Abstract
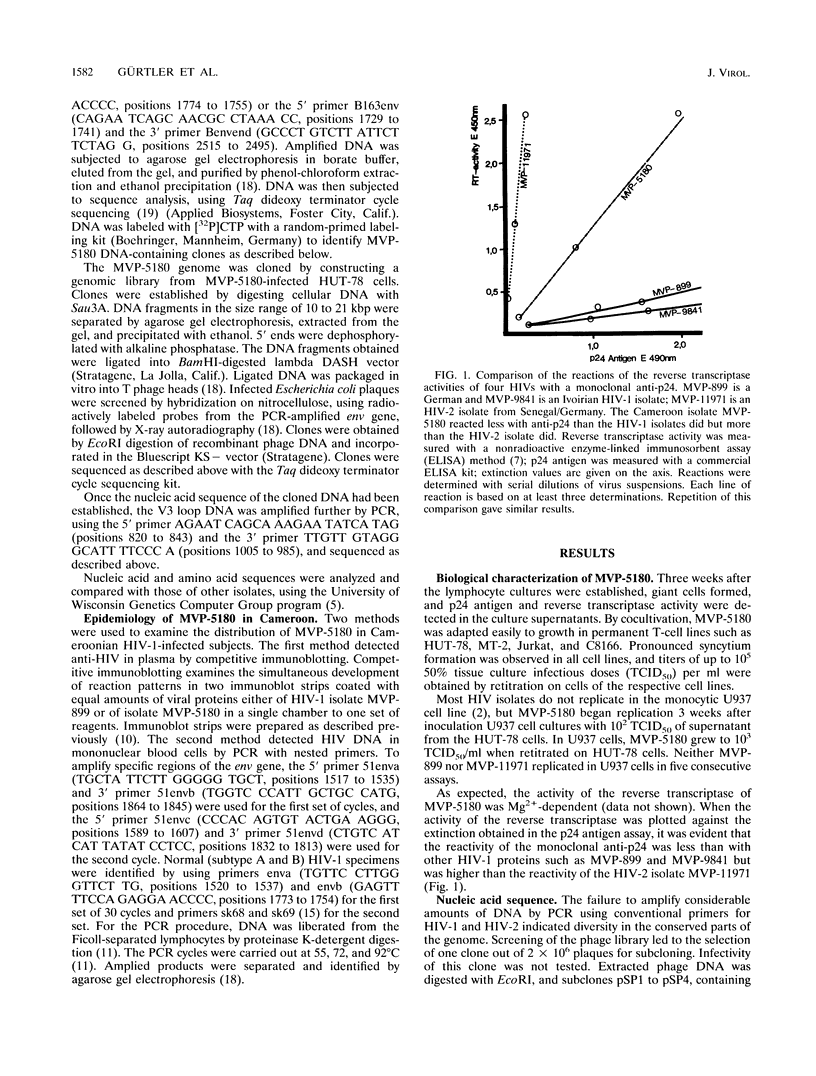
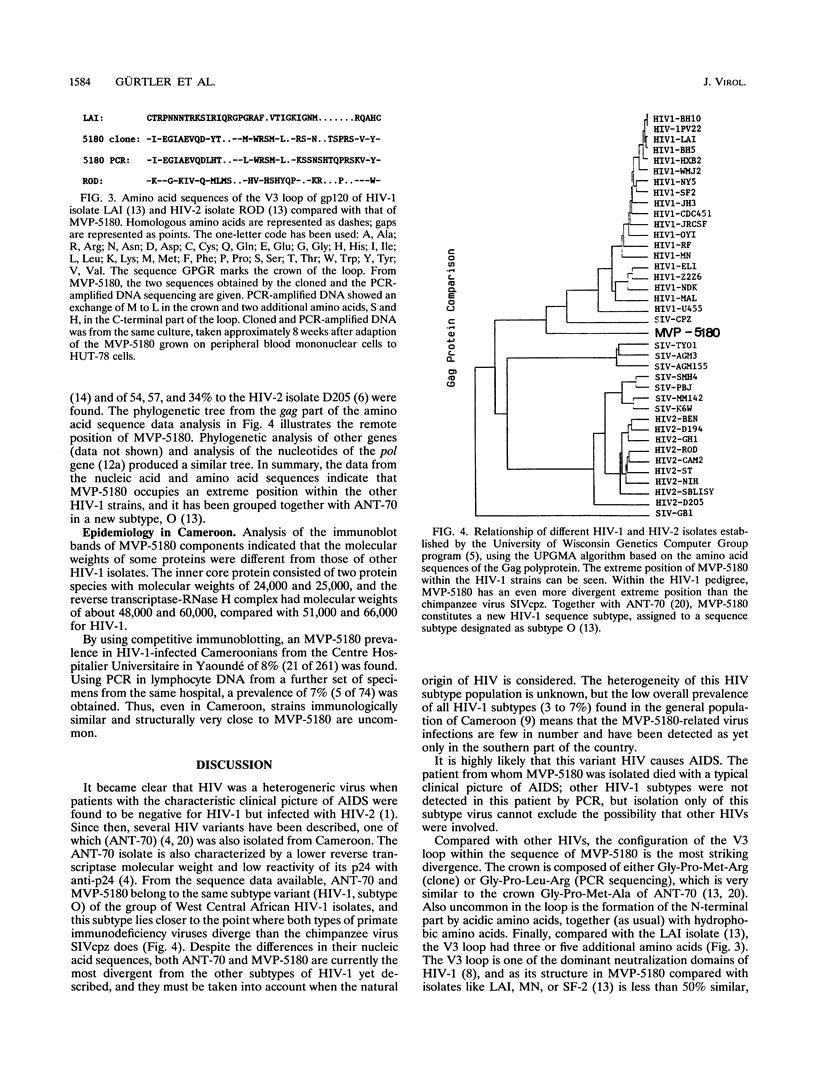
A new subtype (MVP-5180) of human immunodeficiency virus type 1 (HIV-1) was isolated from a Cameroonian AIDS patient. MVP-5180 was grown in several human T-cell lines and the monocytic U937 line. MVP-5180 DNA could not be amplified by nested primer PCR with conventional env primers and could be only very faintly amplified with gag and pol primers. Most German, Ivoirian, and Malawian anti-HIV-1 sera reacted faintly or moderately with Env proteins in an MVP-5180 immunoblot, whereas some Cameroonian sera reacted strongly. Of HIV-1-infected Cameroonians, 8% were identified by serological methods as infected with MVP-5180; 7% were positive when MVP-5180-specific PCR env primers were used. DNA sequence analysis of MVP-5180 showed that its genetic organization was that of HIV-1, with 65% similarity to HIV-1 and 56% similarity to HIV-2 consensus sequences. The env gene of MVP-5180 had similarities to HIV-1 and HIV-2 of 53 and of 49%, respectively. V3 loop analysis identified a crown of Gly-Pro-Met-Arg by using cloned DNA and Gly-Pro-Leu-Arg by using PCR-amplified DNA, neither of which configuration has been described for other HIV strains. In an analysis of relationships, MVP-5180 occupied a position distant to all other HIV-1 strains, including the chimpanzee simian immunodeficiency virus type 1 SIVcpz and the Uganda virus U455, and closer to the HIV-1/HIV-2 divergence node. MVP-5180, together with another Cameroonian isolate, ANT-70, constitutes a group subtype O of the most divergent HIV-1 isolates yet identified. Characterization of MVP-5180 is important for understanding the natural history of the primate immunodeficiency viruses and for the development of vaccines and diagnostics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Moore B. E. Spectrum of biological properties of human immunodeficiency virus (HIV-1) isolates. Virology. 1990 Jan;174(1):103–116. doi: 10.1016/0042-6822(90)90059-z. [DOI] [PubMed] [Google Scholar]
- De Leys R., Vanderborght B., Vanden Haesevelde M., Heyndrickx L., van Geel A., Wauters C., Bernaerts R., Saman E., Nijs P., Willems B. Isolation and partial characterization of an unusual human immunodeficiency retrovirus from two persons of west-central African origin. J Virol. 1990 Mar;64(3):1207–1216. doi: 10.1128/jvi.64.3.1207-1216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich U., Adamski M., Kreutz R., Seipp A., Kühnel H., Rübsamen-Waigmann H. A highly divergent HIV-2-related isolate. Nature. 1989 Dec 21;342(6252):948–950. doi: 10.1038/342948a0. [DOI] [PubMed] [Google Scholar]
- Eberle J., Seibl R. A new method for measuring reverse transcriptase activity by ELISA. J Virol Methods. 1992 Dec 1;40(3):347–356. doi: 10.1016/0166-0934(92)90092-r. [DOI] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Identification of the principal neutralizing determinant of human immunodeficiency virus type 1 as a fusion domain. J Virol. 1991 Jan;65(1):190–194. doi: 10.1128/jvi.65.1.190-194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Calleja J. M., Zekeng L., Louis J. P., Mvondo J. L., Trebucq A., Sokal D., Yanga D., Ndoumou A., Andela D., Salla R. HIV infection in Cameroon: 30 months' surveillance in Yaounde. AIDS. 1992 Aug;6(8):881–882. [PubMed] [Google Scholar]
- Gürtler L. G., Eberle J., Lorbeer B., Deinhardt F. Sensitivity and specificity of commercial ELISA kits for screening anti-LAV/HTLV III. J Virol Methods. 1987 Jan;15(1):11–23. doi: 10.1016/0166-0934(87)90044-9. [DOI] [PubMed] [Google Scholar]
- Murphy E., Korber B., Georges-Courbot M. C., You B., Pinter A., Cook D., Kieny M. P., Georges A., Mathiot C., Barré-Sinoussi F. Diversity of V3 region sequences of human immunodeficiency viruses type 1 from the central African Republic. AIDS Res Hum Retroviruses. 1993 Oct;9(10):997–1006. doi: 10.1089/aid.1993.9.997. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Downing R. G., Roff M., Clegg J. C., Serwadda D., Carswell J. W. Nucleotide sequence of a Ugandan HIV-1 provirus reveals genetic diversity from other HIV-1 isolates. AIDS Res Hum Retroviruses. 1990 Sep;6(9):1073–1078. doi: 10.1089/aid.1990.6.1073. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Peeters M., Honoré C., Huet T., Bedjabaga L., Ossari S., Bussi P., Cooper R. W., Delaporte E. Isolation and partial characterization of an HIV-related virus occurring naturally in chimpanzees in Gabon. AIDS. 1989 Oct;3(10):625–630. doi: 10.1097/00002030-198910000-00001. [DOI] [PubMed] [Google Scholar]
- Rieber E. P., Federle C., Reiter C., Krauss S., Gürtler L., Eberle J., Deinhardt F., Riethmüller G. The monoclonal CD4 antibody M-T413 inhibits cellular infection with human immunodeficiency virus after viral attachment to the cell membrane: an approach to postexposure prophylaxis. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10792–10796. doi: 10.1073/pnas.89.22.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Haesevelde M., Decourt J. L., De Leys R. J., Vanderborght B., van der Groen G., van Heuverswijn H., Saman E. Genomic cloning and complete sequence analysis of a highly divergent African human immunodeficiency virus isolate. J Virol. 1994 Mar;68(3):1586–1596. doi: 10.1128/jvi.68.3.1586-1596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


