Abstract
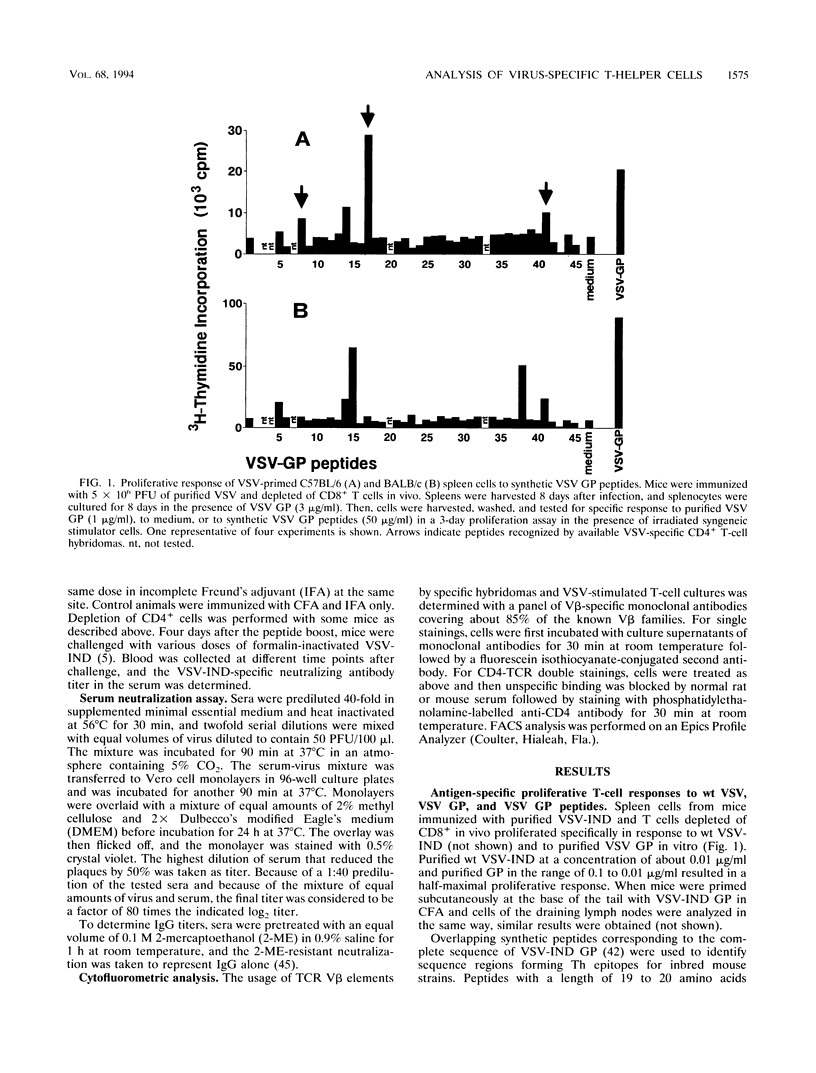
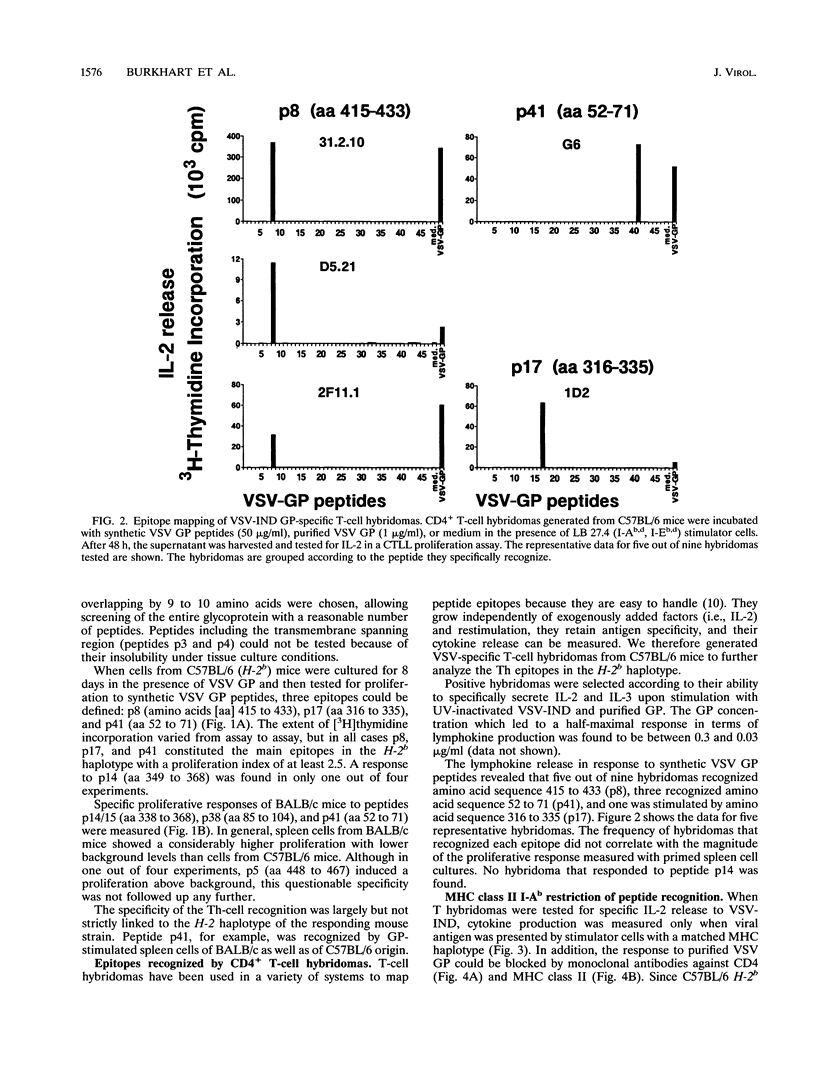
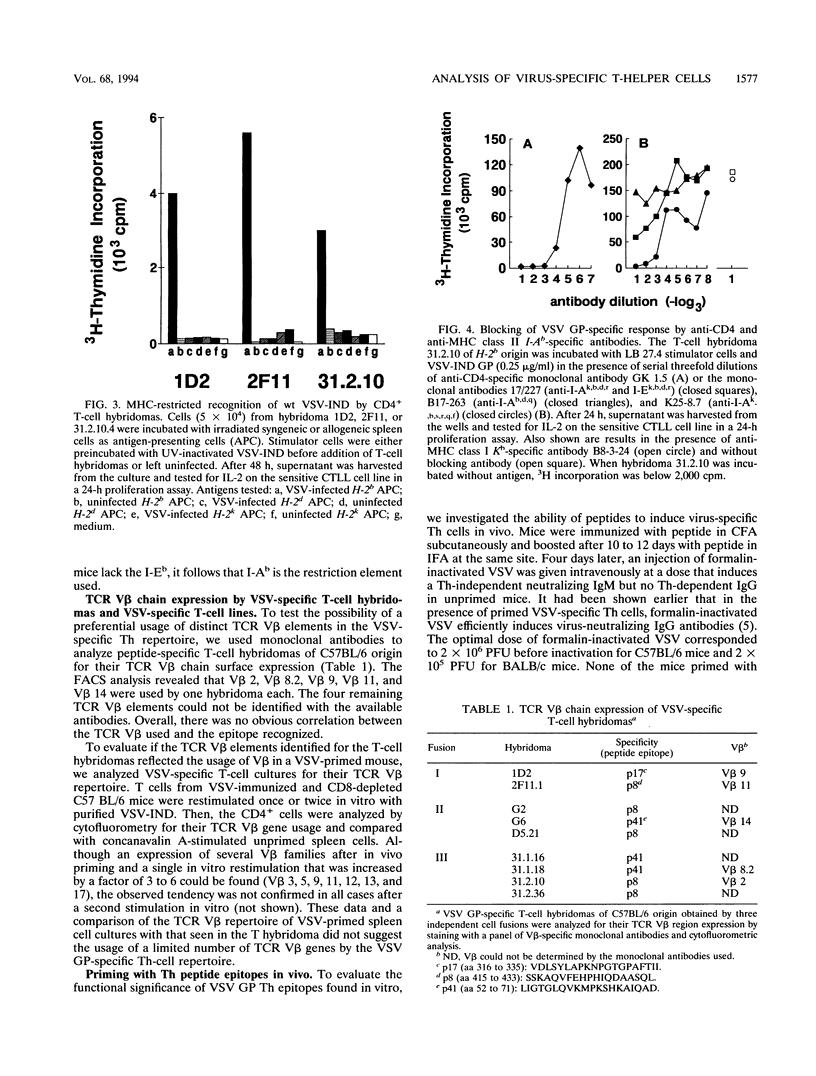
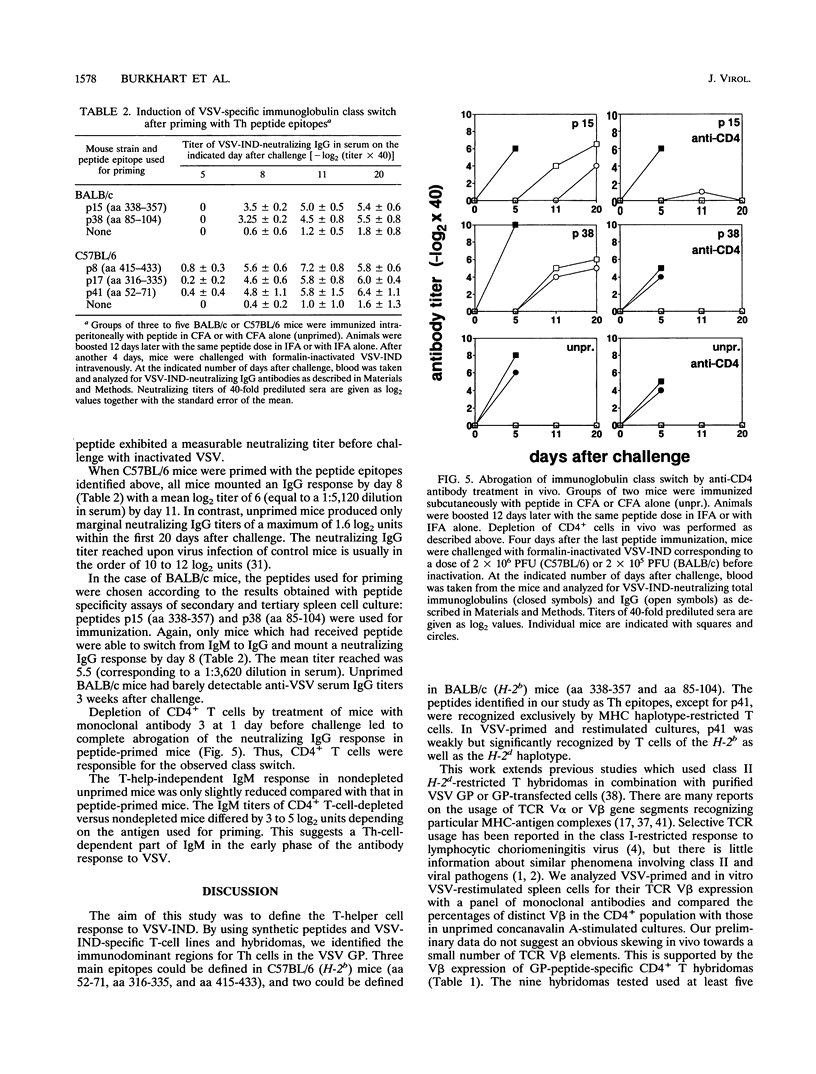
The T-helper (Th) cell epitopes in the glycoprotein (GP) of vesicular stomatitis virus serotype Indiana (VSV-IND) were analyzed with a complete panel of overlapping synthetic peptides. Three Th-cell epitopes in C57BL/6 (H-2b) mice and two epitopes in BALB/c (H-2d) mice were defined by their ability to stimulate in vitro proliferation of virus-primed, CD8+ T-cell-depleted spleen cells in a class II-restricted manner. A series of CD4+, I-Ab-restricted T-cell hybridomas from VSV-primed C57BL/6 mice were characterized by their production of interleukin-2 and interleukin-3 upon stimulation with VSV-IND or purified VSV GP in vitro. Of nine hybridomas derived from three independent fusions, five were specific for amino acids (aa) 415 to 433 (p8) of VSV-IND GP, three recognized aa 52 to 71 (p41), and one reacted against aa 316 to 335 (p17). Fluorocytometric analysis of Th hybridomas or VSV-stimulated T-cell lines with monoclonal antibodies specific for the T-cell receptor V beta chain did not reveal obvious correlations between the T-cell receptor V beta gene segment used and the epitope recognized. All three peptides recognized by H-2b mice and both epitopes recognized by H-2d mice which were characterized in primed T-cell populations were capable of activating specific Th cells in vivo as measured by the induction of antibody class switch from immunoglobulin M (IgM) to IgG. Thus, the epitopes are relevant for VSV GP-specific Th response in vivo and are able to provide functional help for the production of anti-VSV-specific neutralizing IgG antibodies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Mitchell D. J., Timmermann L., Wraith D. C., Tausch G. S., Waldor M. K., Zamvil S. S., McDevitt H. O., Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988 Jul 15;54(2):263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- Acha-Orbea H., Palmer E. Mls--a retrovirus exploits the immune system. Immunol Today. 1991 Oct;12(10):356–361. doi: 10.1016/0167-5699(91)90066-3. [DOI] [PubMed] [Google Scholar]
- Adorini L., Appella E., Doria G., Nagy Z. A. Mechanisms influencing the immunodominance of T cell determinants. J Exp Med. 1988 Dec 1;168(6):2091–2104. doi: 10.1084/jem.168.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebischer T., Oehen S., Hengartner H. Preferential usage of V alpha 4 and V beta 10 T cell receptor genes by lymphocytic choriomeningitis virus glycoprotein-specific H-2Db-restricted cytotoxic T cells. Eur J Immunol. 1990 Mar;20(3):523–531. doi: 10.1002/eji.1830200310. [DOI] [PubMed] [Google Scholar]
- Bachmann M. F., Kündig T. M., Kalberer C. P., Hengartner H., Zinkernagel R. M. Formalin inactivation of vesicular stomatitis virus impairs T-cell- but not T-help-independent B-cell responses. J Virol. 1993 Jul;67(7):3917–3922. doi: 10.1128/jvi.67.7.3917-3922.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzofsky J. A. T-B reciprocity. An Ia-restricted epitope-specific circuit regulating T cell-B cell interaction and antibody specificity. Surv Immunol Res. 1983;2(3):223–229. doi: 10.1007/BF02918417. [DOI] [PubMed] [Google Scholar]
- Binder D., Kündig T. M. Antiviral protection by CD8+ versus CD4+ T cells. CD8+ T cells correlating with cytotoxic activity in vitro are more efficient in antivaccinia virus protection than CD4-dependent IL. J Immunol. 1991 Jun 15;146(12):4301–4307. [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Grey H. M. Autologous peptides constitutively occupy the antigen binding site on Ia. Science. 1988 Nov 18;242(4881):1045–1047. doi: 10.1126/science.3194755. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis E., Ou D., Dietzschold B., Otvos L., Jr, Koprowski H. Rabies virus-specific T cell hybridomas: identification of class II MHC-restricted T-cell epitopes using synthetic peptides. Hybridoma. 1989 Jun;8(3):263–275. doi: 10.1089/hyb.1989.8.263. [DOI] [PubMed] [Google Scholar]
- Charan S., Zinkernagel R. M. Antibody mediated suppression of secondary IgM response in nude mice against vesicular stomatitis virus. J Immunol. 1986 Apr 15;136(8):3057–3061. [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Davidson H. W., Reid P. A., Lanzavecchia A., Watts C. Processed antigen binds to newly synthesized MHC class II molecules in antigen-specific B lymphocytes. Cell. 1991 Oct 4;67(1):105–116. doi: 10.1016/0092-8674(91)90575-j. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Ertl H. C., Dietzschold B., Gore M., Otvos L., Jr, Larson J. K., Wunner W. H., Koprowski H. Induction of rabies virus-specific T-helper cells by synthetic peptides that carry dominant T-helper cell epitopes of the viral ribonucleoprotein. J Virol. 1989 Jul;63(7):2885–2892. doi: 10.1128/jvi.63.7.2885-2892.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink P. J., Matis L. A., McElligott D. L., Bookman M., Hedrick S. M. Correlations between T-cell specificity and the structure of the antigen receptor. Nature. 1986 May 15;321(6067):219–226. doi: 10.1038/321219a0. [DOI] [PubMed] [Google Scholar]
- Francis M. J., Hastings G. Z., Clarke B. E., Brown A. L., Beddell C. R., Rowlands D. J., Brown F. Neutralizing antibodies to all seven serotypes of foot-and-mouth disease virus elicited by synthetic peptides. Immunology. 1990 Feb;69(2):171–176. [PMC free article] [PubMed] [Google Scholar]
- Gobet R., Cerny A., Rüedi E., Hengartner H., Zinkernagel R. M. The role of antibodies in natural and acquired resistance of mice to vesicular stomatitis virus. Exp Cell Biol. 1988;56(4):175–180. doi: 10.1159/000163477. [DOI] [PubMed] [Google Scholar]
- Hunt D. F., Michel H., Dickinson T. A., Shabanowitz J., Cox A. L., Sakaguchi K., Appella E., Grey H. M., Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992 Jun 26;256(5065):1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch N., Hämmerling G. J., Tada N., Kimura S., Hämmerling U. Cross-blocking studies with monoclonal antibodies against I-A molecules of haplotypes b, d and k. Eur J Immunol. 1982 Nov;12(11):909–914. doi: 10.1002/eji.1830121103. [DOI] [PubMed] [Google Scholar]
- Kündig T. M., Castelmur I., Bachmann M. F., Abraham D., Binder D., Hengartner H., Zinkernagel R. M. Fewer protective cytotoxic T-cell epitopes than T-helper-cell epitopes on vesicular stomatitis virus. J Virol. 1993 Jun;67(6):3680–3683. doi: 10.1128/jvi.67.6.3680-3683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A. D., Rothbard J. B., Howland K., Young R. A., Young D. B. Mapping of T cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 1987 May;6(5):1245–1249. doi: 10.1002/j.1460-2075.1987.tb02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Leclercq L., Bismuth G., Thèze J. Antigen-specific helper T-cell clone supernatant is sufficient to induce both polyclonal proliferation and differentiation of small resting B lymphocytes. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6491–6495. doi: 10.1073/pnas.81.20.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol. 1984 Jul;51(1):208–214. doi: 10.1128/jvi.51.1.208-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist T. P., Cobbold S. P., Waldmann H., Aguet M., Zinkernagel R. M. Functional analysis of T lymphocyte subsets in antiviral host defense. J Immunol. 1987 Apr 1;138(7):2278–2281. [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Macfarlan R. I., Dietzschold B., Wiktor T. J., Kiel M., Houghten R., Lerner R. A., Sutcliffe J. G., Koprowski H. T cell responses to cleaved rabies virus glycoprotein and to synthetic peptides. J Immunol. 1984 Nov;133(5):2748–2752. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Chisari F. V., Thornton G. B. Nonoverlapping T and B cell determinants on an hepatitis B surface antigen pre-S(2) region synthetic peptide. J Exp Med. 1986 Aug 1;164(2):532–547. doi: 10.1084/jem.164.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noelle R. J., Snow E. C. Cognate interactions between helper T cells and B cells. Immunol Today. 1990 Oct;11(10):361–368. doi: 10.1016/0167-5699(90)90142-v. [DOI] [PubMed] [Google Scholar]
- Osler A. G. Immunology of reaginic allergy: in vitro studies. Clin Exp Immunol. 1970 Jan;6(1):13–23. [PMC free article] [PubMed] [Google Scholar]
- Osman G. E., Toda M., Kanagawa O., Hood L. E. Characterization of the T cell receptor repertoire causing collagen arthritis in mice. J Exp Med. 1993 Feb 1;177(2):387–395. doi: 10.1084/jem.177.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrarca M. A., Reiss C. S., Diamond D. C., Boni J., Burakoff S. J., Faller D. V. T cell hybridomas define the class II MHC-restricted response to vesicular stomatitis virus infection. Microb Pathog. 1988 Nov;5(5):319–332. doi: 10.1016/0882-4010(88)90033-2. [DOI] [PubMed] [Google Scholar]
- Petri W. A., Jr, Wagner R. R. Reconstitution into liposomes of the glycoprotein of vesicular stomatitis virus by detergent dialysis. J Biol Chem. 1979 Jun 10;254(11):4313–4316. [PubMed] [Google Scholar]
- Rammensee H. G., Falk K., Rötzschke O. MHC molecules as peptide receptors. Curr Opin Immunol. 1993 Feb;5(1):35–44. doi: 10.1016/0952-7915(93)90078-7. [DOI] [PubMed] [Google Scholar]
- Reiner S. L., Wang Z. E., Hatam F., Scott P., Locksley R. M. TH1 and TH2 cell antigen receptors in experimental leishmaniasis. Science. 1993 Mar 5;259(5100):1457–1460. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Expression from cloned cDNA of cell-surface secreted forms of the glycoprotein of vesicular stomatitis virus in eucaryotic cells. Cell. 1982 Oct;30(3):753–762. doi: 10.1016/0092-8674(82)90280-x. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., al-Ramadi B. K., Rothbard J., Janeway C. A., Jr Truncation variants of peptides isolated from MHC class II molecules suggest sequence motifs. Nature. 1992 Oct 1;359(6394):429–431. doi: 10.1038/359429a0. [DOI] [PubMed] [Google Scholar]
- Sette A., Buus S., Appella E., Smith J. A., Chesnut R., Miles C., Colon S. M., Grey H. M. Prediction of major histocompatibility complex binding regions of protein antigens by sequence pattern analysis. Proc Natl Acad Sci U S A. 1989 May;86(9):3296–3300. doi: 10.1073/pnas.86.9.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J. Role of H-2 gene products in the function of T helper cells from normal and chimeric mice in vivo. Immunol Rev. 1978;42:108–137. doi: 10.1111/j.1600-065x.1978.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Stevens T. L., Bossie A., Sanders V. M., Fernandez-Botran R., Coffman R. L., Mosmann T. R., Vitetta E. S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988 Jul 21;334(6179):255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- Swain S. L. T cell subsets and the recognition of MHC class. Immunol Rev. 1983;74:129–142. doi: 10.1111/j.1600-065x.1983.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Tomonari K. A rat antibody against a structure functionally related to the mouse T-cell receptor/T3 complex. Immunogenetics. 1988;28(6):455–458. doi: 10.1007/BF00355379. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Vandepol S. B., Lefrancois L., Holland J. J. Sequences of the major antibody binding epitopes of the Indiana serotype of vesicular stomatitis virus. Virology. 1986 Jan 30;148(2):312–325. doi: 10.1016/0042-6822(86)90328-4. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Rüedi E., Althage A., Hengartner H., Reimann G. Thymic selection of H-2-incompatible bone marrow cells in SCID mice. Differences in T help for induction of B cell IgG responses versus cytotoxic T cells. J Exp Med. 1988 Sep 1;168(3):1187–1192. doi: 10.1084/jem.168.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]


