Abstract
Background Context
In neutral spinal postures with low loading moments the lumbar spine is not inherently stable. Small compromises in paraspinal muscle activity may affect lumbar spinal biomechanics. Proprioceptive feedback from muscle spindles is considered important for control of muscle activity. Because skeletal muscle and muscle spindles are thixotropic, their length history changes their physical properties. The present study explores a mechanism that can affect the responsiveness of paraspinal muscle spindles in the lumbar spine.
Purpose
This study had two aims: to extend our previous findings demonstrating the history dependent effects of vertebral position on the responsiveness of lumbar paraspinal muscle spindles; and to determine the time course for these effects. Based upon previous studies, if a crossbridge mechanism underlies these thixotropic effects, then the relationship between the magnitude of spindle discharge and the duration of the vertebral position will be one of exponential decay or growth.
Study Design/Setting
A neurophysiological study using the lumbar spine of a feline model.
Methods
The discharge from individual muscle spindles afferents innervating lumbar paraspinal muscles in response to the duration and direction of vertebral position were obtained from teased filaments in the L6 dorsal roots of 30 Nembutal-anesthetized cats. The L6 vertebra was controlled using a displacement-controlled feedback motor and was held in each of 3 different conditioning positions for durations of 0, 0.5, 1, 1.5, and 2 seconds. Two of the conditioning positions stretched or shortened the lumbar muscles relative to an intermediate conditioning position. Conditioning positions for all cats ranged from 0.9 – 2.0 mm dorsal and ventralward relative to the intermediate position. These magnitudes were determined based upon the displacement that loaded the L6 vertebra to 50–60% of the cat’s body weight. Conditioning was thought to simulate a motion segment’s position that might be passively maintained due to fixation, external load, a prolonged posture, or structural change. Following conditioning positions that stretched (hold-long) and shortened (hold-short) the spindle, the vertebra was repositioned identically and muscle spindle discharge at rest and to movement was compared with conditioning at the intermediate position.
Results
Lumbar vertebral positions maintained for less than 2 seconds were capable of evoking different discharge rates from lumbar paraspinal muscle spindles despite the vertebra having been returned to identical locations. Both resting spindle discharge and their responsiveness to movement were altered. Conditioning vertebral positions that stretched the spindles decreased spindle activity and positions that unloaded the spindles increased spindle activity upon returning the vertebra to identical original (intermediate) positions. The magnitude of these effects increased as conditioning duration increased to 2 seconds. These effects developed with a time course following a first order exponential reaching a maximal value after approximately 4 seconds of history. The time constant for a hold-short history was 2.6 seconds and for a hold-long history was approximately half of that at 1.1 seconds.
Conclusions
Thixotropic contributions to the responsiveness of muscles spindles in the low back are caused by the rapid, spontaneous formation of stable crossbridges. These sensory alterations due to vertebral history would represent a proprioceptive input not necessarily representative of the current state of intersegmental positioning. As such, they would constitute a source of inaccurate sensory feedback. Examples are presented suggesting ways in which this novel finding may affect spinal physiology.
Introduction
Repositioning accuracy of the lumbar spine is affected by mechanical as well as clinical factors. For example, a slouched sitting posture alters an individual’s ability to accurately reposition their lumbar spine (1). Clinically, low back pain patients have less lumbar repositioning accuracy (2) and need practice to reach accuracies similar to normal subjects (3). Altered paraspinal muscle spindles and central processing were suggested to be the cause for the less refined position sense in low back pain patients (2). The present study explores the presence of a phenomenon in the lumbar spine that can alter proprioceptive signaling from paraspinal muscle spindles.
Skeletal muscle exhibits a rheological property termed thixotropy (reviewed in (4)). This physical property represents a time-dependent change in viscosity and can occur in polymers able to form weak, breakable bonds. At rest thixotropic materials are stable; they become less viscous when deformed by shear forces. Stiffness in extrafusal or intrafusal skeletal muscle fibers is history-dependent. It is affected by the length at which a muscle is passively held and, in the case of intrafusal fibers, by the state of gamma motoneuron discharge (5–7). It is thought that when muscle is held at a constant length, relatively stable actin-myosin crossbridges form spontaneously establishing themselves at the prevailing muscle length. These crossbridges exhibit slower turnover rates compared with the recycling crossbridges that form during active muscle contraction (4–6;8). If muscle length is subsequently changed, the stuck (crossbridged) sliding filaments are unable to slip past each other. If the muscle is shortened the sarcomeres become slack and if lengthened they become taut. For intrafusal fibers, these history dependent effects on stiffness change the responsiveness of muscle spindle afferents. Experiments in both humans and experimental preparations demonstrate that the passive length history of limb muscles alters the resting discharge and sensitivity of these proprioceptive afferents (9) and in turn alters the amplitude of H and monosynaptic reflexes (10;11). In the human cervical spine, head repositioning errors were caused by the immediately preceding history of the posterior neck muscles and were thought to arise from the thixotropic properties of muscle spindles (12).
In resting hindlimb muscle of the cat the effects of muscle history on intrafusal fibers and spindle discharge develop within 2–3 seconds (13). In the lumbar spine of the cat we found that the immediately preceding mechanical history of a lumbar vertebra also affects paraspinal muscles spindles; both their resting discharge and responsiveness to movement are altered in a manner determined by the direction of vertebral movement (14). Compared to holding a lumbar vertebra in an intermediate position, translating it by as little as 1.0 to 2.2 mm in a direction which stretches the muscle spindle and then holding it in that position for 2 to 8 seconds significantly decreases spindle resting discharge and responsiveness upon returning the vertebra to an intermediate position. On the other hand, a similar maneuver but in a direction that shortens the muscle spindle consistently increases resting discharge and responsiveness. These effects fully develop within 2–4 seconds of positioning (14). While these history-dependent changes demonstrate the thixotropic properties of lumbar paraspinal muscle spindles, they do not clearly address the underlying mechanism because the time course for their development using positioning durations less than 2 seconds has not been yet determined.
In human leg muscle, a quantitative analysis has been used to determine whether a crossbridge mechanism is consistent with the increase in extrafusal stiffness ascribed to muscle history (8). The passive length-tension curve of the triceps surae muscles contains an initially steep region followed by a shoulder whose magnitude is dependent upon the duration over which the muscle group was previously held at a shortened length. Hufschmidt and Schwaller (8) show that the relationship between this interval and the shoulder’s magnitude is an exponential function which approaches a maximal or saturating value. This first-order exponential is consistent with an equilibrium model where crossbridge assembly is in a state of flux, the number of crossbridges being determined by the number which attach spontaneously and the number which detach due to muscle movement (8). Maintaining a muscle at constant length would shift the equilibrium toward attachment.
The aim of the present study was to extend our previous findings demonstrating the history dependent effects of vertebral position on lumbar paraspinal muscle spindle discharge (14) and to determine the time course for these effects. Vertebral positions that shortened muscle should tighten and load the spindle, increasing resting spindle discharge upon return to an intermediate position. Conversely, vertebral positions that lengthen muscle should slacken and unload the spindle, decreasing resting spindle discharge upon return to an intermediate position. We reasoned that if a crossbridge mechanism underlies these thixotropic effects in lumbar paraspinal muscles, then the relationship between the magnitude of spindle discharge and the duration of the vertebral position would be one of exponential growth or decay, respectively. Based upon the leg muscle studies in humans (8), we expected the time constant to be on the order of 3–4 seconds.
Methods
Experiments were performed on 30 deeply anesthetized cats treated in accordance with the Guiding Principles in the Care and Use of Animals approved by the American Physiological Society. All procedures have been described previously (14;15). Briefly, deep anesthesia was maintained with pentobarbital sodium (35mg/kg, i.v.) and additional dosages (~5mg/kg, iv) were given when necessary. Cats were mechanically ventilated (model 681, Harvard Apparatus Company, Inc., Millis, MA). A laminectomy was performed removing the caudal half of L4 and all of L5 vertebral segments to expose the L6 dorsal root. Paraspinal tissues of the low back innervated by the L6 and L7 dorsal roots remained intact on both the left and right side. Arterial pH, PCO2 and PO2 were measured every 90 minutes using i-STAT System (i-STAT Corporation, East Windsor, NJ) and were maintained within normal range (pH:7.32–7.43, PCO2: 32–35 mmHg; and PO2: >85 mmHg).
Single unit nerve activity was recorded from 30 muscle spindle afferents in the L6 dorsal root on the right side and having receptive fields in the low back (lumbar multifidus or longissimus muscles). There was likely little or no activity in gamma-motoneuron due to the deep level of anesthesia (16). Bundles of nerve filaments were teased apart and placed on an electrode until a single unit was isolated. Action potentials were identified using a PC based data acquisition system (Spike2, v5, Cambridge Electronic Design Ltd., Cambridge, UK).
While recording from lumbar paraspinal muscle spindles controlled mechanical loads were applied at L6 spinous process in dorsal-ventral direction. Applied loads were controlled using a feedback motor system (model 310, Aurora Scientific Inc., Ontario, Canada). The L6 spinous process was attached to the motor’s drive shaft through a pair of forceps. The lumbar spine was fixed at L4 spinous process and at the iliac crest in a Kopf spinal unit to stabilize the preparation and ensure efficient mechanical loading. The L6 vertebra was translated ventralward and dorsalward which loaded and unloaded the muscle spindles.
Two protocols were used to confirm an afferent was from a muscle spindle. Afferents were classified as muscle spindles based upon their increased discharge to succinlycholine (100 – 400mg/kg, iv) and decreased discharge to electrically induced muscle contraction. Conduction velocities were determined as nerve length divided by conduction latency. Stimulating electrodes were inserted in the vicinity of the L6-7 intervertebral foramen. Conduction distance was approximate and was determined by measuring the length of a thin thread extending from the recording electrode along the dorsal root and spinal nerve to its entrance at the intervertebral foramen. Conduction distances in error by 10 mm would over- or under-estimate conduction velocity by about 10–13 m/s. Mechanical thresholds of paraspinal muscle spindles were obtained using calibrated nylon monofilaments (Stoelting, Ill).
The experimental protocol (see inset, Figure 1) initially established identical motion segment histories prior by moving a vertebra back and forth rapidly (10 mm/s) ten times in a dorsal-ventralward direction. This deconditioning was followed by conditioning which represented a period of controlled history. The L6 vertebra was held in a static position that shortened (hold-short), lengthened (hold-long) or maintained the attached the muscles at an intermediate (hold-intermediate) position for 0, 0.5, 1.0, 1.5, or 2.0 seconds. At the intermediate position, the paravertebral tissues were in a neutral position exerting no net tension against the motor’s drive shaft. The 15 test combinations (3 directions × 5 durations) were randomized to minimize ordering effects. After conditioning, the vertebra was returned to or remained at the intermediate position for 0.5 seconds (static test) and then slowly (0.2 mm/s) moved in a direction (usually ventralward) that loaded the muscle spindle (dynamic test). Each of the 15 protocols was separated by at least 5 minutes.
Figure 1.
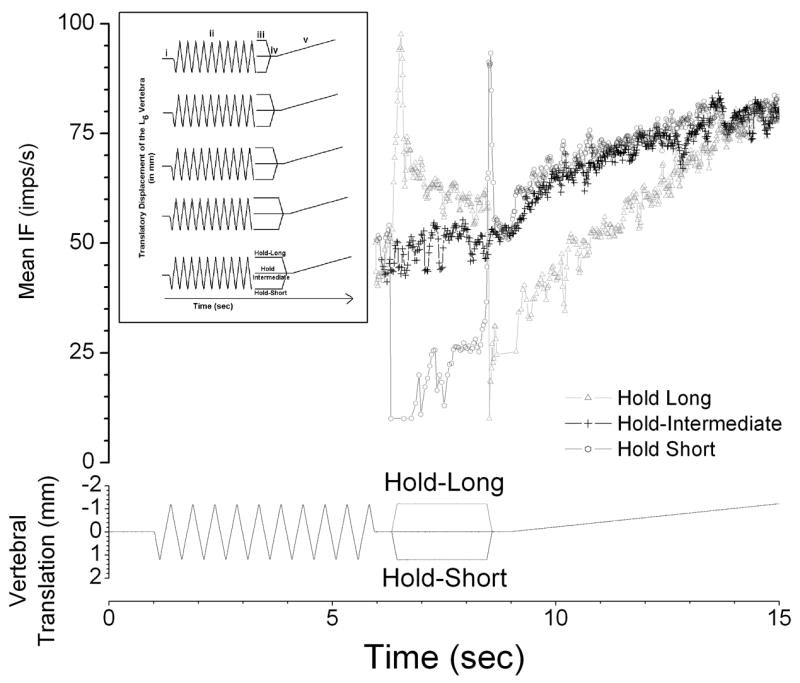
Representative response of one afferent to 2 sec conditioning to the 3 conditioning protocols. Bottom Panel: Loading protocols showing the change in vertebral position relative to the intermediate position. Note that at the beginning of the static test (iv) the vertebra was positioned identically for each of three protocols. Top Panel: average discharge frequency as a running average in 100ms time bins. Values only shown following deconditioning. Note that during conditioning (iii), hold-short (dark gray) decreased the discharge and hold-long (light gray) increased the discharge. Inset; Mechanical loads used in the experiments. Schematic of the experimental protocol showing the 5 conditioning durations and 3 conditioning positions. Roman numerals i, ii, iii, iv, and v, represent the initial, intermediate position, deconditioning, conditioning, static test at the intermediate position, and dynamic test from the intermediate position, respectively. See Methods section for details. The discontinuity at the end of deconditioning is graphic only and did not occur during the experiment as shown in the main figure.
Within a cat and across protocols, the intermediate position and the magnitude of vertebral translation during deconditioning, conditioning, and the dynamic test were identical. The magnitude of the translation was established for each cat by the displacement necessary to load the L6 vertebra at 50–60% of the cat’s body weight (BW). Displacements ranged between 0.9 and 2.0 mm [1.4 (0.3) mm; mean (SD)], similar to that used in our previous study (14).
Spindle activity was quantified as mean instantaneous frequency (MIF) for the static test and mean frequency (MF) for the dynamic test. MIF was calculated by averaging the reciprocal of each time interval between consecutive action potentials. MF was calculated by number of action potential divided by fixed duration. Muscle spindle responsiveness during the static and dynamic tests was characterized as a change (Δ) by subtracting MIF or MF after conditioning in the hold-intermediate position from the MIF or MF after conditioning in the hold-short (ΔMIFshort or ΔMFshort) or hold-long (ΔMIFlong or ΔMFlong) position. A positive value indicated an increase in muscle spindle responsiveness and conversely, a negative value indicated a reduction in muscle spindle responsiveness. When values were close to zero, conditioning produced little change in muscle spindle responsiveness.
A sample size of 30 provided 80% power at the 0.05 level of significance to detect a 5 imp/s difference between the conditioning durations. Five imps/s was considered to be a minimally important difference because this change in muscle spindle discharge frequency likely represents a 1.25 mm change of muscle length (17). The effect of conditioning duration on ΔMIF or ΔMF was compared with a one-way analysis of variance (ANOVA) using a randomized complete block design. Post-hoc pairwise comparisons were performed by the Bonferroni method when conditioning duration was statistically significant at the 0.05 level of significance. Assumptions of normality and homogeneity of variance were examined using residual plots. Spindle responses are reported as means (lower 95% confidence limit, upper 95% confidence limit) unless otherwise indicated. Other values are represented as mean (SD) unless otherwise noted. Statistical analyses were conducted using SAS (version 8, SAS Institute, Cary, NC).
Linear and exponential regressions of conditioning duration on changes in spindle discharge were performed for the static test. The regressions were performed using Origin (v7.5, OriginLab Corporation, Northampton, MA). The function used in the linear regression was:
where y is either ΔMIFlong or ΔMIFshort, a is the y intercept and b is the slope.
The function used in exponential regression for hold short was the same as that used by Hufschmidt and Schwaller (8):
Because resting spindle discharge decreased in response to hold-long, an exponential decay function was used for the regression:
where y is either ΔMIFlong or ΔMIFshort, yo is an offset, x is the conditioning duration, and s is the ΔMIF at saturation and t is the time constant.
Results
All afferents were activated by succinylcholine and silenced by bipolar muscle stimulation. The receptive field of each of 30 paraspinal muscle spindle afferents was located in either the lumbar multifidus (n = 6) or longissimus (n = 24) muscles. Most receptive fields were in deeper parts of the paraspinal muscles close to the L6-7 facet joint (Fig. 2). Mechanical threshold ranged between 0.03 and 75.9 gm [7.4 gm (SD 15.6)] similar to those found in our previous study (14). Conduction velocities ranged between 34.3 and 69.8 m/s [51.9 m/s (SD 9.5)]. Ventralward translation of the L6 vertebra loaded 24 muscle spindles and dorsalward translation loaded the remaining 6 spindles.
Figure 2.
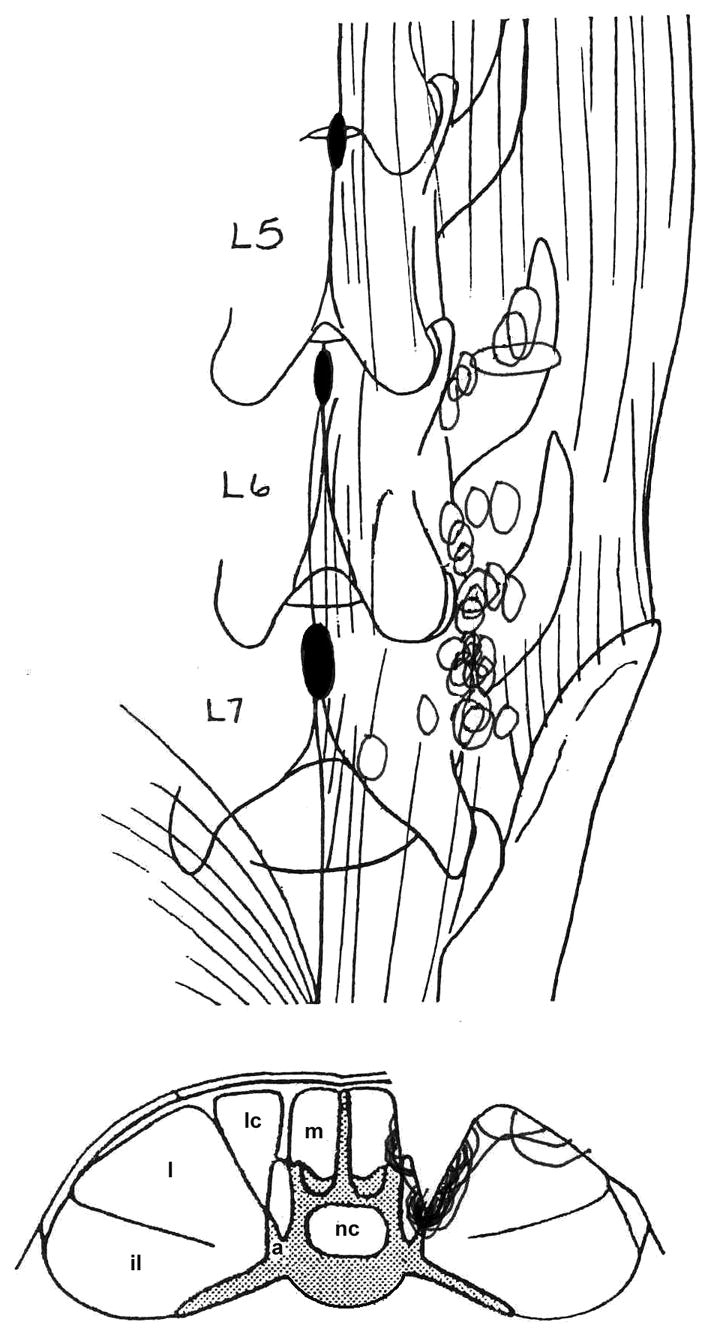
Location of the most sensitive portion of the receptive fields for each paraspinal muscle. spindle. Top: dorsal view of the lumbar spine from the 5th lumbar vertebra (L5)to the superior portion of the sacrum. Bottom, representative cross-section through the lumbar spine. il, iliocostalis; l, longissiumus; lc, lumbococcygeus; m, multifidus; a, accessory process; nc, neural canal.
Hold-long compared with hold-intermediate conditioning decreased resting muscle spindle discharge (Figure 3, filled symbols). For the static test, ΔMIFlong was −6.1 (−10.1, −2.1), −11.6 (−14.1, −9.0), −16.1 (−20.0, −12.2), −19.2 (−23.9, −14.6) and −20.6 (−25.4, −15.7) imp/s for the 0, 0.5, 1.0, 1.5, 2.0 second conditioning durations, respectively. None of the 95% confidence intervals crossed 0 imp/s. The average decrease for the 0.5 to 2 second conditioning duration was −16.9 imp/s. The hold-long conditioning duration significantly affected the magnitude of resting muscle spindle discharge (F4, 116=25.66, p<0.001). Post-hoc pairwise comparisons indicated significant differences in ΔMIFlong between the control duration (0 seconds) and all other conditioning durations (p<0.01) as well as between the 0.5 and 1.5 or 2.0 second conditioning durations (p<0.001). The differences between the 0.5 and 1.0 and the 1.0 and 2.0 second conditioning durations were not significant (p = 0.07, 0.08, respectively). In addition, ΔMIFlongs between 1.0 and 1.5 and between 1.5 and 2.0 second conditioning durations were not significantly different (p = 0.59 and 1.0, respectively).
Figure 3.
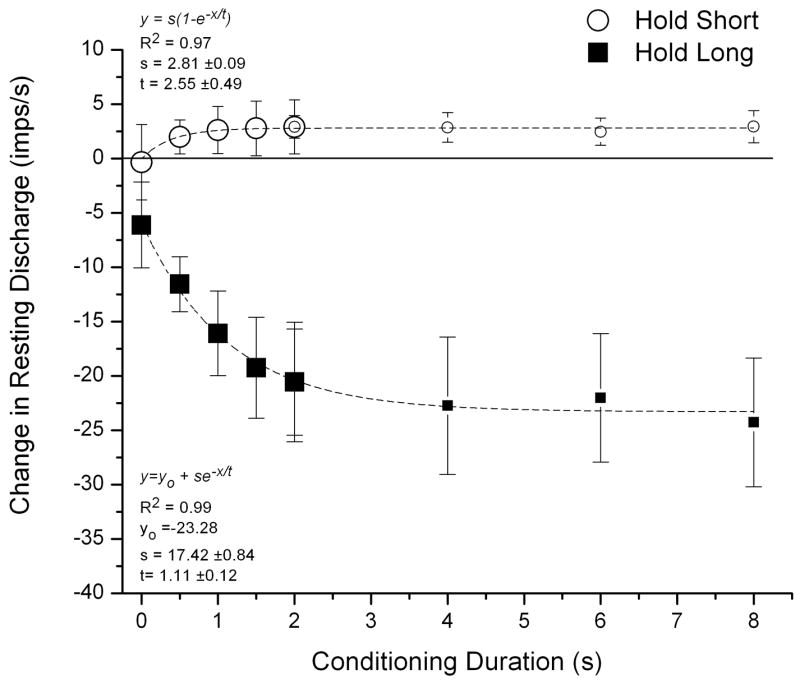
Static test: effect of conditioning direction and conditioning duration on paraspinal muscle spindle responses. Large symbols represent data from the current study. Small symbols represent data from Ge et al. (14) adjusted to make the mean ΔMIF at the 2 second duration equal from the 2 studies. The Y-axis represents the change in spindle response after hold long or hold short compared with hold intermediate. Each symbol represents the mean ±95% confidence interval for 30 observations. Dashed lines represent the fit to the means for the saturating exponential growth (upper plot) and decay (lower plot) functions, where y is either ΔMIFlong or ΔMIFshort, yo is an offset, x is the conditioning duration, and s is the ΔMIF at saturation and t is the time constant in seconds. R2 represents the coefficient of determination for the fit.
Hold-short compared with hold-intermediate conditioning increased resting muscle spindle but to a substantially smaller absolute magnitude compared with hold-long (Figure 3, open symbols). For the static test, ΔMIFshort was −0.3 (−3.8, 3.1), 2.0 (0.4, 3.6), 2.6 (0.5, 4.8), 2.8 (0.3, 5.3) and 2.9 (0.4, 5.4) imp/s for the 5 conditioning durations, respectively (Fig. 3). None of the 95% confidence intervals crossed 0 imp/s, except for the 0 sec duration. The average increase over the durations from 0.5 to 2 seconds was 2.6 Hz. However, the hold-short conditioning duration did not significantly affect ΔMIFshort (F4, 116 =2.27, p=0.07).
Muscle spindle responsiveness to passive vertebral movement decreased in response to hold-long compared to hold-intermediate conditioning (Figure 4, filled symbols). For the dynamic test, MF decreased by −0.7 (−1.2, −0.2), −2.6 (−3.5, −1.7), −4.4 (−5.6, −3.3), −5.7 (−7.1, −4.3) and −6.8 (−8.4, −5.3) imp/s for the 5 conditioning durations, respectively. Similar to the static test, none of the 95% confidence intervals crossed 0 imp/s. The average decrease was −4.9 imp/s over the conditioning durations from 0.5 to 2 seconds. Hold-long conditioning duration significantly affected muscle spindle responsiveness (F4, 116 =49.98, p=0.001). Post-hoc pairwise comparisons indicated significant differences between all durations (p<0.003), except between the 1.0 and 1.5 second duration (p=0.09) and between the 1.5 and 2.0 second duration (p=0.26). In contrast, hold-short conditioning increased MF by 0.9 (0.4, 1.4), 0.9 (0.4, 1.5), 1.0 (0.3, 1.6), 1.3 (0.6, 1.9) and 1.0 (0.4, 1.6) imp/s for those 5 conditioning durations, respectively (Fig. 4). None of the 95% confidence intervals crossed 0 imp/s. The average decrease was 1.0 imp/s over the durations from 0.5 to 2 seconds. For the dynamic test, ΔMFshort was not statistically significant among the 5 levels of conditioning durations (F4, 116 =0.37, p=0.83).
Figure 4.
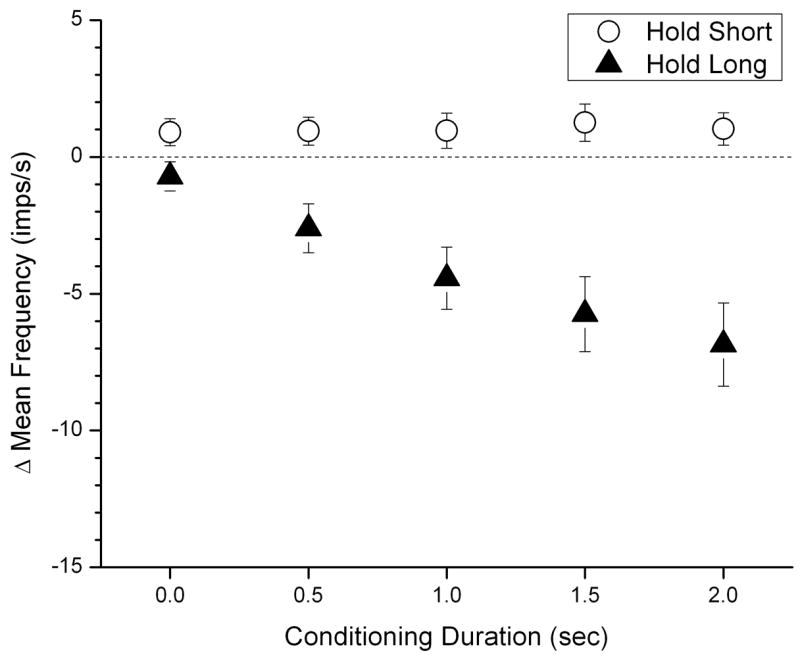
Dynamic Test: effect of conditioning direction and conditioning duration on paraspinal muscle spindle responses. The Y-axis represents the change in spindle response after hold long or hold short compared with hold intermediate. Each symbol represents the mean ±95% confidence interval of 30 observations.
Table 1 reports the correlation coefficients (R-values) for the linear and exponential regressions of conditioning duration on resting spindle discharge during the static test. Regressions include data obtained from a previous study (14) where longer conditioning durations were used (2, 4, 6, 8 as well as 0 seconds). A constant value was added to ΔMIF at each of the latter conditioning durations so as to render ΔMIF at 2 seconds comparable to the current study as shown in Figure 3. The exponential regression better described the relationship between the duration of vertebral history and the change in resting spindle than the linear regression. For response to hold-long positioning, it took approximately 1.1 seconds of hold-long positioning to reach 67% of final decrease in resting spindle discharge whereas the time constant was nearly twice that (2.6s) for hold-short positioning.
Table 1.
Correlation coefficients for regression of the change in spindle discharge during the static test on conditioning duration.
| CONDITIONING DIRECTION | ||
|---|---|---|
| Hold-short | Hold-long | |
| Linear regression | 0.49 | −0.79 |
| Exponential regression | 0.98 | −1.00 |
Figure 5 illustrates the time course of the dynamic test arising from the effects of vertebral history. ΔMFshort and ΔMFsong are shown in relation to 10% increments in the maximal displacement, the displacement which had loaded the spine to 50–60 % body weight (see Methods). The process is nonlinear and the changes are the highest at the beginning of dynamic test. As vertebral translation approaches the maximal displacement (ie, at 100%), the effects of vertebral history are gradually abolished.
Figure 5.
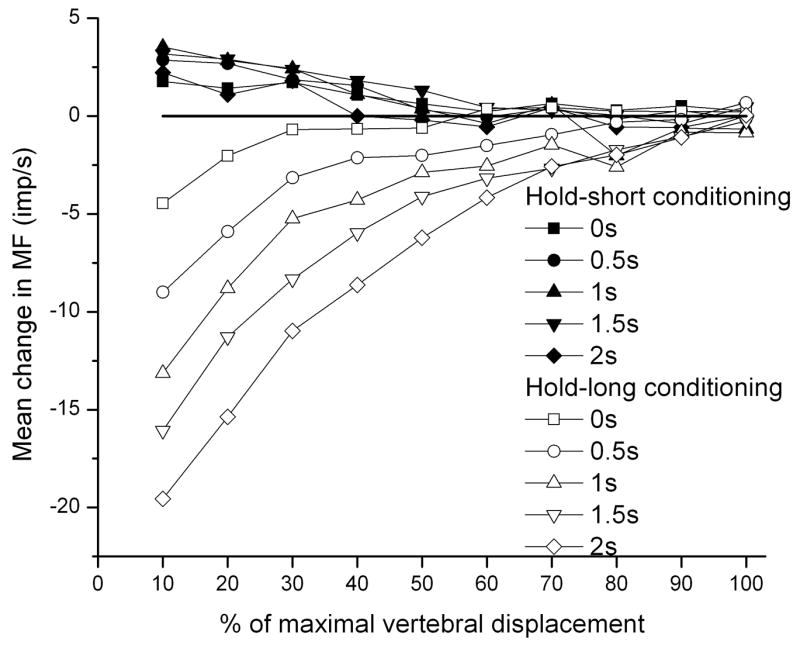
Effect of conditioning direction and conditioning duration on muscle spindle responses to movement over the time course of the dynamic test in 10% increments. The x-axis is normalized by the maximal displacement used for each spindles based on the displacement that loaded the spine 50–60 % body weight. The velocity of displacement in dynamic test was same for all spindles (0.2 mm/s). The displacements were between 0.9 and 2.0 mm.
Discussion
The results indicated that lumbar vertebral positions maintained for less than 2 seconds were capable of evoking different discharge rates from lumbar paraspinal muscle spindles despite the vertebra having been returned to its original location. Both spindle resting discharge and their responsiveness to movement were altered. Conditioning vertebral positions that stretched the spindles decreased spindle afferent activity and positions that unloaded the spindles increased spindle afferent activity upon returning the vertebra to identical original (intermediate) positions. The magnitude of these effects increased as conditioning duration increased to 2 seconds. When combined with a previous study (14), the data indicated that these effects developed with a time course following a first order exponential reaching a maximal value after approximately 4 seconds of history. The time constant for a hold-short history was 2.6 seconds and for a hold-long history was approximately half of that at 1.1 seconds. These sensory alterations due to vertebral history would represent a proprioceptive input not necessarily representative of the current state of segmental positioning in the lumbar spine.
The exact mechanical mechanism by which muscle stretch itself activates muscle spindle afferents is not known (18). Muscle spindle afferents respond to deformation of their sensory terminals, a deformation transmitted by mechanical attachments between cell membrane of a sensory terminal and an intrafusal muscle fiber (18;19). While the nature of the attachments and their relationship to membrane conductance changes has not been determined, it is well accepted and incorporated into muscle spindle models that an intrafusal fiber’s stiffness contributes to the timing and fidelity with which passive stretch can be transmitted to and deform the sensory terminal (20;21). This mechanical property is likely conferred by both crossbridge formation and passive, structural elements (non-crossbridge) but the history dependent effects on this property are likely due to the spontaneous formation of stable, non-recycling crossbridges between actin and myosin (4–6;8;22). When intrafusal fibers are held stretched non-recycling cross-bridges would form at this longer length. Upon being returned to a shorter position, the crossbridges restrain the myofilaments from sliding thus slackening or kinking the intrafusal fiber and reducing its stiffness. Subsequent stretching would decrease muscle spindle responsiveness (relative to not having previously been held stretched) until the slackness had been removed, similar to that observed in Figure 5 for hold-long. Conversely, holding intrafusal fibers in a shortened position would allow cross-bridge formation at the shorter length, stiffen the fiber and increase spindle afferent discharge.
Our data are consistent with an active, stable crossbridge mechanism for three reasons. First, contrasting changes in muscle spindle responsiveness depended upon directionally opposite movements of the vertebra. These effects, in particular for hold-long, were abolished when vertebral movement during the dynamic test had traversed the conditioning position. This suggests the detachment of structures that formed at the conditioning length. It is difficult to reconcile how purely passive, structural elements incapable of changing their chemical energy state could affect the directionally specific changes in intrafusal fiber stiffness. Second, there was an asymmetry to the absolute magnitude of the history-dependent effect, ie to ΔMIF and ΔMF. The absolute magnitude was less for hold-short than for hold-long conditioning, again difficult to reconcile with a purely passive, structural explanation. During the return from hold-short conditioning to the intermediate position, the increase in spindle discharge was greatest at the beginning of the ramp and became less as the intermediate position was reached (see Figure 1). We speculate that during the return (which represented a lengthening of the paraspinal muscles relative to the hold-short conditioning position) the crossbridge equilibrium shifted towards increasing detachment. While there is evidence, albeit controversial, that intrafusal fiber stretch can induce the formation of non-stable, recycling crossbridges [see (18) for review], this mechanism is not consistent with our data showing that muscle spindle discharge actually decreased during the lengthening from the hold-short conditioning position.
Third, the exponential decay and growth relationship between conditioning duration and the change in resting spindle discharge directly supports the stable crossbridge model developed by Hufschmidt and Schwaller (8). The time constants in the present experiment were on the same order of magnitude as the 3.4 to 4.2 seconds found by Hufschmidt and Schwaller for the thixotropic increase in leg muscle stiffness. In the present experiments the time constants were 1.5 to 3 times shorter than those for the extrafusal fibers. While this may simply represent experimental variation, it is also possible that crossbridge kinetics in the intrafusal fibers is different from that in extrafusal fibers due to either structural and/or functional considerations. For example, the myosin heavy chain composition of intrafusal fibers is quite different from that of extrafusal fibers (23) as are the shortening velocities of intrafusal compared to extrafusal fibers (see (18;24)). Taken together the data suggest that thixotropic properties in the lumbar spine are caused by the rapid, spontaneous formation of stable crossbridges.
PERSPECTIVE
Panjabi classified the physiological systems that contribute to the vertebral column’s biomechanical stability into 3 subsystems: passive, active, and neural control (25). The passive subsystem, comprised of vertebrae, facet joints, intervertebral discs, ligaments, tendon, and passive muscle properties, does not require high energy compounds to generate mechanical forces. The active subsystem, comprised of the contractile apparatus of paravertebral muscles, generates intersegmental and regional forces through ATP hydrolysis. The neural control subsystem, through the integrative action of the central nervous system and the final common output pathways of alpha- and gamma-motoneurons, engages the active subsystem by using mechanical, chemical, and thermal sensory feedback combined with descending feedforward signals from higher centers. The combined activity of these subsystems contributes to a control system that regulates muscle activity to help maintain spinal stability in the face of changing static loads (eg, external forces) and dynamic loads (e.g., inertial loads applied to or by the vertebral column).
In neutral spinal postures with low loading moments the lumbar spine’s mechanical stability is least. Muscle activity is low and passive structures operate on the toe region of their force-displacement curves (26;27). During these times efferent signals from the neural subsystem must be timed and distributed appropriately between the smaller intersegmental muscles (e.g., multifidus, semispinalis) and the larger multisegmental muscles (e.g., longissimus, iliocostalis) otherwise vertebral segments are at risk for buckling (28;28;29). Even very small increases in activity of lumbar multifidus, iliocostalis and thoracic longissimus muscles at L2-L4 (1–3% of MVC; maximal voluntary contraction) appear sufficient to restore lumbar spine stability when loading moments are increased to as high as 75% of body weight (26). Thus, small compromises in the activity of the active subsystem may affect lumbar spine biomechanics. These considerations have led to the idea that damage to structures of the vertebral column and the risk of injury to the spine can be great during easy, non-demanding tasks curves (26).
The effects of muscle history would represent a previously unrecognized factor influencing the active and neural control subsystems of the spinal column. It provides a potential source of inaccuracy in proprioceptive signaling of vertebral kinematics. In the present experiments, conditioning was thought to simulate a motion segment’s position that might be passively maintained due to fixation, external load, a prolonged posture, or structural change. In particular, vertebral positions that lengthened the paraspinal muscles reversibly but substantially desensitized the spindle apparatus. While alterations in segmental reflexes would be expected, especially based upon previous studies from the limbs (9–11), higher levels of motor control may be affected as well. The excitability of the human motor cortex is decreased by thixotropic decreases in muscle spindle input (30). Clinically, we speculate that position-sensitive, spine-mediated changes in proprioceptive feedback may contribute to the mechanism by which awkward or sustained postures of the low back contribute to work-related injuries (31). In addition, the basis for the suggestion that walking after long drives reduces the risk of back injury (32) may involve a removal or resetting of the neural feedback system. Might rehabilitation or strengthening programs help the neuromuscular control system compensate either mechanically and/or neurologically for sensory feedback anomalies arising from muscle spindle’s thixotropic property? Because neuromuscular control of the spine is considered crucial for both spinal mobility and stability, the potential impact of thixotropy on spinal physiology warrants further investigation.
Acknowledgments
The authors thank Mr. Tom Cobb for technical assistance. This project is supported by NIH grant R01NS46818. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR15433-01 from the National Center for Research Resources, National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Joel G. Pickar, Palmer Center Chiropractic Research Davenport, IA.
Weiqing Ge, Palmer Center for Chiropractic Research, wge@ysu.edu.
Reference List
- 1.Dolan KJ, Green A. Lumbar spine reposition sense: The effect of a ‘slouched’ posture. Man Ther. 2006;11(3):202–207. doi: 10.1016/j.math.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Brumagne S, Cordo P, Lysens R, Verschueren S, Swinnen S. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine. 2000;25(8):989–994. doi: 10.1097/00007632-200004150-00015. [DOI] [PubMed] [Google Scholar]
- 3.Descarreaux M, Blouin JS, Teasdale N. Repositioning accuracy and movement parameters in low back pain subjects and healthy control subjects. Eur Spine J. 2005;14(2):185–191. doi: 10.1007/s00586-004-0833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol. 1993;41:705–721. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- 5.Brown MC, Goodwin GM, Matthews PBC. After-effects of fusimotor stimulation on the response of muscle spindle primary afferent endings. J Physiol. 1969;205:677–694. doi: 10.1113/jphysiol.1969.sp008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill DK. Tension due to interaction between the sliding filaments in resting striated muscle - the effect of stimulation. J Physiol. 1968;199:637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan DL, Prochazka A, Proske U. The after-effects of stretch and fusimotor stimulation on the responses of primary endings of cat muscle spindles. J Physiol. 1984;356:465–477. doi: 10.1113/jphysiol.1984.sp015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hufschmidt A, Schwaller I. Short-range elasticity and resting tension of relaxed human lower leg muscles. J Physiol. 1987:391451–465. doi: 10.1113/jphysiol.1987.sp016749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles and errors of limb position sense in man. J Neurophysiol. 1988;59(4):1220–1230. doi: 10.1152/jn.1988.59.4.1220. [DOI] [PubMed] [Google Scholar]
- 10.Gregory JE, Morgan DL, Proske U. Changes in size of the stretch reflex of cat and man attributed to aftereffects in muscle spindles. J Neurophysiol. 1987;58(3):628–640. doi: 10.1152/jn.1987.58.3.628. [DOI] [PubMed] [Google Scholar]
- 11.Gregory JE, Mark RF, Morgan DL, Patak A, Polus B, Proske U. Effects of muscle history on the stretch reflex in cat and man. J Physiol. 1990;424:93–107. doi: 10.1113/jphysiol.1990.sp018057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owens EF, Jr, Henderson CN, Gudavalli MR, Pickar JG. Head repositioning errors in normal student volunteers: a possible tool to assess the neck’s neuromuscular system. Chiropr Osteopat. 2006;14:5. doi: 10.1186/1746-1340-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles. J Neurophysiol. 1986;56(2):451–461. doi: 10.1152/jn.1986.56.2.451. [DOI] [PubMed] [Google Scholar]
- 14.Ge W, Long CR, Pickar JG. Vertebral position alters paraspinal muscle spindle responsiveness in the feline spine: effect of positioning duration. J Physiol. 2005:569655–665. doi: 10.1113/jphysiol.2005.095281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickar JG. An in vivo preparation for investigating neural responses to controlled loading of a lumbar vertebra in the anesthetized cat. J Neurosci Methods. 1999;89:87–96. doi: 10.1016/s0165-0270(99)00060-6. [DOI] [PubMed] [Google Scholar]
- 16.Collins JG, Kendig JJ, Mason P. Anesthetic actions within the spinal cord:contributions to the state of general anesthesia. Trends Neurosci. 1995;18(12):549–553. doi: 10.1016/0166-2236(95)98377-b. [DOI] [PubMed] [Google Scholar]
- 17.Matthews PBC. Mammalian muscle receptors and their central actions. Baltimore: The Williams & Wilkin Co; 1972. [Google Scholar]
- 18.Hunt CC. Mammalian muscle spindle:peripheral mechanisms. Physiol Rev. 1990;70(3):643–663. doi: 10.1152/physrev.1990.70.3.643. [DOI] [PubMed] [Google Scholar]
- 19.Boyd IA. Films on the muscle spindle. In: Boyd IA, Gladden MH, editors. The Muscle Spindle. New York: Stockton Press; 1985. pp. 159–171. [Google Scholar]
- 20.McMahon TA. Muscles, Reflexes, and Locomotion. Princeton: Princeton Univ. Press; 1984. [Google Scholar]
- 21.Mileusnic MP, Brown IE, Lan N, Loeb GE. Mathematical models of proprioceptors. I. Control and transduction in the muscle spindle. J Neurophysiol. 2006;96(4):1772–1788. doi: 10.1152/jn.00868.2005. [DOI] [PubMed] [Google Scholar]
- 22.Proske U, Morgan DL. Do cross-bridges contribute to the tension during stretch of passive muscle? J Muscle Res Cell Motil. 1999;20(5–6):433–442. doi: 10.1023/a:1005573625675. [DOI] [PubMed] [Google Scholar]
- 23.Walro JM, Kucera J. Why adult mammalian intrafusal and extrafusal fibers contain different myosin heavy-chain isoforms. Trends Neurosci. 1999;22(4):180–184. doi: 10.1016/s0166-2236(98)01339-3. [DOI] [PubMed] [Google Scholar]
- 24.Boyd IA. The isolated mammalian muscle spindle. Trends Neurosci. 1980;3(11):258–265. [Google Scholar]
- 25.Panjabi MM. The stabilizing system of the spine. Part 1. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992;5(4):383–389. doi: 10.1097/00002517-199212000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Cholewicki J, McGill SM. Mechanical stability of the in vivo lumbar spine: implications for injury and chronic low back pain. Clin Biomech. 1996;11(1):1–15. doi: 10.1016/0268-0033(95)00035-6. [DOI] [PubMed] [Google Scholar]
- 27.White AA, Panjabi MM. Clinical Biomechanics of the Spine. 2. Philadelphia: J.B. Lippincott; 1990. [Google Scholar]
- 28.Mayer TG, Tencer AF, Kristoferson S, Mooney V. Use of noninvasive techniques for quantification of spinal range-of-motion in normal subjects and chronic low-back dysfunction patients. Spine. 1984;9(6):588–595. doi: 10.1097/00007632-198409000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Cholewicki J, Panjabi MM, Khachatryan A. Stabilizing function of trunk flexor-extensor muscles around a neutral spine posture. Spine. 1997;22(19):2207–2212. doi: 10.1097/00007632-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 30.Stuart M, Butler JE, Collins DF, Taylor JL, Gandevia SC. The history of contraction of the wrist flexors can change cortical excitability. J Physiol. 2002;545(Pt 3):731–737. doi: 10.1113/jphysiol.2002.032854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernard BP, editor. DHHS (NIOSH) Publication No. 97–141. 1997. Musculoskeletal disorder and workplace factors. A critical review of epidemiologic evidence for work-related musculoskeletal disorders of the neck, upper extremity and low back; pp. x–xv. [Google Scholar]
- 32.Wilder DG, Aleksiev AR, Magnusson ML, Pope MH, Spratt KF, Goel VK. Muscular response to sudden load. A tool to evaluate fatigue and rehabilitation. Spine. 1996;21(22):2628–2639. doi: 10.1097/00007632-199611150-00013. [DOI] [PubMed] [Google Scholar]


