Abstract
Initiation of breast-feeding within 1 h after birth has been associated with reduced neonatal mortality in a rural Ghanaian population. In South Asia, however, breast-feeding patterns and low birth weight rates differ and this relationship has not been quantified. Data were collected during a community-based randomized trial of the impact of topical chlorhexidine antisepsis interventions on neonatal mortality and morbidity in southern Nepal. In-home visits were conducted on d 1-4, 6, 8, 10, 12, 14, 21, and 28 to collect longitudinal information on timing of initiation and pattern of breast-feeding. Multivariable regression modeling was used to estimate the association between death and breast-feeding initiation time. Analysis was based on 22,838 breast-fed newborns surviving to 48 h. Within 1 h of birth, 3.4% of infants were breast-fed and 56.6% were breast-fed within 24 h of birth. Partially breast-fed infants (72.6%) were at higher mortality risk [relative risk (RR) = 1.77;95% CI = 1.32-2.39] than those exclusively breast-fed. There was a trend (P = 0.03) toward higher mortality with increasing delay in breast-feeding initiation. Mortality was higher among late (≥24 h) compared with early (<24 h) initiators (RR = 1.41; 95% CI = 1.08-1.86) after adjustment for low birth weight, preterm birth, and other covariates. Improvements in breast-feeding practices in this setting may reduce neonatal mortality substantially. Approximately 7.7 and 19.1% of all neonatal deaths may be avoided with universal initiation of breast-feeding within the first day or hour of life, respectively. Community-based breast-feeding promotion programs should remain a priority, with renewed emphasis on early initiation in addition to exclusiveness and duration of breast-feeding.
Introduction
Approximately 4 million newborns die annually, the majority in developing countries (1); one-third of these deaths are due to infections. Numerous evidence-based interventions exist to reduce neonatal mortality in low-resource settings (2-5), but delivery of these interventions at scale remains an ongoing research and program challenge. Exclusive and continued breast-feeding has been well established as one of the most important interventions to reduce postneonatal and child mortality (6-9). Among 23 interventions considered by the Bellagio Child Survival Study Group, scale-up of exclusive breast-feeding of infants for 6 mo and continued feeding until 1 y could prevent an estimated 1.3 million child deaths per year (10). Breast-feeding has been shown to reduce the risk of respiratory infections (9), diarrhea (11), and neonatal sepsis (12,13). A pooled analysis of data from 3 countries has shown that either predominately or exclusively breast-fed infants are at substantially lower risk for infant mortality than non-breast-fed infants (11).
The focus of most breast-feeding promotion programs has been on exclusive breast-feeding through 6 mo of age, delaying the age at weaning, and efforts to define the optimal recommendations for breast-feeding practices in settings of high HIV prevalence. Fewer data are available on the impact of breast-feeding patterns and timing of initiation on neonatal mortality. In Egypt, initiation within 72 h markedly reduced diarrhea incidence in the first 6 mo of life (7). The WHO Collaborative Study Team (8) estimated that breast-feeding had the greatest benefit on mortality in the first 2 mo of age compared with later ages. A subanalysis of the Ghana data included in the WHO analysis demonstrated that delayed breast-feeding initiation time was a crucial risk factor for neonatal mortality and authors estimated that up to 16% of neonatal deaths could be prevented by increasing the proportion of infants that receive breast milk within 24 h of birth (14). In South Asia, although there have been some demonstrated improvements in the proportion of infants receiving breast milk within the first day of life (15-18), discarding colostrum or delaying breast-feeding remains common in many settings (16,18). Demonstrating a similar benefit of early breast-feeding as observed in Ghana could provide additional support for renewed focus on breast-feeding programs in general and increase emphasis on early initiation as an important aspect of these programs. In this manuscript, we provide an analysis of prospectively collected data on breast-feeding practices and neonatal mortality, concurrently collected within the context of recently completed evaluations of chlorhexidine (CHX)5 antisepsis interventions in southern Nepal (3,19).
Materials and Methods
Parent trial and data collection
Data for this analysis were collected during a large community-based, placebo-controlled randomized trial of the effect of 2 CHX interventions (newborn skin and umbilical cord cleansing) on neonatal mortality and morbidity. Details of implementation and results of these trials have been published previously (3,19). Briefly, between August 2002 and January 2006, 23,662 live-born infants in the Nepal Nutrition Intervention Project of Sarlahi District, Nepal were eligible to participate in either a comparative phase or post-trial scale-up phase of the study. Identification, follow-up, and data collection activities remained identical in both phases. Pregnancies were identified at approximately mid-pregnancy, study procedures were explained, and oral informed consent obtained. Women received iron folic acid supplements, deworming, weekly vitamin A supplementation, a clean birthing kit, and basic counseling on nutrition and antenatal and postnatal care. Information on household socioeconomic status, parental education, and birth history was obtained. Notification of live-born infants to study workers was facilitated by local female staff who visited infants as soon as possible after birth and then followed up during the neonatal period on a standard schedule (d 1-4, 6, 8, 10, 12, 14, 21, and 28). In this population, over 90% of newborns are delivered at home.
At the initial visit after birth (“birth assessment”), mothers were asked if their infant had been breast-fed and, if so, how much time had passed between birth and initiation. At each of the follow-up visits, workers asked mothers if their child had breast-fed within the previous 24 h. A more extensive interview completed at 2 wk of age provided further information on breast-feeding practices, including timing of initiation and prelacteal or complementary feeding practices. Additional data collected during the 11 visits included measurement of birth weight, longitudinal measures of vital status of the child, signs of omphalitis and other morbidities, and a range of care practices during the first weeks of life. Gestational age for infants was estimated as time since last menstrual period estimated from maternal report at enrollment and the d 1 visit. All infants were followed until discharge at 28 d, out-migration, or death.
Definition of exposure and outcome
Live-born infants surviving to 48 h of life whose mother reported breast-feeding and for whom a breast-feeding initiation time could be estimated were included in the analysis. Similar to previous analyses (14), early deaths were excluded to minimize reverse causation bias (i.e. underestimation of the effect of breast-feeding by including infants who never breast-feed as a result of serious illness leading to death). Breast-feeding initiation time in hours was estimated from the 3 main sources of breast-feeding information (birth assessment, follow-up visits on d 1-4, and d 14 care practices interview), giving priority to responses made with minimal recall time. Thus, the primary source of information was the birth assessment interview. If this visit occurred before breast-feeding had been initiated or data on this form were missing, the exposure variable was then estimated from longitudinal information regarding breast-feeding in the prior 24 h, collected repeatedly during the follow-up visits occurring through the first 2 wk of life. The 3rd source of information, collected at d 14, was used only in the following situations: 1) to more precisely estimate the hour of birth if the response matched the 24-h interval estimated from the longitudinal data generated through follow-up visits; or 2) as the primary source of information if both the birth assessment data and prospective follow-up data were unavailable. The primary exposure variable was categorized as follows: within 1 h of birth, ≥1 to <24 h, ≥24 to <48 h, ≥48 h to <72 h, and ≥72 h. The interval included and excluded the lower and upper bounds, respectively, of the interval. A 2nd exposure variable was created to compare early initiators, defined as initiation breast-feeding within 24 h, to late initiators (≥24 h).
Analysis
The primary outcome, mortality within 28 d, was estimated within each stratum of both breast-feeding initiation exposure variables and compared using relative risks obtained from binomial regression modeling with a log link function. Kaplan-Meier survival curves were constructed for each stratum of the categorical variable. Multivariate models were adjusted for a range of variables known to be associated with mortality risk in this population, including treatment allocations in the parent trial. A composite variable defining “possible severe disease” within the first 2 d was created. Infants were defined as such if 2 or more of the following conditions were present: 1) difficulty breathing; 2) stiffening of the back or convulsions; 3) dysentery; 4) 5 or more watery stools within 24 h; 5) severe chest in-drawing; 6) axillary temperature >37.8°C; 7) respiratory rate <70 breaths/min. To further examine for residual reverse-causation bias, analyses were repeated after removing infants who were positive for this variable. Population attributable fractions were estimated from the adjusted multivariate models to estimate the proportion of deaths that might be prevented given universal coverage of breast-feeding within 1 or 24 h. Statistical analyses were conducted using STATA version 9.2 (Stata).
This study received ethical approval from the Nepal Health Research Council and the Committee on Human Research of the Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, and is registered at Clinicaltrials.gov (NCT00109616).
Results
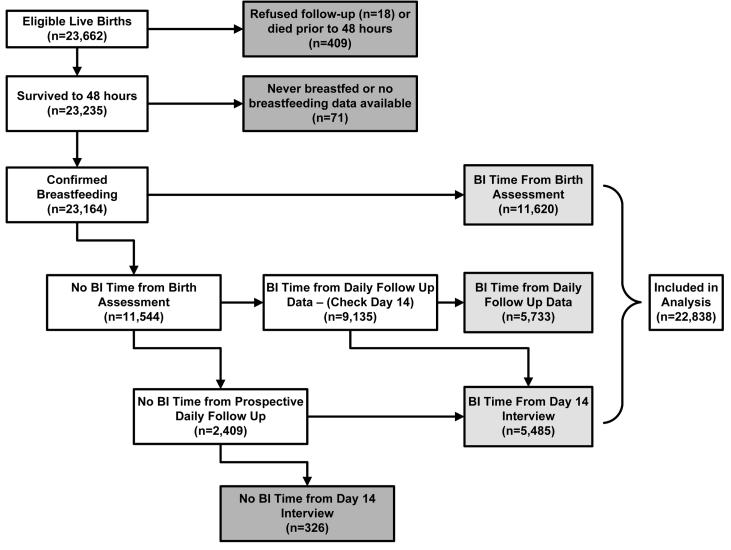
There were 23,662 live-born infants during the study period and a total of 759 neonatal deaths (neonatal mortality rate = 32.1/1000 live births). Many (409, 53.9%) of the neonatal deaths occurred in the first 48 h and were excluded from the analysis. Among 23,235 infants surviving to 48 h, there were 71 for whom breast-feeding data were not available (53 of these infants subsequently died). There were a total of 23,164 (99.7%) infants for whom we could confirm that breast-feeding was ever initiated and the actual time to breast-feeding was estimable for 22,838 (98.6%) infants (Fig. 1). The median time to breast-feeding was 18.4 h and the mean was 22.7 ± 22.1 h. Only 771 infants (3.4%) were breast-fed within the first hour after birth, but breast-feeding within the first 24 (56.6%) or first 48 (83.1%) h was more common. Breast-feeding was established within 72 h for 97.2% of breast-fed infants.
FIGURE 1.
Flowchart of infants with defined breast-feeding initiation time and included in the analysis.
Partial breast-feeding (i.e. combined breast-feeding with other milk-based fluids and/or solids) was the most common established breast-feeding pattern in this setting (72.6% of infants). Goat and/or buffalo milk are often provided to the newborn infant in Sarlahi, especially during the first 24-48 h after birth. Partially breast-fed infants were at substantially higher mortality risk than those who were exclusively breast-fed (relative risk [RR] = 1.77 [95% CI = 1.32, 2.39]). Infants who were breast-fed in the first 24 h were more likely to be exclusively breast-fed (42.3%) than late initiators (8.0%, OR = 8.47 [95% CI 7.81, 9.18]).
Both the pattern of breast-feeding (exclusive vs. partial) and initiation time varied substantially between the 2 major ethnic groups. Infants of pahadi households (those originating from the hills region of Nepal, 28.8% of households) were more likely to be breast-fed exclusively (58.4 vs. 14.8%; OR = 8.09 [95% CI = 7.57, 8.64]) and be early initiators (92.9 vs. 41.9%; OR = 18.2 [95% CI = 16.4, 20.1]) than those from madeshi households (those originating from the plains region of Nepal). Similarly, mothers of pahadi infants were more likely to report feeding their infant(s) colostrum (90.9 vs. 78.1%; OR = 2.86 [95% CI = 2.61 - 3.13]).
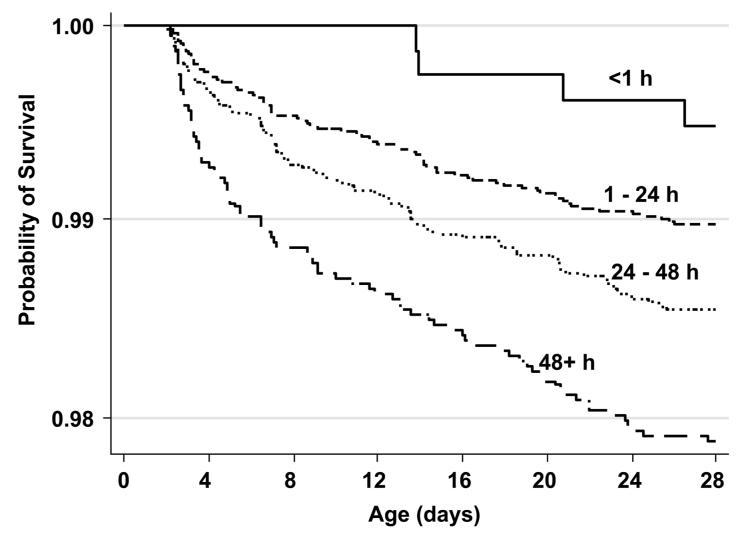
Among the 22,838 infants included in the analysis, there were 297 deaths after 48 h and prior to 28 d. There was a trend toward significantly higher risk of mortality among infants who were breast-fed later compared with those who received breast milk within the first hour of life (Table 1; Fig. 2). Compared with those fed within the first hour after birth, mortality risk was 2.80, 4.08, and 4.19 times higher among infants first breast-fed after d 1, 2, or 3 of life, respectively. Mortality risk was 2.56 (95% CI = 0.96, 6.85) times higher comparing all those fed after vs. before 1 h after birth. Late initiators (≥24 h) were 1.74 (95% CI = 1.39, 2.19) times more likely to die during the neonatal period than early initiators (<24 h).
TABLE 1.
Mortality risk by breast-feeding initiation time (BI time) among breast-fed infants surviving to 48 h1
| BI time,2h | n | Died, n | Rate, n/1000 | RR3 | 95% CI |
|---|---|---|---|---|---|
| Overall | |||||
| <1 | 771 | 4 | 5.2 | 1.00 | |
| 1-24 | 12,148 | 123 | 10.1 | 1.95 | 0.72, 5.27 |
| 24-48 | 6063 | 88 | 14.5 | 2.80 | 1.03, 7.60 |
| 48-72 | 3212 | 68 | 21.2 | 4.08 | 1.49, 11.15 |
| ≥72 | 644 | 14 | 21.7 | 4.19 | 1.39, 12.67 |
| Pahadi | |||||
| <24 | 6029 | 45 | 7.5 | 1.00 | |
| ≥24 | 461 | 10 | 21.7 | 2.91 | 1.47, 5.73 |
| Madeshi | |||||
| <24 | 6696 | 79 | 11.8 | 1.00 | |
| ≥24 | 9293 | 157 | 16.9 | 1.43 | 1.09, 1.87 |
n = 22,838.
Inclusive and exclusive for the lower and upper bound of each interval, respectively.
Trend: χ2 = 32.12; P < 0.0001.
FIGURE 2.
Risk of mortality of infants in Nepal by time of breast-feeding initiation (Kaplan-Meier survival curves).
Given the substantial differences in pattern and timing of breast-feeding between pahadi and madeshi households, estimates of mortality risk comparing early vs. late initiators is also shown separately by ethnic status. The interaction term for ethnic group status was marginally significant (P = 0.06).
The relationship between mortality risk and breast-feeding initiation time was adjusted for multiple covariates, including low birth weight (LBW, <2500 g) status, prematurity (<37 wk), cord and skin cleansing treatment allocation in the parent trial, maternal literacy, sex, maternal hand-washing, previous death of a sibling, ethnicity, parity, and maternal report of fever in the 7 d prior to delivery (Table 2). The most important confounders of the main relationship were birth weight and prematurity; after adjustment for these variables, the relationship between initiation time and mortality was not significant for any individual category, although there was a trend (P = 0.03) toward increasing risk with delayed initiation time. Furthermore, when comparing early to late initiators, adjusted risk of mortality was 1.41 (95% CI = 1.08, 1.86) times higher among those initiating after the first day of life. Sex of the newborn, maternal literacy, and treatment allocation in the parent trial, parity, and maternal report of fever in the 7 d prior to delivery did not confound this association.
TABLE 2.
Adjusted mortality risk by breast-feeding initiation time (BI time) among breast-fed infants surviving to 48 h1
| RR3 | 95% CI | RR | 95% CI | |
|---|---|---|---|---|
| BI time,2 h | ||||
| <1 | 1.00 | |||
| 1-24 | 1.43 | 0.52-3.89 | ||
| 24-48 | 1.78 | 0.64-5.00 | ||
| 48-72 | 2.43 | 0.86-6.90 | ||
| ≥72 | 2.06 | 0.62-6.82 | ||
| <24 | 1.00 | |||
| ≥24 | 1.41 | 1.08-1.86 | ||
| Covariates | ||||
| <2500 g | 4.14 | 3.16-5.42 | 4.16 | 3.18-5.44 |
| <37 wk | 2.24 | 1.74-2.88 | 2.25 | 1.74-2.89 |
| Female | 1.02 | 0.80-1.30 | 1.02 | 0.80-1.30 |
| Maternal literacy | 0.99 | 0.70-1.39 | 0.99 | 0.70-1.39 |
| CHX cord cleansing | 0.91 | 0.71-1.18 | 0.91 | 0.70-1.17 |
| CHX skin cleansing | 0.92 | 0.71-1.19 | 0.92 | 0.71-1.18 |
| Maternal hand washing | 0.64 | 0.42-0.98 | 0.64 | 0.42-0.98 |
| Previous death of sibling | 1.34 | 0.94-1.91 | 1.35 | 0.95-1.91 |
| Madeshi household | 0.88 | 0.60-1.26 | 0.90 | 0.62-1.31 |
| Parity | 0.98 | 0.91-1.06 | 0.98 | 0.91-1.06 |
| Maternal fever prior to outcome | 2.09 | 1.36-3.20 | 2.10 | 1.37-3.22 |
n = 22,838.
Inclusive and exclusive for the lower and upper bound of each interval, respectively.
Trend: χ2 = 4.94; P = 0.03.
The adjusted model was estimated separately by ethnic status (Table 3). There was little change from the ethnic group-specific, nonadjusted estimates from Table 1, suggesting that after the effect modification of ethnic group was taken into account, the relationship between mortality and initiation time was not confounded by LBW or preterm birth. For pahadi newborns, the adjusted risk of mortality was 3.00 [95% CI = 1.50-5.98] times higher among late initiators compared with early initiators. Among madeshi newborns, the magnitude of the relationship and the statistical strength of evidence was lower, but late initiators in this subgroup remained 1.32 [95% CI = 1.00-1.74] times more likely to die than early initiators.
TABLE 3.
Adjusted mortality risk by breast-feeding initiation time (BI time) among breast-fed infants surviving to 48 h by ethnic group1
| Pahadi | Madeshi | |||
|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | |
| BI time,2 h | ||||
| <24 | 1.00 | 1.00 | ||
| ≥24 | 3.00 | 1.50-5.98 | 1.32 | 1.00-1.74 |
| Covariates | ||||
| <2500 g | 3.90 | 2.19-6.95 | 4.53 | 3.35-6.12 |
| <37 wk | 2.25 | 1.15-4.39 | 2.25 | 1.72-2.94 |
| Maternal hand washing | 0.78 | 0.37-1.66 | 0.59 | 0.35-1.00 |
| Previous death of sibling | 1.02 | 0.46-2.27 | 1.40 | 1.06-1.86 |
n = 22,231.
Inclusive and exclusive for the lower and upper bounds of each interval, respectively.
Infant illness status was defined only for infants for whom daily morbidity data were available within 48 h of life (82.3%). In analyses restricted to this subset of infants, the multivariate model was further adjusted by this variable and there was little evidence for confounding; after adjustment, the RR estimate for mortality comparing early vs. late initiators increased only slightly from 1.41 to 1.44 (95% CI = 1.08-1.92). Whereas infants who were ill prior to 48 h after birth (n = 741, 4.0%) were more likely to die (RR = 1.76 [95% CI =1.07-2.91]), removing them from the analysis led to no substantial change in the association of early vs. late breast-feeding initiation on neonatal mortality (1.35 [95% CI = 1.02-1.78]). This suggests that any residual reverse-causation bias in this analysis is likely to be minimal.
Discussion
Data presented here provide evidence that early initiation of breast-feeding among newborns in a rural southern Nepal population is associated with a reduced risk of mortality during the neonatal period. There was a strong dose-response relationship with mortality risk increasing with later initiation times in both crude and adjusted models and overall initiation after 24 h was associated with a 41% (8-86%) increase in mortality risk after adjustment for confounding variables. Using estimates from the adjusted model, ∼41.3% of neonatal deaths after 48 h might be prevented if breast-feeding was initiated within 1 h of birth. Assuming that early breast-feeding has no effect on deaths prior to 48 h (53.9% of deaths), universal initiation within 1 h might prevent 19.1% of neonatal deaths in this setting.
The major strength of this study was the large sample size (>22,000 newborns), which was more than twice the size of a previous reported analysis of breast-feeding initiation time and neonatal mortality risk (14). Furthermore, the intensive follow-up schedule in the parent trial (11 home visits during the neonatal period) allowed for rapid initial in-home visits around the time of birth and prospective observation of breast-feeding practices and vital status during the neonatal period. This improved measurement accuracy of timing of both the outcome (death) and exposure (breast-feeding initiation time) and minimized the likelihood of recall bias or differential misclassification of breast-feeding practices among deaths and surviving newborns.
There have been few previous efforts to examine this relationship (8). The most comprehensive prior analyses from Ghana estimated that up to 22 and 16% of all neonatal deaths could be prevented with universal coverage of breast-feeding within 1 and 24 h of birth, respectively (14). Although the magnitude of the relationship estimated here in adjusted models was slightly lower, the overall conclusions are similar. The LBW rate in our study setting (29.8%) compared with the Ghana population (7.4%) is likely the most important difference between the settings. Small size at birth has been previously shown to be directly related to lower likelihood of breast-feeding (20,21) and although these data support the evidence provided by the Ghana study, they also demonstrate that in settings with high prevalence of LBW, the benefit of early initiation of human milk may not be as strong as in those with lower prevalence.
There were a number of limitations to our study. We attempted to take into account the potential for reverse causation bias (22) by restricting our analyses to infants surviving to 2 d, similar to the previous study (14). Although we also examined the impact of removing infants with signs of morbidity within the first 48 h from the analysis and found no direct evidence for residual reverse-causation bias, we cannot discount the possibility that some caretakers delayed breast-feeding because of preexisting morbid conditions that were not fully characterized by our data. Also, we could not define this variable for 17.7% of the newborns in the dataset and thus did not include the variable as a confounder in our final multivariate model. We do not think, however, that the model without this adjustment overestimates the primary relationship. Rather, when this variable was included in the multivariate model to examine this possibility, the parameter estimates for breast-feeding initiation time were slightly higher than in the more parsimonious model that excluded this variable. We did not collect detailed information on maternal nutritional status, weight gain during pregnancy, or other potential maternal variables that may have further confounded the relationship.
There was some indication that the importance of this practice differed between 2 ethnic groups with distinct breast-feeding patterns, complicating efforts to generalize these results to the wider Nepali context or beyond to South Asian populations in general. However, ethnic group-specific population attributable fractions are similar (data not shown) and the adjusted estimates in the madeshi communities, whose timing and pattern of breast-feeding are more representative of a larger population in southern Nepal, northern India, and Bangladesh, were similar to the population-averaged adjusted RR estimate of 1.41 (1.08-1.86). Breast-feeding patterns are tightly correlated with timing of initiation of breast-feeding in this population. If promotion programs can improve the time to initiation in this setting, exclusive breast-feeding rates will also rise, because partially breast-fed infants normally receive non-breast milk substitutes only during the period prior to initiation.
The most recent Demographic Health Surveillance study in Nepal (18) estimated that ∼85% of newborns are breast-fed within 24 h of birth; the same indicator for communities in the Terai region of Nepal is 75%. These estimates are substantially higher than we estimated through our intensive follow-up schedule of home visits during the neonatal period. The difference potentially reflects the dangers of using long recall periods to classify breast-feeding initiation status and suggests that, despite reported improvements at the national level, continued emphasis on early breast-feeding is necessary. Furthermore, the potential benefit to neonatal survival is greater when initiated within 1 h of birth; the parallel Demographic Health Surveillance estimate for Nepal was 35% (18), indicating that continued efforts to promote improved breast-feeding practices in Nepal might play a substantial role in reducing neonatal mortality and achieving Millennium Development Goal 4.
Footnotes
Supported by grants from the NIH, Bethesda, Maryland (HD 44004, HD 38753), by the Bill and Melinda Gates Foundation, Seattle, Washington (810-2054), and by Cooperative Agreements between Johns Hopkins University and the Office of Health and Nutrition, US Agency for International Development, Washington DC (HRN-A-00-97-00015-00, GHS-A-00-03-000019-00). Commodity support was provided by Procter and Gamble Company, Cincinnati, Ohio.
- BI time
- breast-feeding initiation time (in hours)
- CHX
- chlorhexidine
- LBW
- low birth weight (<2500 g)
- RR
- relative risk
Literature Cited
- 1.Lawn JE, Cousens S, Zupan J. Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L, Lancet Neonatal Survival Steering Team Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365:977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 3.Mullany LC, Darmstadt GL, Khatry SK, Katz J, LeClerq SC, Shrestha S, Adhikari R, Tielsch JM. Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomised trial. Lancet. 2006;367:910–8. doi: 10.1016/S0140-6736(06)68381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klemm RD, Labrique A, Christian P, Rashid R, Shamim AA, Katz J. Micronutrient Forum Abstracts. Istanbul, Turkey: Apr 16-18, 2007. Efficacy of newborn vitamin A supplementation in reducing infant mortality in rural Bangladesh: the JiVitA-2 trial. [Google Scholar]
- 5.Rahmathullah L, Tielsch JM, Thulasiraj RD, Katz J, Coles C, Devi S, John R, Prakash K, Sadanand AV, et al. Impact of supplementing newborn infants with vitamin A on early infant mortality: community based randomised trial in southern India. BMJ. 2003;327:254–9. doi: 10.1136/bmj.327.7409.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Victora CG, Smith PG, Vaughan JP, Nobre LC, Lombardi C, Teixeira AM, Fuchs SM, Moreira LB, Gigante LP, et al. Evidence for protection by breast-feeding against infant deaths from infectious diseases in Brazil. Lancet. 1987;330:319–22. doi: 10.1016/s0140-6736(87)90902-0. [DOI] [PubMed] [Google Scholar]
- 7.Clemens J, Elyazeed RA, Rao M, Savarino S, Morsy BZ, Kim Y, Wierzba T, Naficy A, Lee YJ. Early initiation of breastfeeding and the risk of infant diarrhea in rural Egypt. Pediatrics. 1999;104:e3. doi: 10.1542/peds.104.1.e3. [DOI] [PubMed] [Google Scholar]
- 8.WHO. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355:451–5. [PubMed] [Google Scholar]
- 9.Arifeen S, Black RE, Antelman G, Baqui A, Caulfield L, Becker S. Exclusive breastfeeding reduces acute respiratory infection and diarrhea deaths among infants in Dhaka slums. Pediatrics. 2001;108:E67. doi: 10.1542/peds.108.4.e67. [DOI] [PubMed] [Google Scholar]
- 10.Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS, Bellagio Child Survival Study Group How many child deaths can we prevent this year? Lancet. 2003;362:65–71. doi: 10.1016/S0140-6736(03)13811-1. [DOI] [PubMed] [Google Scholar]
- 11.Bahl R, Frost C, Kirkwood BR, Edmond K, Martines J, Bhandari N, Arthur P. Infant feeding patterns and risks of death and hospitalization in the first half of infancy: multicentre cohort study. Bull World Health Organ. 2005;83:418–26. [PMC free article] [PubMed] [Google Scholar]
- 12.Bhutta ZA, Yusuf K. Early-onset neonatal sepsis in Pakistan: a case control study of risk factors in a birth cohort. Am J Perinatol. 1997;14:577–81. doi: 10.1055/s-2007-994338. [DOI] [PubMed] [Google Scholar]
- 13.Ashraf RN, Jalil F, Zaman S, Karlberg J, Khan SR, Lindblad BS, Hanson LA. Breast feeding and protection against neonatal sepsis in a high risk population. Arch Dis Child. 1991;66:488–90. doi: 10.1136/adc.66.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edmond KM, Zandoh C, Quigley MA, Amenga-Etego S, Owusu-Agyei S, Kirkwood BR. Delayed breastfeeding initiation increases risk of neonatal mortality. Pediatrics. 2006;117:e380–6. doi: 10.1542/peds.2005-1496. [DOI] [PubMed] [Google Scholar]
- 15.Syed U, Asiruddin S, Helal MS, Mannan II, Murray J. Immediate and early postnatal care for mothers and newborns in rural Bangladesh. J Health Popul Nutr. 2006;24:508–18. [PMC free article] [PubMed] [Google Scholar]
- 16.Barnett S, Azad K, Barua S, Mridha M, Abrar M, Rego A, Khan A, Flatman D, Costello A. Maternal and newborn-care practices during pregnancy, childbirth, and the postnatal period: a comparison in three rural districts in Bangladesh. J Health Popul Nutr. 2006;24:394–402. [PMC free article] [PubMed] [Google Scholar]
- 17.Darmstadt GL, Kumar V, Singh P, Singh V, Yadav R, Mohanty S, Bharti N, Gupta S, Baqui AH, et al. Countdown to 2015: Tracking Progress in Child Survival. London, UK: Dec 14, 2005. Community mobilization and behavior change communications promote evidence-based essential newborn care practices and reduce neonatal mortality in Uttar Pradesh, India [poster] [cited 3 Oct 2007]. Available from: http://cs.server2.textor.com/alldocs/G%20Darmstadt.doc. [Google Scholar]
- 18.Ministry of Health and Population (MOHP) [Nepal] New ERA, and Macro International . Nepal Demographic and Health Survey 2006. Ministry of Health and Population, New ERA, and Macro International Inc; Kathmandu (Nepal): 2007. [Google Scholar]
- 19.Tielsch JM, Darmstadt GL, Mullany LC, Khatry SK, Katz J, LeClerq SC, Shrestha S, Adhikari R. Impact of newborn skin-cleansing with chlorhexidine on neonatal mortality in southern Nepal: a community-based, cluster-randomized trial. Pediatrics. 2007;119:e330–40. doi: 10.1542/peds.2006-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barros FC, Victora CG, Vaughan JP, Smith PG. Birth weight and duration of breast-feeding: are the beneficial effects of human milk being overestimated? Pediatrics. 1986;78:656–61. [PubMed] [Google Scholar]
- 21.Adair LS, Popkin BM. Low birth weight reduces the likelihood of breast-feeding among Filipino infants. J Nutr. 1996;126:103–12. doi: 10.1093/jn/126.1.103. [DOI] [PubMed] [Google Scholar]
- 22.Bauchner H, Leventhal JM, Shapiro ED. Studies of breastfeeding and infection: how good is the evidence? JAMA. 1986;256:887–92. [PubMed] [Google Scholar]