organophosphate insecticides represent one of the most widely used classes of pesticides with high potential for human exposure in both rural and residential environments.
Objective
In the present study, we investigated the effects of pirimiphos-methyl (0, 2-diethylamino-6-methylpirimidin-4-yl O, O-dimethyl phosphorothioate), an organophosphothioate pesticide, on male rat reproductive performances.
Methods
A total of 24 adult Wistar rats were divided into 4 groups of 6 animals each and orally treated with 0, 41.67, 62.5 or 125 mg/kg of pirimiphos-methyl for 90 days.
Results
Results from the study showed a significant increase (p<0.05) in feed consumption, body weight gain, relative testis and epidiydimis weights and intra-testicular cholesterol level in rats receiving the test substance at doses of 62.5 or 125mg/kg whereas a significant decrease (p<0.05) in serum total protein, sperm density and motility, fertility and parturition indices and pups sex-ratio (M/F) was recorded in animals treated with 125 mg/Kg of pirimiphos methyl. Histological findings also indicated enlargement of interstitial space, inhibition of spermatogenesis, rarefaction of Leydig cells and oedema in testes compared to control animals.
Conclusion
It could then be concluded that pirimiphos-methyl (62.5 and 125mg/kg) is detrimental to the reproductive potentials of male rats.
Keywords: pirimiphos-methyl, spermatogenesis, fertility, male rats
Introduction
Organophosphorous insecticides represent one group of pesticides that is widely used and has been shown to have toxic effects in human and animals 1, 2, 3, 4, 5, 6. The primary toxicity associated with acute exposure to organophosphate insecticides is cholinergic crisis resulting from acetylcholinesterase inhibition 7, 8. However, these compounds have numerous other compound-specific chronic effects including delayed polyneuropathy, immunotoxicity, carcinogenesis and endocrine, developmental and reproductive toxicity 9, 10, 11, 12, 13. Pirimiphos-methyl (0,2-diethylamino-6-methylpirimidin-4-yl O, O-dimethyl phosphorothioate) is a broad spectrum, non-cumulative organophosphorous pesticide responsible for the hydrolysis of body choline esters including acetylcholine at cholinergic synapses 14, 15, 16. The inhibition of these enzymes increases the availability of acetylcholine which in turn can stimulate cholinergic receptors producing both nicotinic and muscarinic effects in the organism such as muscle contraction and secretion in many glands 16.
Pirimiphos methyl is rapidly absorbed, metabolised and excreted in rats and dogs. In both species, 2-ethyl amino-4-hydroxy-6-methyl pyrimidine is the major metabolite 17. The reproductive organs have been shown to be among the most vulnerable organs to organophosphorous insecticides 18, 19, 20. Pirimiphosmethyl is a cheap pesticide widely used in the world and particularly in Africa to protect food against pests. Therefore, there is a possibility that this compound could affect the reproductive health of humans and wildlife in their natural habitats.To the best of our knowledge, little is known on the effects of pirimiphos-methyl on the male reproductive system. The present work was therefore undertaken in order to access the effect of this pesticide on some biochemical (total proteins, total cholesterol), fertility (sperm density and motility, fertility indices) and histological parameters.
Materials and methods
Animals
Healthy Wistar adult male rats weighing between 250 and 300g were used in this study. Animals were housed in wire cages (50 cm long X 25 cm wide X 25 cm high), six per cage in a centralized animal care facility maintained at 22 to 25°C with a relative humidity of 76 ± 5%. Animals were kept in a 12 h light-dark cycle and provided ad libitum with water and a laboratory diet.
Chemical
Actellic (SYNGENTA, United Kingdom) is an insecticide and acaricide whose active principle is pirimiphos-methyl (0, 2-diethylamino-6-methylpirimidin-4-yl O, O-dimethyl phosphorothioate) concentrated at 450g/l. Pirimiphos-methyl has a molecular weight of 305.3 and it is soluble in water, acetone and alcohol. It has no characteristic odour and the purity is 99%.
Experimental design
Animals were randomly divided into four groups of 6 rats each and treated as follows: Group 1 was orally administered with 10 ml/Kg of distilled water whilst Groups 2–4 received per os Pirimiphos-methyl at a dose equivalent to 41.67, 62.5 or 125 mg/ kg body weight for 90 days. The doses used in the study were selected from a pilot study and represent 1/30, 1/20 and 1/10 of LD50 value obtained in mice (1250 mg/ kg) (personal communication). During the treatment, body weight and food consumption were measured at 2 days intervals.
Fertility test
Each male was allowed to undergo mating with 2 females (3–4 months old) of proven fertility during the last 10 days of treatment (as from day 80 of treatment). The vaginal smear was examined for the presence of sperm as a criterion of successful insemination and each sperm-positive female was caged separately and observed after 21–24 days for deliver. Furthermore, pups were examined for litter size, litter weight, sex and viability. Fertility index [(number of pregnant animals / number of females mated) X 100], gestation index [number of pups born alive / number of total pups born] X 100 and parturition index [number of females delivered / number of pregnant animals] X 100 were also calculated according to the method of Kennedv et al.21 and Wernik et al.22.
Serum and organ collections
At the end of treatment (91th day), blood was collected by cardiac puncture from each rat under ether anaesthesia. Serum was prepared and stored at −20°C prior to analysis.
After killing the rats by overdose of ether, organs like testes, epididymis, ventral prostate, vas deferens, seminal vesicles, liver and kidneys were carefully removed, free of adipose tissue, blotted dry and weighed separately. The left testes was then homogenised in a known volume of cold distilled water and aliquots of supernatant were kept at − 20°C prior to biochemical estimations.
Sperm density and motility
Immediately after the sacrifice, the right cauda epididymis of each animal was minced in 10 ml of warm (36°) NaCl solution for motility evaluation. 20 µl of this mixture were used to count motile and non-motile sperms (Olympus, X40). The percentage of motile spermatozoa was determined using the following formula: Percentage of mobile spermatozoa = [Number of mobile spermatozoa / Total number of counted spermatozoa] X 100
The sperm density was determined in the right cauda epididimis using a Bürker haemocytometer.
Biochemical analysis
Total protein contents of serum and testes were determined by the methods of Biuret (Gornal et al. (23)) and Bradford (24) respectively. Serum and intra-testicular total cholesterol were determined using a commercially available kit from Human Gesellschaft für Biochemical und Diagnostica mbH (Wiesbaden-Germany).
Tissue preparation and histopathology
The left testis was fixed in Bouin's fluid for 1 week, embedded in paraffin, cut at 5 ì m and stained with Harris haematoxylin and eosin. The tissue sections were observed under a light microscope (Olympus, X 40) for semineferous tubule morphology and cellular harmony.
Statistical analysis
Values are presented as Mean ± SD. ANOVA was performed for comparison with post-hoc Duncan test. A p value of <0.05 was considered statistically significant. Statistical analyses were performed with the aid of SPSS for Windows software programme (Release 10.1).
Results
Feed consumption
Treatment of rats with Pirimiphos-methyl (PM) at doses of 62.5 and 125 mg/kg b. w. for 90 days significantly increased (p<0.05) feed consumption compared to control (Table 1).
Table 1.
Effects of pirimiphos-methyl on feed consumption, body and organ weights
| Parameters | Pirimiphos-methyl (mg/kg) | |||
| 0 (control) | 41.67 | 62.5 | 125 | |
| (n=6) | (n=6) | (n=6) | (n=6) | |
| Feed consumption (g/kg/day) |
77.90±22.70a | 96.37±26.09ab | 106.68±21.09b | 114.39±27.52b |
| Final body weight (g) | 338.25±68.97a | 302.33±40.67ab | 286.75±28.13ab | 262.60±17.33b |
| Body weight gain (%) | 8.45±4.32a | 13.63±7.66ab | 15.10±8.19bc | 17.67±10.99bc |
| Organ weights (g/100g of bw) |
AOW | ROW | AOW | ROW | AOW | AOW | ROW | |
| Testes | 3.13±0.29a | 0.89±0.13a | 2.79±0.20b | 0.93±0.14ab | 2.71±0.20b | 0.94±0.06ab | 2.73±0.08b | 1.04±0.07b |
| Epididymis | 0.94±0.10a | 0.29±0.04a | 0.94±0.11a | 0.31±0.04ab | 0.88±0.05a | 0.30±0.03ab | 0.92±0.71a | 0.34±0.03b |
| Vas deferens | 0.19±0.02ab | 0.05±0.01a | 0.19±0.04ab | 0.06±0.01a | 0.21±0.01a | 0.06±0.08a | 0.17±0.03b | 0.05±0.01a |
| Ventral prostate | 0.4±0.09a | 0.11±0.04a | 0.37±0.16a | 0.11±0.04a | 0.42±0.07a | 0.14±0.03a | 0.35±0.04a | 0.13±0.02a |
| Seminal vesicles | 1.23±0.25a | 0.37±0.11a | 0.90±0.17b | 0.29±0.05a | 0.95±0.22ab | 0.32±0.06a | 0.86±0.21b | 0.32±0.07a |
| Liver | 9.85±1.43a | 2.95±0.31a | 9.11±1.09ab | 3.01±0.18a | 8.48±0.80ab | 2.96±0.29a | 7.86±0.95b | 2.98±0.03a |
| Kidneys | 1.75±0.39ab | 0.51±0.05a | 1.89±0.21a | 0.62±0.08bc | 1.87±0.22a | 0.64±0.03b | 1.50±0.15b | 0.56±0.03ac |
a,b,c : On the same line, values affected by the same letter no differ significantly (P>0.05).
All values: Mean ± SD
n: number of rats
AOW: Absolute Organ Weights
ROW: Relative Organ Weights
bw: Body weight
Body and organ weights
As shown in Table 1, the final body weight decreased significantly (p<0.05) in rats receiving 125 mg/kg of pirimiphos-methyl whereas the body weight gain increased (p<0.05) in rats treated with either 62.5 or 125 mg/kg b. w.
At all doses, the absolute weight of testes and seminal vesicles were decreased significantly (p<0.05) compared to control. Only the dose of 125 mg/kg b.w. reduced (p<0.05) the liver weight. Additionally, the absolute weights of the kidney and vas deferens decreased (p<0.05) in rats treated with 125 mg/kg b.w. compared to those receiving PM at 41.67 and 62.5 mg/kg b.w. The treatment had no effect on epididimis and ventral prostate absolute weights (Table 1).
The relative weight of ventral prostate, seminal vesicles, liver and vas deferens remained unchanged whilst the relative weight of testes and epididymis was significantly higher (p<0.05 ) in rats treated with 125 mg/kg of pirimiphos-methyl. A significant increase (p<0.05) of the relative weight of kidneys was recorded in rats fed with 41.67 and 62.5 mg/kg of pirimiphos-methyl compared to control. A highly positive correlation (r = + 0.92) was found between the relative weight of testes and epididymis. The relative weight of kidneys was also significantly higher (p<0.05) in the 41.67 and 62.5 mg/kg groups compared to control animals. Additionally, the relative weight of kidneys was significantly lower (p<0.05) in rats fed with the highest dose of PM when compared to 62.5 mg/kg group (Table 1).
Sperm density and motility
Sperm density per cauda epididymis and per gram of tissue as well as sperm motility were significantly lowered (p<0.05) in animals treated with 125 mg/kg compared to control group (Table 2).
Table 2.
Effects of pirimiphos-methyl on sperm characteristics, biochemical and fertility parameters Pirimiphos-methyl (mg/kg of body weight)
| Parameters | 0 (control) | 41.67 | 62.5 | 125 |
| (n=6) | (n=6) | (n=6) | (n=6) | |
| Sperm characteristics | ||||
| Sperm density (x106) | ||||
| Per cauda epididymis | 126.7±3.14a | 108.91±32.29ac | 107.66±31.06ac | 76.0814.81bc |
| Per g tissue | 573.10±141.9a | 508.17±171.15ac | 554.07±128.3a | 87.83±49.69bc |
| Motility (%) | 94.39±3.89a | 87.18±7.61ac | 85.82±11.75ac | 80.08±15.82bc |
| Biochemical parameters | ||||
| Serum proteins (mg/ml) | 2.0±0.46a | 1.89±0.39a | 1.76±0.23b | 1.91±0.3a |
| Testes proteins (µg/mg) | 46.69±6.37a | 49.10±8.26a | 49.03±6.72a | 45.58±6.51a |
| Serum cholesterol (mg/ml) | 1.96±0.37ab | 1.86±0.37ab | 1.86±0.32ab | 1.52±0.38b |
| Testes cholesterol (µg/mg) | 0.25±0.12a | 0.31±0.17ac | 0.45±0.16ac | 0.44±0.13bc |
| Fertility parameters | ||||
| Fertility index (%) | 91.66±20.41a | 75.27±27.38ab | 76.0±26.37ab | 50.0±28.02b |
| Parturition index (%) | 91.66±20.41a | 70.38±27.8ab | 66.66±25.81ab | 50.0±24.2b |
| Gestation index (%) | 92.59±28.43a | 87.38±15.54a | 86.35±21.43a | 84.0±37.77a |
| Average birth weight (g) | 4.35±0.8a | 4.09±0.6a | 3.91±0.65a | 3.18±0.54a |
| Average litter size | 7.0±2.6a | 8.0±1.63a | 7.42±2.37a | 7.2±2.93a |
| Pups sex-ratio (M/F) (%) | 46.67±8.62a | 39.10±19.63a | 30.05±9.58a | 26.10±16.81b |
a,b,c : On the same line, values affected by the same letter no differ significantly (P>0.05).
All values: Mean ± SD
n: number of rats
Biochemical parameters
The effects of pirimiphos-methyl (PM) on biochemical parameters are outlined in Table 2.
There was a significant difference (p<0.05) between the serum total protein of rats treated with 62.5 mg/kg of pirimiphos-methyl and the other groups while a non significant trend to an increase was observed in the total protein contents of the testes. Results of the study also revealed a decrease (p<0.05) in the serum cholesterol level (125 mg/kg) in the one hand, and an increase (p<0.05) in intra-testicular total cholesterol (62.5 and 125 mg/kg) compared to control in the other hand.
Fertility
Fertility index, parturition index and sex-ratio (M/F) recorded in females mated with male receiving orally PM (125 mg/kg) for 90 days were significantly reduced (P<0.05) compared to control. Litter size, gestation index and average birth weight were not significantly affected (Table 2).
Histological findings
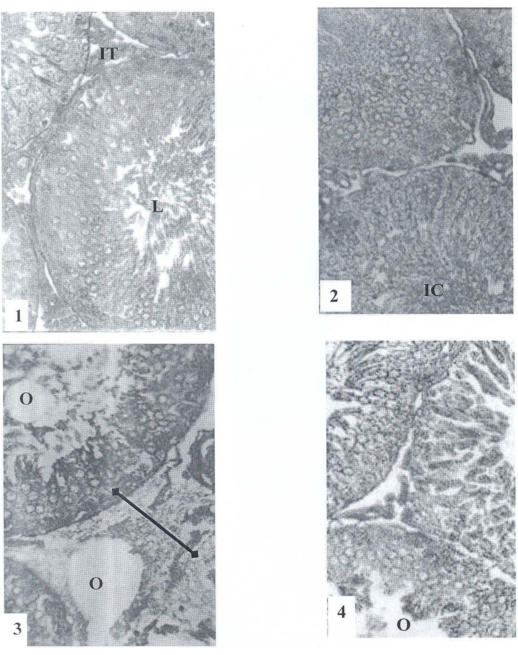
In the treated testes (62.5 and 125 mg/kg), the lumen of the seminiferous tubules was devoided of spermatozoa in contrast to the control group where it was fully packed with sperms. There were also immature cells in the lumen (41.67 mg/kg), enlargement of interstitial tissue (oedema) (62.5mg/kg), damaged sertolian cells (125 mg/kg) and scarcity of Leydig cells (125 mg/kg) (Figure 1).
Figure 1.
Testes of control and PM treated rats for 90 days.
Discussion
Pirimiphos-methyl is an organophosphate pesticide widely used in developing countries for the protection of cultures and food-stuffs. From the results of the present study, it appears that after the oral treatment of rats with pirimiphos-methyl (PM), the feed consumption significantly increased in a dose-dependent manner. This hyperphagia may be related to the effects of this active principle (PM) on the central structures involved in the control of feed intake. Psychoyos25, Fox26 and Ganong27 have linked hyperphagia with lesions of the ventromedian nucleus of the hypothalamus. It could then be suggested that PM may stimulate this centre thus increasing the feed intake and consequently the body weight gain observed in the work.
Administration of PM (125 mg/kg) resulted in an increase in the relative weights of testes and epididymis. This observation contrasts with the findings of Poul and Dagorn28 who reported a decrease in the relative weights of testes from rats treated with 12.5 and 25 mg/kg of bromophenophos,. This difference could be attributed to the accumulation of water (oedematic status) induced by PM as observed in the cross-section of the treated testes. Blanchet and co-workers29 have reported that orgaphosphorus possess neurotoxic effects evidenced by accumulation of water in various tissues including testes.
One of the objectives of this study was to detect the effects of PM on some biochemical and fertility parameters. After treatment of rats for 90 days with various doses of PM, the total cholesterol level was decreased in the serum and increased in the testis. It is well known that cholesterol is the main precursor for steroidogenesis and it is produced mostly in the liver from LDL and HDL.30, 31 The decrease observed in the serum may be correlated to the detrimental effect of PM on the liver as shown by the significant reduction of its weight. On the contrary, the increase of cholesterol level in the testes may be a result of its non utilization leading to the reduction of the production of testosterone, the main hormone involved in the control of fertility of animals including rats. Many other organophosphorus such as Dimethoate blocks steroid hormone biosynthesis in the Leydig cells 32.
The epididymal sperm reserve was decreased in rats treated with PM. Similar observations were recorded by Thonneau33 in rats force-fed with quinalphos, and by Sobarzo and Bustos-Obregün 19 in mice acutely treated with parathion. This decrease of sperm concentration in the epididymis may be due to a number of factors: the destruction of testis architecture observed on testicular histology, the inhibition of testosterone biosynthesis as mentioned above or the suppression of brain's release hormones (FSH and LH). Indeed, Short34 and Gwynne35 showed that organophosphorous and carbamate pesticides reduce acethylcholinesterase (enzyme) activity, and block nerve impulses. This effect may be linked to the suppression of the brain's release of hormones which are follicle stimulating hormone (FSH) and lutinizing (LH) hormone leading to the reduction of sperms production in the testes.
The present study also showed possible deterioration in sperm motility of the rats fed with PM, compared to the controls. Similar results were obtained by Kamijima et al. 36 on pesticides sprayers exposed to organophosphate insecticides. This could be due to retention of cytoplasmic droplet in sperms as shown by Akbarsha et al. 37 in rat's cauda epididymal spermatozoa after treatment with cytotoxic and xenobiotic agents.
Reduction of fertility, gestation and parturition indices, average birth weight and the pup's sex-ratio (M/F) was obtained when mating females with treated males. Savitz et al. 38, Petrelli and Mantovani (39); Petrelli et al. 40 also noted a decrease of the fertility in spouses of men exposed to pesticides. Moreover, Whorton 41; Ryan et al. 42 and Figa-Talamanca 43 reported a decrease in pups sex-ratio (M/F) respectively in spouses of banana workers, exposed to 1, 2 Dibromopropane, and in spouses of pesticides manufacturers and pesticides sprayers. On the other hand, Blakly et al. 44 reported the decrease of foetal weight and increase of fetal abnormalities in females mice exposed to tordon 202 C. The decrease of fertility in this study could be due to the decrease of sperm production, and to teratospermia due to retention of cytoplasmic droplets like mentioned above or to the posimplantation loss.
In conclusion, our results have showed that the administration of Pirimiphos-methyl, a widely use organophosphate insecticide, by oral route to adult male rats at the doses of 62.5 and 125 mg/kg/day during 90 days disrupted spermatogenesis and reduced the fertility.
Finaly, it is recommended that the use of pirimiphos-methyl must be limited due to its hazardous effect.
References
- 1.De-Bleecker D, Van-Den-Neucker F Colradyn. Intermediate syndrome in organophosphorous poisoning; a prospective study. Crit Care Med. 1993;21:1706–1711. doi: 10.1097/00003246-199311000-00020. [DOI] [PubMed] [Google Scholar]
- 2.National Research Council, author. Pesticides in the diets of infants and Children. Washington: National Academy Press; 1993. [PubMed] [Google Scholar]
- 3.World Health Oganization, author. Guidlines for poison control. Geneva: WHO in collaboration with UNEP and ILO; 1997. p. 3. [Google Scholar]
- 4.Littovitz TL, Klein-Schwartz W, Dyer KS, Shannon M, Lee S, Powers M. 1997 annual report of the American Association of Poison control centers Toxic Exposure Surveillance System. Am J Emerg Med. 1998;16:443–497. doi: 10.1016/s0735-6757(98)90000-6. [DOI] [PubMed] [Google Scholar]
- 5.Tsatsakis A, Manousakis A, Anastasaki M, et al. Clinical and toxicological data in fenthion and omethoate acute poisoning. J Environ Sci health. 1998;33:657–670. doi: 10.1080/03601239809373170. [DOI] [PubMed] [Google Scholar]
- 6.Eskenazi B, Bradman A, Castorina R. Exposure of children to organophosphate pesticides and their potential adverse health effects. Environmental Health Perspectives. 1999;107(suppl. 3):409–419. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pope CN. Organophosphorous pesticides: do they all have the same mechanism of toxicity? J Toxicol Environm Healh B, Crit Rev. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- 8.Cabello G, Valenzuela M, Vilava A, et al. A rat mammary Tunor Model Induced by the Organophosphorous pesticides Parathion and Malathion, possibly through Acetyl-cholinesterase inhibition. Environmental Health perspectives. 2001;109(5):471–479. doi: 10.1289/ehp.01109471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crammer JS, Avery DL, Grady RR, Kitay JI. Postnatal endocrine dysfunction resulting from prenatal exposure to carbofuran, diazinon or chlordane. J Environ Pathol Toxicol. 1978;2:357–369. [PubMed] [Google Scholar]
- 10.Reuber MD. Carcinogenicity and toxicity of malathion and malaxon. Environ Res. 1985;37:119–153. doi: 10.1016/0013-9351(85)90054-4. [DOI] [PubMed] [Google Scholar]
- 11.Sultatos LG. Mammalian toxicology of organophosphorous pesticides. J Toxicol Environ Health. 1994;43:271–289. doi: 10.1080/15287399409531921. [DOI] [PubMed] [Google Scholar]
- 12.Astroff AB, FreshWater KJ, Eigenberg DA. Comparative organophosphate-induced effects observed in adult and neonatal Sprague Dawley rats during the conduct of multigeneration toxicity studies. Reprod Toxicol. 1998;12:619–645. doi: 10.1016/s0890-6238(98)00044-6. [DOI] [PubMed] [Google Scholar]
- 13.Tamura H, Maness SC, Reishman K, Dorman DC, Gray LE, Gaido KW. Androgen Receptor antagonism by the organophosphate insecticide fenitrothion. Toxicological Sciences. 2001;60:56–62. doi: 10.1093/toxsci/60.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Klaassen C. Non metallic environmental toxicants: air polluants, solvents and vapors and pesticides. In: Goodman Gilman A, Rall TW, Nies AS, Taylor P, editors. The pharmacological basis of Therapeutics. New-York: Pergamon Press Inc; 1990. pp. 1615–1635. [Google Scholar]
- 15.Silman I, Futerman A. Modes of attachment of acetylcholinesterase to the surface membrane. Eur J Biochem. 1987;170:11–20. doi: 10.1111/j.1432-1033.1987.tb13662.x. [DOI] [PubMed] [Google Scholar]
- 16.Taylor P. Anticholinesterase agents. In: Goodman Gilman A, Rall TW, Nies AS, Taylor P, editors. The pharmacological Basis of therapeutics. New-York: Pergamon Press Inc; 1990. pp. 131–147. [Google Scholar]
- 17.Mills IE. Pirimiphos-methyl : blood concentrations and tissue retention in rat. Report No CTL/P/247. Imperial Chemical industries, central toxicology laboratory. 1976 11p. [Google Scholar]
- 18.Cox C. “Masculinity at risk”. Journal of Pesticide Reform. 1996;2:2–7. [Google Scholar]
- 19.Sobarzo C, Bustoos-Obregón E. Sperm quality in mice acutely treated with parathion. Asian Journal of Andrology. 2000;2:147–150. [PubMed] [Google Scholar]
- 20.ustos-Obregón E, Gonzállez-Hormanzábal P. Testicular ddamage elicited by malathion. Int J Morphol. 2003;2:155–159. [Google Scholar]
- 21.Kennedv GL, Jr, John P, Frawley J, Calandra JC. Multigeneration reproductive effects of three pesticides in rats. Toxicol appl pharmacol. 1973;25:589–596. doi: 10.1016/0041-008x(73)90029-x. [DOI] [PubMed] [Google Scholar]
- 22.Wernik T, Lamman BM, Franx JL. Chronic toxicity Teratologic and reproduction studies with hair dyes. Toxicol appl pharmacol. 1975;32:450–460. doi: 10.1016/0041-008x(75)90110-6. [DOI] [PubMed] [Google Scholar]
- 23.Gornal AG, Bardwil GS, David MM. Determination of serum proteins by mean of Biuret reactions. Biochemistry. 1949;177:751–766. [PubMed] [Google Scholar]
- 24.Bradford MN. A rapid and sensitive method for the quantification of microgram quantity of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 25.Psychoyos A. La reproduction. In: Meyer P, editor. Physiologie Humaine. Paris: Flammarion Médecine-Sciences; 1983. pp. 471–493. [Google Scholar]
- 26.Fox SI. Human Physiology. Sixth edition. Boston: Mc. Graw-Hill; 1999. pp. 612–614. [Google Scholar]
- 27.Ganong WF. Physiologie medicale. Paris: Masson; 2001. pp. 408–411. [Google Scholar]
- 28.Poul JM, Dagorn M. Etude de la toxicité du bromophénophos administre par voie orale pendant 3 mois chez le rat. Rec Méd Vét. 1982;158:363–368. [Google Scholar]
- 29.Blanchet G, Carpenter P, Lallement G. Vulnérabilité du système nerveux central vis-à-vis des neurotoxiques organophosphorés. Médecine Armées. 1991;19:403–407. [Google Scholar]
- 30.Robel P. La stéroïdogénèse : les enzymes et la régulation de leur expression génomique. In: Thibaullt C, Levasseur MC, editors. La reproduction chez les mammifères et l'homme. Nouvelle édition. Paris: INRA Editions; 2001. pp. 146–154. [Google Scholar]
- 31.Johnson M, Everitt B J. Reproduction. De Boek Université s.a.; 2002. 298p. [Google Scholar]
- 32.Walsh LP, Stocco DM. The effects of roundup and dimethoate on steroidogenesis acute regulatory (StAR) protein in mouse MA-10 Lydig cells. Proceedings of the Society for the Study of Reroduction; Society for the study of reproduction; 8–11 August 1998; College Station, Texas Madison, WI. 1998. p. 182. [Google Scholar]
- 33.Thonneau P, Multigner L, Ducot B, Clavert A, Spir A. Pesticides and male fertility current knowledge and epidemiological approach. In: Hamammah R, editor. Research in Male gamete: production and quality. INSERM; 1996. pp. 159–165. [Google Scholar]
- 34.Short P, Colborn T. Pesticides use in the U.S. and polity implications. Toxicology and Industrial Healt. 1999;15:240–275. doi: 10.1191/074823399678846736. [DOI] [PubMed] [Google Scholar]
- 35.Gwynne L. Mixed messages: Pesticides that confuse hormones. UK: Pesticide Action Network (PAN); 2000. Briefing paper; 6p. [Google Scholar]
- 36.Bakley PM, Kim JS, Firneisz GD. The effects of paternal subacute exposure to tordon 202 C on foetal growth and development in CD-1 mice. Teratology. 1989;39:237–241. doi: 10.1002/tera.1420390305. [DOI] [PubMed] [Google Scholar]
- 37.Akbarsha MA, Latha PNL, Murugaian P. Retention of cytoplasmic droplet by rat cauda epididymal spermatozoa after treatment with cytotoxic and xenobiotic agents. J Reprod Fertil. 2000;120:385–390. doi: 10.1530/jrf.0.1200385. [DOI] [PubMed] [Google Scholar]
- 38.Savitz D, Arbukle T, Kaczor D, Curtis KM. Male pesticide exposure and pregnancy outcome. American Journal of Epidemiology. 1997;146:1025–1036. doi: 10.1093/oxfordjournals.aje.a009231. [DOI] [PubMed] [Google Scholar]
- 39.Petrelli G, Mantovani A. Environmental risk factors and male fertility and reproduction. Contraception. 2002;65:297–300. doi: 10.1016/s0010-7824(02)00298-6. [DOI] [PubMed] [Google Scholar]
- 40.Petrelli G, Figà-Talamanca I, Lauria L, Mantovani A. Spontaneous abortion in spouses of greenhouse workers exposed to pesticides. Environmental Health Perspective Medecine. 2003;8:77–81. doi: 10.1007/BF02897919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whorton MD, Kranss RM, Marshall S, Milby TH. Infertility in male pesticide workers. Lancet. 1977;2:1259–1261. doi: 10.1016/s0140-6736(77)92665-4. [DOI] [PubMed] [Google Scholar]
- 42.Ryan JJ, Amirora Z, Carrier G. Sex-ratios des enfants de producteurs de pesticides russes exposés aux dioxines. Environnemental health perspectives. 2000;110:699–701. [Google Scholar]
- 43.Figa-Talamanca I, Traina ME, Urbani E. Occupational exposure to metals, solvents and pesticides. Recent evidence on male reproductive effects and Biological markers. Occupationnal Medicine. 2001;51:174–188. doi: 10.1093/occmed/51.3.174. [DOI] [PubMed] [Google Scholar]
- 44.Bakley PM, Kim JS, Firneisz GD. The effects of paternal subacute exposure to tordon 202 C on foetal growth and development in CD-1 mice. Teratology. 1989;39:237–241. doi: 10.1002/tera.1420390305. [DOI] [PubMed] [Google Scholar]