Abstract
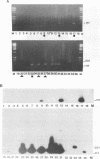
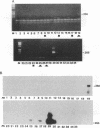
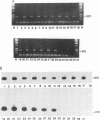
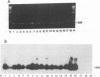
Monocytes are one site of carriage of the human cytomegalovirus (HCMV) genome in healthy human carriers. However, as there are conflicting data detailing the level of HCMV gene expression during persistence in these cells, we have analyzed monocytes for evidence of viral immediate-early, early, and late transcription by using reverse transcription followed by PCR. We were unable to find evidence of HCMV lytic gene transcription in freshly isolated peripheral blood monocytes from HCMV-seropositive subjects. However, as differentiation of monocytes to monocyte-derived macrophages results in increased permissiveness to infection with HCMV in vitro, we examined whether such differentiation could result in reactivation of endogenous viral gene expression. Here we show that in vitro differentiation of monocytes does result in expression of endogenous HCMV immediate-early genes. Although this differentiation led to reactivation of endogenous viral immediate-early expression, we were unable to detect any early or late viral transcription. Cocultivation experiments correlated with this level of gene induction, as no productive infection was detected. These data strongly suggest a mechanism of persistence of HCMV in the peripheral blood that is independent of HCMV lytic gene expression and that initial phases of lytic gene expression in monocytes can be induced by differentiation of these cells to monocyte-derived macrophages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. P. Transfusion-associated cytomegalovirus infections. Rev Infect Dis. 1983 Nov-Dec;5(6):977–993. doi: 10.1093/clinids/5.6.977. [DOI] [PubMed] [Google Scholar]
- Akrigg A., Wilkinson G. W., Oram J. D. The structure of the major immediate early gene of human cytomegalovirus strain AD169. Virus Res. 1985 Mar;2(2):107–121. doi: 10.1016/0168-1702(85)90242-4. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- De Witte T., Schattenberg A., Van Dijk B. A., Galama J., Olthuis H., Van der Meer J. W., Kunst V. A. Prevention of primary cytomegalovirus infection after allogeneic bone marrow transplantation by using leukocyte-poor random blood products from cytomegalovirus-unscreened blood-bank donors. Transplantation. 1990 Dec;50(6):964–968. doi: 10.1097/00007890-199012000-00013. [DOI] [PubMed] [Google Scholar]
- Diosi P., Moldovan E., Tomescu N. Latent cytomegalovirus infection in blood donors. Br Med J. 1969 Dec 13;4(5684):660–662. doi: 10.1136/bmj.4.5684.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew W. L., Mintz L., Hoo R., Finley T. N. Growth of herpes simplex and cytomegalovirus in cultured human alveolar macrophages. Am Rev Respir Dis. 1979 Feb;119(2):287–291. doi: 10.1164/arrd.1979.119.2.287. [DOI] [PubMed] [Google Scholar]
- Einhorn L., Ost A. Cytomegalovirus infection of human blood cells. J Infect Dis. 1984 Feb;149(2):207–214. doi: 10.1093/infdis/149.2.207. [DOI] [PubMed] [Google Scholar]
- Gilbert G. L., Hayes K., Hudson I. L., James J. Prevention of transfusion-acquired cytomegalovirus infection in infants by blood filtration to remove leucocytes. Neonatal Cytomegalovirus Infection Study Group. Lancet. 1989 Jun 3;1(8649):1228–1231. doi: 10.1016/s0140-6736(89)92330-1. [DOI] [PubMed] [Google Scholar]
- Gnann J. W., Jr, Ahlmén J., Svalander C., Olding L., Oldstone M. B., Nelson J. A. Inflammatory cells in transplanted kidneys are infected by human cytomegalovirus. Am J Pathol. 1988 Aug;132(2):239–248. [PMC free article] [PubMed] [Google Scholar]
- Greenaway P. J., Wilkinson G. W. Nucleotide sequence of the most abundantly transcribed early gene of human cytomegalovirus strain AD169. Virus Res. 1987 Feb;7(1):17–31. doi: 10.1016/0168-1702(87)90055-4. [DOI] [PubMed] [Google Scholar]
- Gönczöl E., Andrews P. W., Plotkin S. A. Cytomegalovirus replicates in differentiated but not in undifferentiated human embryonal carcinoma cells. Science. 1984 Apr 13;224(4645):159–161. doi: 10.1126/science.6322309. [DOI] [PubMed] [Google Scholar]
- Hart P. H., Vitti G. F., Burgess D. R., Whitty G. A., Royston K., Hamilton J. A. Activation of human monocytes by granulocyte-macrophage colony-stimulating factor: increased urokinase-type plasminogen activator activity. Blood. 1991 Feb 15;77(4):841–848. [PubMed] [Google Scholar]
- Ibanez C. E., Schrier R., Ghazal P., Wiley C., Nelson J. A. Human cytomegalovirus productively infects primary differentiated macrophages. J Virol. 1991 Dec;65(12):6581–6588. doi: 10.1128/jvi.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M. C. Latent infection and the elusive cytomegalovirus. Rev Infect Dis. 1983 Mar-Apr;5(2):205–215. doi: 10.1093/clinids/5.2.205. [DOI] [PubMed] [Google Scholar]
- Kothari S., Baillie J., Sissons J. G., Sinclair J. H. The 21bp repeat element of the human cytomegalovirus major immediate early enhancer is a negative regulator of gene expression in undifferentiated cells. Nucleic Acids Res. 1991 Apr 25;19(8):1767–1771. doi: 10.1093/nar/19.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathey J. L., Spector S. A. Unrestricted replication of human cytomegalovirus in hydrocortisone-treated macrophages. J Virol. 1991 Nov;65(11):6371–6375. doi: 10.1128/jvi.65.11.6371-6375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubon H., Ghazal P., Hennighausen L., Reynolds-Kohler C., Lockshin C., Nelson J. Cell-specific activity of the modulator region in the human cytomegalovirus major immediate-early gene. Mol Cell Biol. 1989 Mar;9(3):1342–1345. doi: 10.1128/mcb.9.3.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Bankier A. T., Landini M. P., Brown C. M., Barrell B. G., Rüger B., Mach M. Identification and procaryotic expression of the gene coding for the highly immunogenic 28-kilodalton structural phosphoprotein (pp28) of human cytomegalovirus. J Virol. 1988 Jul;62(7):2243–2250. doi: 10.1128/jvi.62.7.2243-2250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale D., Chikkappa G., Wang G., Santella D. Hydrocortisone promotes survival and proliferation of granulocyte-macrophage progenitors via monocytes/macrophages. Exp Hematol. 1989 Dec;17(11):1110–1115. [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Nelson J. A., Oldstone M. B. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science. 1985 Nov 29;230(4729):1048–1051. doi: 10.1126/science.2997930. [DOI] [PubMed] [Google Scholar]
- Shelbourn S. L., Kothari S. K., Sissons J. G., Sinclair J. H. Repression of human cytomegalovirus gene expression associated with a novel immediate early regulatory region binding factor. Nucleic Acids Res. 1989 Nov 25;17(22):9165–9171. doi: 10.1093/nar/17.22.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelbourn S. L., Sissons J. G., Sinclair J. H. Expression of oncogenic ras in human teratocarcinoma cells induces partial differentiation and permissiveness for human cytomegalovirus infection. J Gen Virol. 1989 Feb;70(Pt 2):367–374. doi: 10.1099/0022-1317-70-2-367. [DOI] [PubMed] [Google Scholar]
- Sinclair J. H., Baillie J., Bryant L. A., Taylor-Wiedeman J. A., Sissons J. G. Repression of human cytomegalovirus major immediate early gene expression in a monocytic cell line. J Gen Virol. 1992 Feb;73(Pt 2):433–435. doi: 10.1099/0022-1317-73-2-433. [DOI] [PubMed] [Google Scholar]
- Stamminger T., Puchtler E., Fleckenstein B. Discordant expression of the immediate-early 1 and 2 gene regions of human cytomegalovirus at early times after infection involves posttranscriptional processing events. J Virol. 1991 May;65(5):2273–2282. doi: 10.1128/jvi.65.5.2273-2282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Thomsen D. R., Stinski M. F. Structural analysis of the major immediate early gene of human cytomegalovirus. J Virol. 1984 Jan;49(1):190–199. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderberg C., Larsson S., Bergstedt-Lindqvist S., Möller E. Definition of a subset of human peripheral blood mononuclear cells that are permissive to human cytomegalovirus infection. J Virol. 1993 Jun;67(6):3166–3175. doi: 10.1128/jvi.67.6.3166-3175.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Wiedeman J., Hayhurst G. P., Sissons J. G., Sinclair J. H. Polymorphonuclear cells are not sites of persistence of human cytomegalovirus in healthy individuals. J Gen Virol. 1993 Feb;74(Pt 2):265–268. doi: 10.1099/0022-1317-74-2-265. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J., Sissons J. G., Borysiewicz L. K., Sinclair J. H. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991 Sep;72(Pt 9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- Tolpin M. D., Stewart J. A., Warren D., Mojica B. A., Collins M. A., Doveikis S. A., Cabradilla C., Jr, Schauf V., Raju T. N., Nelson K. Transfusion transmission of cytomegalovirus confirmed by restriction endonuclease analysis. J Pediatr. 1985 Dec;107(6):953–956. doi: 10.1016/s0022-3476(85)80201-8. [DOI] [PubMed] [Google Scholar]
- Toorkey C. B., Carrigan D. R. Immunohistochemical detection of an immediate early antigen of human cytomegalovirus in normal tissues. J Infect Dis. 1989 Nov;160(5):741–751. doi: 10.1093/infdis/160.5.741. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S., Kobayashi Y., Goto Y., Okumura H., Nakae S., Konno T., Tada K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982 Apr;42(4):1530–1536. [PubMed] [Google Scholar]
- Tsui F. W., Siminovitch L. Isolation, structure and expression of mammalian genes for histidyl-tRNA synthetase. Nucleic Acids Res. 1987 Apr 24;15(8):3349–3367. doi: 10.1093/nar/15.8.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker B. G., Wilton S., Rice G. P. Phorbol ester-induced differentiation permits productive human cytomegalovirus infection in a monocytic cell line. J Immunol. 1988 Mar 1;140(5):1625–1631. [PubMed] [Google Scholar]
- Yeager A. S., Grumet F. C., Hafleigh E. B., Arvin A. M., Bradley J. S., Prober C. G. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981 Feb;98(2):281–287. doi: 10.1016/s0022-3476(81)80662-2. [DOI] [PubMed] [Google Scholar]
- Zeevi M., Nevins J. R., Darnell J. E., Jr Nuclear RNA is spliced in the absence of poly(A) addition. Cell. 1981 Oct;26(1 Pt 1):39–46. doi: 10.1016/0092-8674(81)90031-3. [DOI] [PubMed] [Google Scholar]
- Zerbini M., Musiani M., La Placa M. Effect of heat shock on Epstein-Barr virus and cytomegalovirus expression. J Gen Virol. 1985 Mar;66(Pt 3):633–636. doi: 10.1099/0022-1317-66-3-633. [DOI] [PubMed] [Google Scholar]
- Zerbini M., Musiani M., La Placa M. Stimulating effect of heat shock on the early stage of human cytomegalovirus replication cycle. Virus Res. 1986 Dec;6(3):211–216. doi: 10.1016/0168-1702(86)90070-5. [DOI] [PubMed] [Google Scholar]
- de Graan-Hentzen Y. C., Gratama J. W., Mudde G. C., Verdonck L. F., Houbiers J. G., Brand A., Sebens F. W., van Loon A. M., The T. H., Willemze R. Prevention of primary cytomegalovirus infection in patients with hematologic malignancies by intensive white cell depletion of blood products. Transfusion. 1989 Nov-Dec;29(9):757–760. doi: 10.1046/j.1537-2995.1989.29990070177.x. [DOI] [PubMed] [Google Scholar]