Summary
There are many forms of oxalosis, with deposition of oxalate crystals in various organs, including arteries. In this retrospective study we describe deposition of calcium oxalate crystals within atherosclerotic plaques in coronary arteries of 4 patients, a site of oxalate deposition not previously reported. We suggest the phrase “atherosclerotic oxalosis” for this finding.
Background
Systemic oxalosis may be hereditary or acquired. In these cases, calcium oxalate deposits have been reported in numerous tissues, including the media of arteries. In any category, calcium oxalate deposition has not been described within atherosclerotic plaques in any arteries.
Methods
As part of a retrospective clinicopathologic study, 80 hearts were obtained from the National Neurologic AIDS Bank in an effort to study coronary atherosclerosis in patients infected with HIV. The population consisted of 66 HIV-positive and 14 HIV-negative patients with an average age of 47 years; 79% were males. Proximal coronary arteries were serially-sectioned and processed routinely. Sections were studied by H&E staining, and in selected cases, von Kossa stain, and alizarin red S under different conditions, including polarized light, to allow distinction of calcium phosphate from calcium oxalate. Medical histories, autopsy reports, and general autopsy slides were reviewed.
Results
In 4 patients (3 with AIDS) calcium oxalate crystals were observed within atherosclerotic plaques in the coronary arteries. Similar deposits were seen in the thyroid gland and other organs, but not in the kidneys. None of the patients had chronic renal failure.
Conclusion
The calcium oxalate crystal deposits observed in the atherosclerotic plaques in coronary arteries of these 4 patients are unique in two ways: 1) these deposits have not previously been described in atherosclerotic plaques, 2) the patients did not demonstrate any of the recognized patterns of oxalosis. We suggest the phrase “atherosclerotic oxalosis” to describe this finding.
Keywords: Oxalosis, hyperoxaluria, calcium oxalate, atherosclerosis, coronary artery, HIV
Introduction
Oxalosis is the accumulation of crystalline deposits of calcium oxalate in tissues. Precipitation of calcium oxalate occurs when the solubility of this highly insoluble salt is exceeded. There are several subclassifications of oxalosis, collectively involving a wide distribution of tissues. It is the purpose of this document to describe what we believe to be a new site of involvement not previously reported, the atherosclerotic plaque. We report four cases in which oxalate crystals were observed in coronary artery atherosclerotic plaques. The patients were being studied as part of an investigation into the relationship of coronary atherosclerosis and HIV/AIDS.
Methods
Heart specimens were obtained from the National Neurologic AIDS Bank as part of a study of coronary atherosclerosis in HIV infected patients. The population consisted of 66 HIV-positive and 14 HIV-negative patients with an average age of 47; 79% were male. Coronary arteries were fixed in formalin, decalcified when necessary, and cut serially every 2–3 mm. The proximal 2 cm of the left anterior descending, 2 cm of the left circumflex, 3 cm of the right coronary artery, and the entirety of the left main coronary artery were routinely processed, then embedded in paraffin wax. All microscopy slides were stained with hematoxylin and eosin (H&E) and reviewed by 3 observers (GAF, MCF, RGM).
Upon histological review of the coronary arteries, we observed oxalate in 4 cases. Calcium oxalate was identified as prismatic, light yellow-brown crystals birefringent in both polarized and semipolarized light (Fig 1). The presence of phosphate was confirmed histochemically by von Kossa stain.
Figure 1.
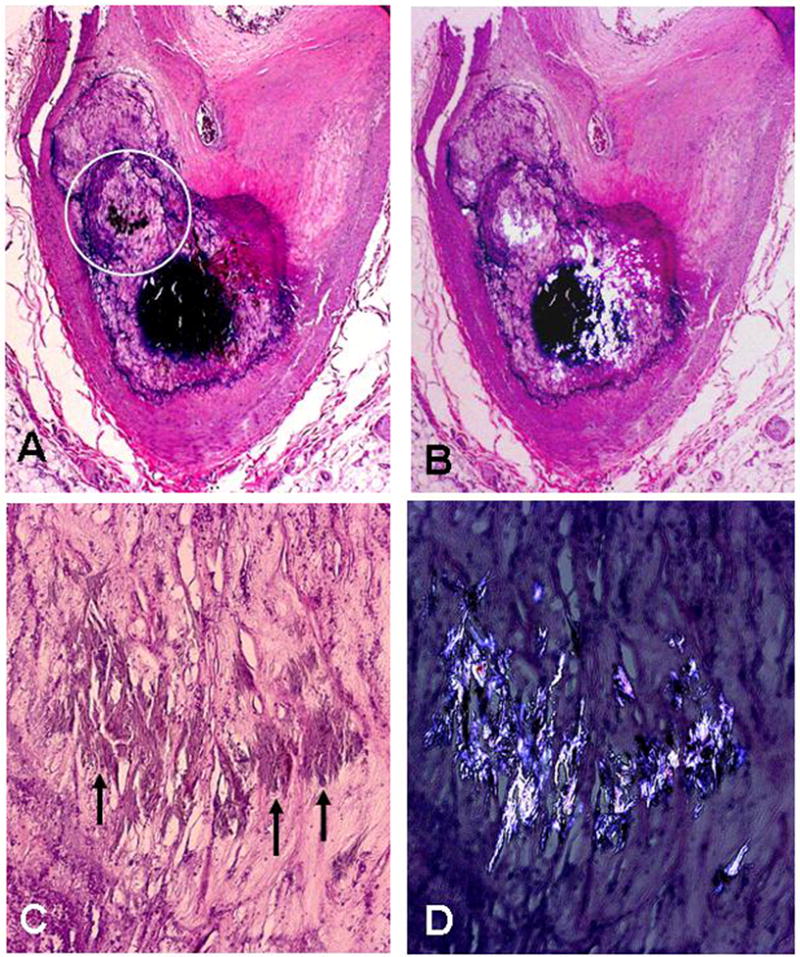
Calcified atherosclerotic plaque with oxalate crystals within the large calcific nodule: A) Low power H&E stain showing marked calcification of plaque; the circle indicates region shown in panels C & D (x40); B) Section shown in panel A with partially-polarized light showing oxalate crystals within the large calcific nodule (x40); C) and D) close up of oxalate crystals (arrows) inside of the calcified region of the atherosclerotic plaque without and with polarized light (x100).
Oxalate was differentiated from phosphate by a variety of stains using alizarin red S1. Staining at pH 4.2 indicates calcium phosphate but not oxalate, while at pH 7 both calcium phosphate and oxalate stain. Dissolution of calcium phosphate in 2M acetic acid followed by alizarin red S stain at pH 7 reveals only the calcium oxalate. Upon dissolution of both calcium phosphate and calcium oxalate in 0.1N HCl, neither calcium phosphate nor oxalate will be present to stain (Fig 2).
Figure 2.
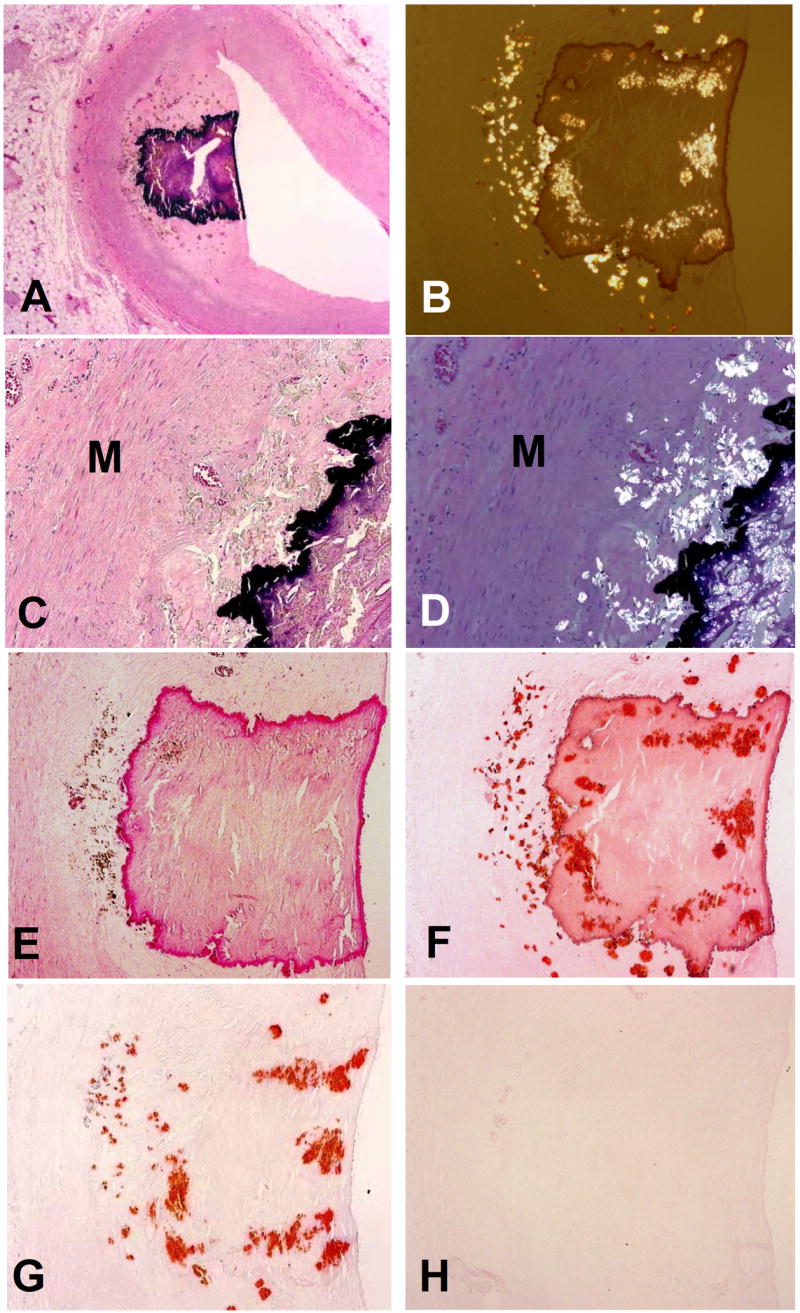
Atherosclerotic plaque with both calcium phosphate and calcium oxalate deposits: A) H&E stain showing calcified plaque; note brown dots that are oxalate crystals around the basophilic calcified nodule in the plaque (x12.5); B) under partially polarized light, the oxalate crystals can be seen in and around the large calcific nodule (H&E x40); C&D) Higher magnification of H&E stained section shows brown staining crystals that polarize, in intima but not media (M)(x100); E) Alizarin red S stain at pH 4.2 stains only calcium phosphate (x40); F) Alizarin red S stain at pH 7.0 stains both calcium phosphate and calcium oxalate (x40); G) Alizarin red S stain after treatment of slide with acetic acid that dissolves calcium phosphate stains only oxalate (x40), and G) Alizarin red S stain after treatment of slide with hydrochloric acid stains neither phosphate nor oxalate – both have been dissolved by the acid (x40).
Medical histories, autopsy reports, and histological slides were reviewed in the 4 cases in which the presence of oxalate was confirmed.
Report of Cases
Case 1
The patient, a 49 year old male, had a 21 year history of HIV and an 11 year history of AIDS prior to death from pneumonia and respiratory complications. Highly active antiretroviral therapy (HAART), including multiple protease inhibitors had been prescribed. Additionally, the patient developed secondary progressive multifocal leukoencephalopathy (PML). Coronary arteries showed oxalate crystal deposits within atherosclerotic plaques. Oxalate crystals were also clearly present in a mediastinal lymph node, thyroid follicles, the myocardium, and the media of the aorta and coronary arteries, but not the kidneys (Fig 3,4). No renal insufficiency was reported or observed; the patient’s creatinine and BUN levels were consistently normal as recently as two months before his death.
Figure 3.
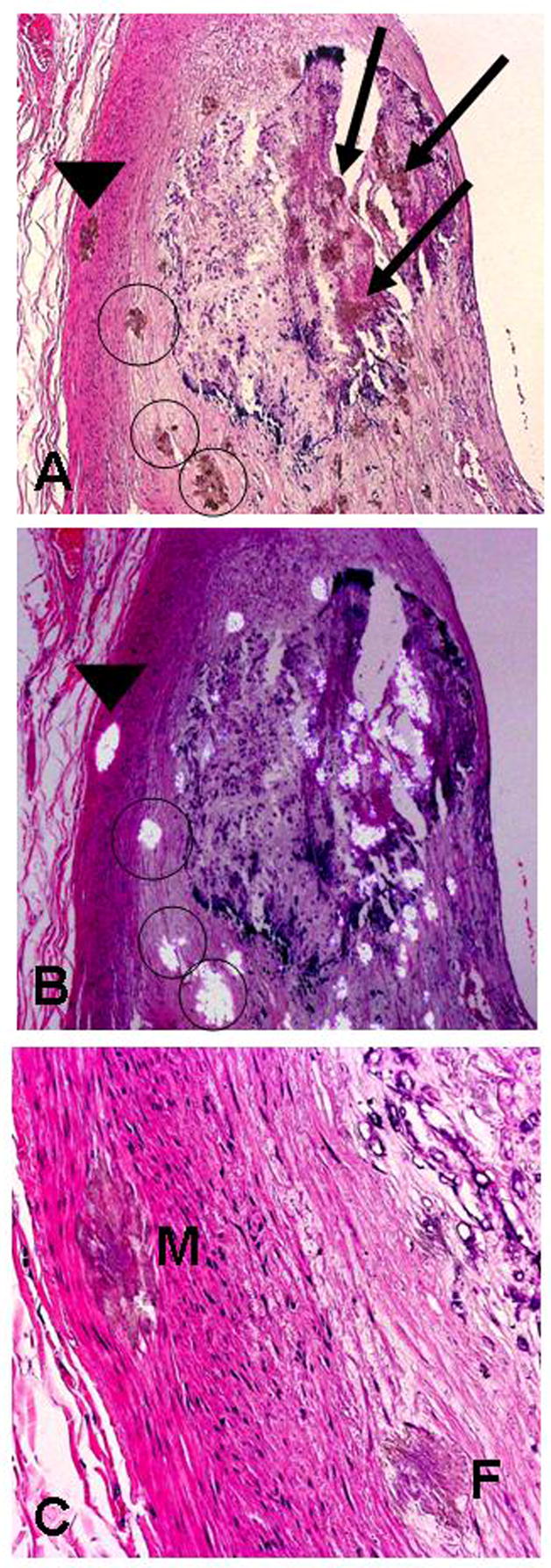
Coronary artery with oxalate deposits in media (M) as well as within the calcified and non-calcified regions of an atherosclerotic plaque: A) H&E stained section showing oxalate deposits in the media (arrowhead) (M), within the calcified region of the plaque (arrows), and in the non-calcified region (circles) of the plaque (x40); B) same section as A with partially-polarized light, and C) H&E at greater magnification showing oxalate crystals in media and fibrotic portion of plaque (F) (x200).
Figure 4.
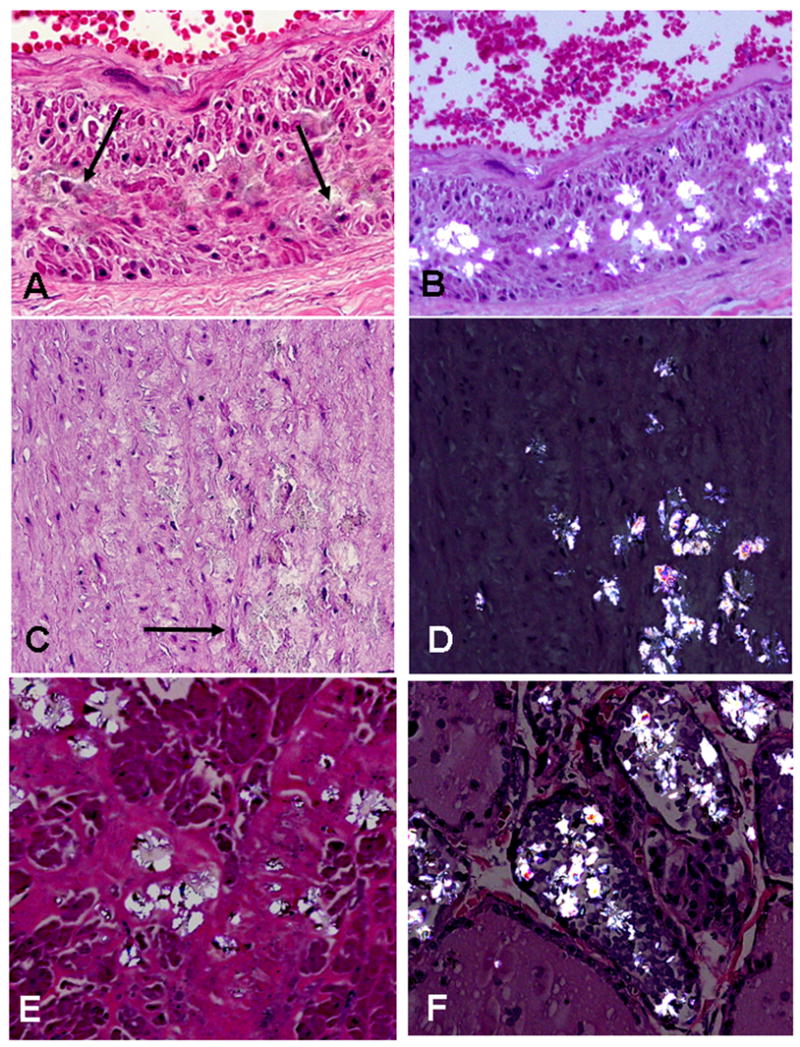
Figure legend: A & B) H&E stain of oxalate deposits in media of a normal coronary artery (arrows) (A = x200, B = x100 with partially polarized light); C & D: H&E stain of deposits in media of aorta (arrow) (C & D = x200, D under polarized light); E) oxalate crystals in myocardium (x100 with partially polarized light), and F) oxalate crystals in thyroid follicles (x200 under partial polarized light).
Case 2
A 52 year old male with a 14 year history of HIV and a 4 year history of AIDS died of end stage liver disease secondary to hepatitis C. In addition, the patient suffered from Kaposi’s sarcoma. HAART had been administered. The patient was also being treated for neurosyphilis and had a history of cryptococcal meningitis. The subject was in acute renal failure at the time of death, though no kidney disease was apparent histologically. His creatinine and BUN levels were slightly elevated on the day of his death, yet had been within the normal ranges only two days prior. Oxalate deposits were found in coronary atherosclerotic plaques and thyroid follicles. No oxalate was present in the myocardium or lymph nodes. While the patient did have minor calcifications in the renal medulla, no oxalate crystals could be seen histologically in the kidneys.
Case 3
A 36 year old male died of sepsis following a 5 year history of AIDS and a 13 year history of HIV. HAART, including multiple protease inhibitors, had been administered. Three months prior to death the patient exhibited normal levels of creatinine and only slightly elevated BUN levels (44 mg/dL). The patient had no history of renal or liver disease. Oxalate crystals were abundant in calcified atheromatous lesions of the coronary arteries. Minute oxalate deposits were discovered in the vasculature of the testis. Oxalate deposits were not discovered in the kidneys.
Case 4
A 78 year old male with a history of ischemic heart disease died of complications from metastatic prostate adenocarcinoma and leukemia. The patient was HIV-negative. Adenocarcinoma was metastatic to the liver, lungs and spleen. Additionally, the leukemia had extensively infiltrated the bone marrow, spleen, lymph nodes, and adrenal glands. The heart demonstrated old subendocardial infarcts and leukemic infiltrate. Extensive atherosclerosis was present in the coronary arteries and aorta, as well as calcinosis of the internal elastic lamina of coronary arteries. Oxalate crystals were seen in heavily calcified atherosclerotic plaques in coronary arteries. Calcium oxalate was also present in thyroid follicles but not in the kidneys. Although leukemic infiltrate was discovered in the kidneys, the patient had no history of renal disease.
Discussion
Oxalosis can be hereditary or acquired. Hereditary oxalosis, or primary hyperoxaluria, is a general term for at least three rare autosomal recessive disorders. Types I and II primary hyperoxaluria are alterations of glycoxalate metabolism resulting in the production of excess oxalate ions. Type III primary hyperoxaluria is caused by increased oxalate absorption by the gastrointestinal tract2. All three types are characterized by chronic renal failure and usually death at a young age. In fact, prior to the advent of hepatorenal transplantation, 80% of patients with primary hyperoxaluria died by age 203.
Acquired, or secondary, oxalosis exists in five sub-categories. Exogenous oxalosis is a well established side effect of the ingestion of oxalate, ethylene glycol, xylitol infusion, and methoxyflurane anesthesia. Enteric oxalosis describes the increased absorption of normal dietary oxalate in enteric diseases. Uremic oxalosis is associated with chronic renal insufficiency. Vitamin intake has also been linked to oxalosis. Dietary lack of cofactors such as pyridoxine and thiamine pyrophosphate has been implicated in deficiency oxalosis3. Additionally, excessive intake of vitamin C has been found to cause oxalosis4. The system-wide manifestations of the categories of oxalosis described above are similar as they all involve alterations in systemic oxalate metabolism. There is a subcategory to characterize the presence of oxalate deposits in the absence of oxalate metabolism alterations. This so-called dystrophic oxalosis has been associated with acute tissue injury, such as in the eye, or infection by oxalate producing micro-organisms, such as Aspergillus niger3.
The involvement of several organs is typical in oxalosis not of the dystrophic type. Due to alterations in oxalate metabolism, deposits of calcium oxalate crystals are found first in the kidney and commonly in myocardium and thyroid follicles. In fact, the kidney, being the primary site of oxalate excretion, is always implicated. The findings described in this report are therefore unique for two reasons. Oxalosis of an atherosclerotic plaque has not previously been described. In addition, the oxalosis we observed, while not confined to a single organ, specifically did not involve the kidney.
All of the four cases described above share at least one rare commonality – the presence of calcium oxalate deposits in coronary atherosclerotic plaques, which, to our knowledge, has not previously been described. In addition, oxalosis in the absence of chronic renal insufficiency is a peculiar finding. All four of these patients suffered from chronic disease, including three with advanced AIDS. Yet, beyond this commonality, there is apparently no clear relationship between all four cases. These findings therefore raise several questions. Why has oxalate deposition in atherosclerotic plaques, seen four times in a single study, never been previously identified? If man’s only outlet for endogenous oxalate is renal excretion3, why is there no renal involvement in these four cases? How should this type of oxalosis be classified?
Identification of Calcium Oxalate
The identification of oxalate by its optical properties is well established, most famously characterized by polarization microscopy. However, in the absence of polarization and specific chemical testing, randomly oriented and fragmented crystals may be difficult to interpret. The most frequent orientations in histological material have refractive indices very near that of the mounting medium3. This contributes to poor visibility in ordinary light microscopy, which may account for a failure to identify oxalate when unsuspected. In addition, the von Kossa silver nitrate procedure, a popularly employed technique to identify calcium deposits, in fact stains the phosphate anion rather than the calcium cation. Due to the low solubility of calcium oxalate, it is expected that oxalate would have a lower threshold of positivity in von Kossa staining. Accordingly, the von Kossa procedure inconsistently stained oxalate in our trials (3/5 stains). Furthermore, if stained, the oxalate deposits no longer polarize, rendering them indistinguishable from ordinary phosphate deposits. Other chemical tests for calcium oxalate can be employed, such as Yasue’s silver nitrate-rubeanic acid method, Pizzolato’s mercurous nitrate method, and the napthalhydroxamic acid method3. As an alternative, we employed an array of stains using alizarin red S. Staining with alizarin red S has distinct advantages beyond its ease and reliability. We employed an algorithm described by Proia et al to distinguish calcium oxalate from other calcium deposits, such as phosphate and carbonate1. While we have not performed ultrastructural microanalysis, the histologic and histochemical tests utilized in this study have been shown to confidently identify crystalline deposits of calcium oxalate5.
Oxalate Deposition and Renal Function
Also of primary interest is the apparent lack of correlation between the oxalosis we observed and renal involvement. The link between renal insufficiency and both renal tubular and myocardial oxalosis is well established6. Additionally, studies of patients on hemodialysis indicate that the duration of renal failure contributes to the extent of oxalosis7. Indeed, at the time of death these patients underwent acute system-wide organ failure. However, there was no history of chronic renal disease in any of the four cases. Recent BUN and creatinine levels confirmed this. Autopsy slides indicated no extensive renal disease and, more importantly, no oxalate deposition in renal tubules.
Classification of Oxalosis
The absence of oxalate in the kidneys of our patients is most surprising, given that the primary site of oxalate deposition in virtually all types of systemic oxalosis is the kidney. Kidney involvement is characteristic in cases of exogenous oxalosis, enteric oxalosis, hereditary oxalosis, and oxalosis in vitamin deficiencies. One rare and notable exception exists, so-called dystrophic oxalosis, defined as oxalate deposition without apparent disturbance in systemic oxalate metabolism3. Dystrophic oxalosis has been reported in AIDS patients in the globe of the eye8, the eyelids9, and a renomedullary insterstitial cell tumor10. Notably, 3 of our 4 cases had AIDS and were being treated with HAART.
An argument can be made that deposition of oxalate crystals in atherosclerotic plaques of coronary arteries is a form of dystrophic oxalosis. However, the oxalosis we observed was not limited to coronary arteries, but was in all cases also seen in the follicles of the thyroid as well as other sites, as described in each case. Therefore, the lesions we observed, not confined to a single organ, do not conform to the definition of dystrophic oxalosis. Since the pattern of involvement we observed has not been reported before, it is tempting to speculate that the oxalosis we observed represents an alternative oxalate metabolic pathway other than those currently described involving the kidney.
Cardiovascular Oxalosis
Oxalosis of the heart is a well studied phenomenon. Myocardial oxalosis has been linked to heart block11 and restrictive cardiomyopathy in primary hyperoxaluria12. In secondary uremic oxalosis, Salyer et al reported myocardial lesions with involvement of intramural coronaries in the absence of atherosclerosis. Arterial wall oxalosis has also been described in primary and secondary oxalosis in association with peripheral gangrene13. Arbus and Sniderman describe the deposits in the middle third of the media of large vessels. None of the above, however, describes intimal involvement or any association to atherosclerosis. What is unique about our findings is the presence of calcium oxalate within atherosclerotic plaques in the coronary arteries. Therefore, we feel that the lesions we observed are a distinct form of cardiovascular involvement in oxalosis - atherosclerotic oxalosis.
Figure 5.
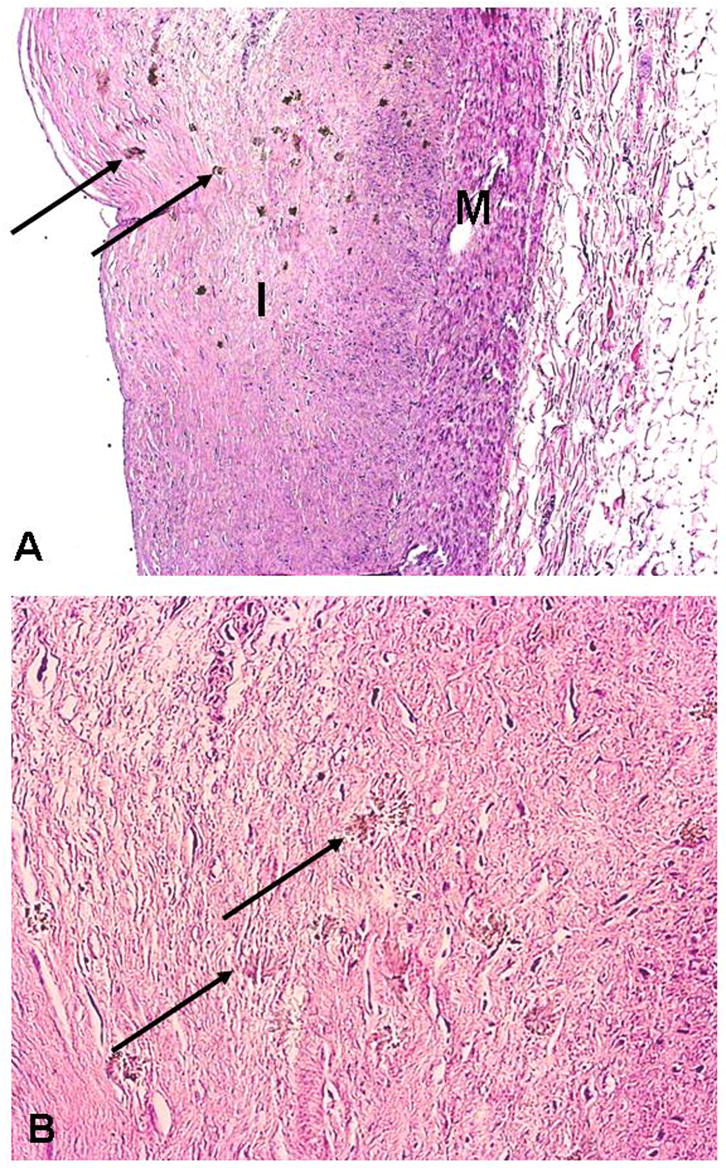
Oxalate deposits in non-calcified atherosclerotic plaque: A) H&E stain of fibrotic plaque in intima(I) with numerous small brown granules that represent oxalate crystals (arrows) (M = media, x40); B) same section at greater magnification showing crystals within the fibrous plaque (arrows) (x 200).
Acknowledgments
This work supported by a generous endowment from the Piansky Family Trust (MCF) and by NIH K24 AI056933 (JSC)
The National Neurological AIDS Bank (NS-38841) provided tissue samples and Dr. Singer’s support.
Footnotes
This work was done while Mr. Fishbein and Mr. Micheletti were visiting student research scientists at UCLA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Proia AD, Brinn NT. Identification of calcium oxalate crystals using alizarin red S stain. Arch Pathol Lab Med. 1985;109(2):186–9. [PubMed] [Google Scholar]
- 2.Silver MD, Gotlieb AI, et al. Cardiovascular pathology. New York: Churchill Livingstone; 2001. [Google Scholar]
- 3.Chaplin AJ. Histopathological occurrence and characterisation of calcium oxalate: a review. J Clin Pathol. 1977;30(9):800–11. doi: 10.1136/jcp.30.9.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mashour S, Turner JF, Jr, et al. Acute renal failure, oxalosis, and vitamin C supplementation: a case report and review of the literature. Chest. 2000;118(2):561–3. doi: 10.1378/chest.118.2.561. [DOI] [PubMed] [Google Scholar]
- 5.Symmans PJ, Brady K, et al. Calcium oxalate crystal deposition in epithelioid histiocytes of granulomatous lymphadenitis: analysis by light and electronmicroscopy. Histopathology. 1995;27(5):423–9. doi: 10.1111/j.1365-2559.1995.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 6.Salyer WR, Hutchins GM. Cardiac lesions in secondary oxalosis. Arch Intern Med. 1974;134(2):250–2. [PubMed] [Google Scholar]
- 7.Fayemi AO, Ali M, et al. Oxalosis in hemodialysis patients: a pathologic study of 80 cases. Arch Pathol Lab Med. 1979;103(2):58–62. [PubMed] [Google Scholar]
- 8.Pecorella I, McCartney AC, et al. Histological study of oxalosis in the eye and adnexa of AIDS patients. Histopathology. 1995;27(5):431–8. doi: 10.1111/j.1365-2559.1995.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 9.Pecorella I, Ciardi A, et al. Histological findings in the eyelids of AIDS patients. Acta Ophthalmol Scand. 1999;77(5):564–7. doi: 10.1034/j.1600-0420.1999.770517.x. [DOI] [PubMed] [Google Scholar]
- 10.Pecorella I, Lucas SB, et al. Calcium oxalate precipitates in a renomedullary interstitial cell tumor. Pathol Oncol Res. 2003;9(1):47–8. doi: 10.1007/BF03033714. [DOI] [PubMed] [Google Scholar]
- 11.Coltart DJ, Hudson RE. Primary oxalosis of the heart: a cause of heart block. Br Heart J. 1971;33(2):315–9. doi: 10.1136/hrt.33.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulze MR, Wachter R, et al. Restrictive cardiomyopathy in a patient with primary hyperoxaluria type II. Clin Res Cardiol. 2006;95(4):235–40. doi: 10.1007/s00392-006-0362-2. [DOI] [PubMed] [Google Scholar]
- 13.Arbus GS, Sniderman S. Oxalosis with peripheral gangrene. Arch Pathol. 1974;97(2):107–10. [PubMed] [Google Scholar]


