Abstract
Vascular smooth muscle cells (SMC) maintained in high glucose are more responsive to IGF-I than SMC maintained in normal glucose due to a difference in the Shc phosphorylation response. In this study we aimed to determine the mechanism by which glucose regulates the sensitivity of SMC to IGF-I. For Shc to be phosphorylated in response to IGF-I it must be recruited to tyrosine-phosphorylated sites on Src homology 2 domain-containing phosphatase (SHP) substrate-1 (SHPS-1). The association of integrin-associated protein (IAP) with SHPS-1 is required for SHPS-1 tyrosine phosphorylation. When SMC were grown in 5 mm glucose, the amount of intact IAP was reduced, compared with SMC grown in 25 mm glucose. This reduction was due to proteolytic cleavage of IAP. Proteolysis of IAP resulted in loss of its SHPS-1 binding site, which led to loss of SHPS-1 phosphorylation. Analysis of the conditioned medium showed that there was more protease activity in the medium from SMC cultured in 5 mm glucose as compared with 25 mm. Inhibition of matrix metalloprotease-2 synthesis using RNA interference or its activity using a specific protease inhibitor protected IAP from cleavage. This protection was associated with an increase in IAP-SHPS-1 association, increased recruitment and phosphorylation of Shc, and increased cell growth in response to IGF-I. Our results show that the enhanced response of SMC in 25 mm glucose to IGF-I is due to the protection of IAP from proteolytic degradation, thereby increasing its association with SHPS-1 and allowing the formation of the SHPS-1-Shc signaling complex.
ALTERATIONS IN THE nutrient environment, e.g. changes in glucose concentrations or levels of amino acids or fatty acids can affect growth, proliferation, and survival of cells (1,2). Understanding how changes in these nutrients affects the response of cells is likely to be important in understanding the mechanisms that lead to diseases such as diabetes and its associated complications. We have determined that when smooth muscle cells (SMC) are grown in medium containing high levels (25 mm) of glucose, they are significantly more responsive to IGF-I than SMC grown in medium containing physiologically normal levels of glucose (5 mm) (3).
Our studies have shown that the response of SMC, grown in medium containing high glucose (12 mm or higher), to IGF-I requires phosphorylation of the adaptor protein Shc, which in turn couples activation of the IGF-I receptor (IGF-IR) to activation of the MAPK pathway and cell proliferation (4). We have determined that the absence of a response of SMC to IGF-I in normal glucose can be attributed to a lack of Shc phosphorylation (3). For Shc to be phosphorylated, it must first be recruited to the cell membrane-associated protein, Src homology 2 (SH2) domain-containing phosphatase (SHP) substrate-1 (SHPS-1, SIRPα) (4). Failure to recruit Shc to SHPS-1 results in no Shc phosphorylation and reduced MAPK activation (4). After IGF-IR activation, SHPS-1 is phosphorylated on at least two tyrosine residues in its cytoplasmic domain (5). This generates binding sites for the SH2 domains of SHP-2. Shc, through its association with SHP-2, is then recruited to SHPS-1 (5), and Shc is subsequently phosphorylated by Src kinase (6). For SHPS-1 to be phosphorylated and therefore form the SHPS-1-SHP-2-Shc signaling complex, its extracellular domain must be bound to another transmembrane protein, integrin-associated protein (IAP) (7). Disruption of IAP-SHPS-1 binding using a blocking antibody blocks SHPS-1 phosphorylation and consequently MAPK activation and cell proliferation (7). Because IAP-SHPS-1 association is required for these signaling events to occur in response to IGF-I, we hypothesized that the difference in Shc phosphorylation between normal and high glucose exposure might be due to a difference in the formation of the IAP-SHPS-1-Shc signaling complex.
RESULTS
Glucose Regulation of SHPS-1-Shc Association
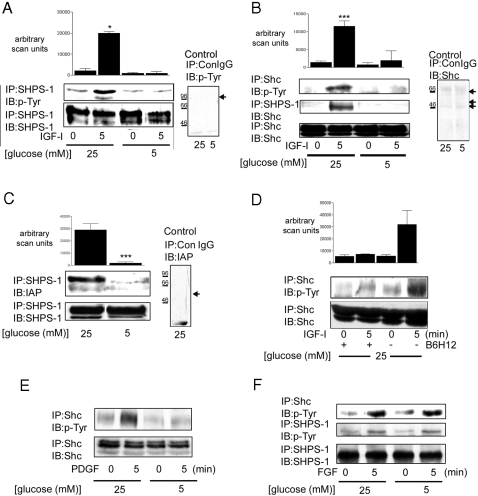
We have previously reported that the increase in Shc phosphorylation in response to IGF-I was significantly greater when SMC were grown in 25 mm as compared with 5 mm glucose (3). The difference in Shc phosphorylation could not be accounted for by a difference in the extent of IGF-IR phosphorylation or abundance of the IGF-IR (3). Because we have reported previously that SHPS-1 phosphorylation and the subsequent recruitment of Shc to phosphorylated SHPS-1 is required for Shc phosphorylation, we compared these two events in response to IGF-I in SMC grown in 5 and 25 mm glucose. There was a significant (7.7 ± 2.5-fold, mean ± sd; n = 3; P < 0.05) increase in SHPS-1 phosphorylation in response to IGF-I in SMC grown in 25 mm glucose, but there was no significant increase in SHPS-1 phosphorylation when SMC were grown in 5 mm (Fig. 1A). Consistent with the difference in SHPS-1 phosphorylation (4), there was significantly less Shc recruited to SHPS-1 in 5 mm glucose compared with SMC grown in 25 mm glucose [a 2.0 ± 1.5-fold increase (mean ± sd; n = 3; P value nonsignificant) compared with an 8.02 ± 0.8-fold increase (mean ± sd; n = 3; P < 0.005)] (Fig. 1B). Consistent with our previous report, this was associated with a significant difference in Shc phosphorylation under the two different glucose conditions (Fig. 1B).
Figure 1.
Glucose Regulation of Shc Recruitment to SHPS-1, SHPS-1 Phosphorylation, and SHPS-1 Association with IAP
Cells were grown to confluency in either 25 or 5 mm glucose before overnight incubation in SFM with the appropriate glucose concentration. IGF-I (100 ng/ml) was added for the lengths of times indicated. A, SHPS-1 phosphorylation was determined by immunoprecipitating (IP) cell lysates with an anti-SHPS-1 antibody and then immunoblotting (IB) with an anti-phosphotyrosine antibody (p-Tyr) (top panel). The membrane was then stripped and reprobed with an anti-SHPS-1 antibody (bottom panel). To control for nonspecific precipitation of SHPS-1, cell lysates from SMC grown in 5 and 25 mm glucose was immunoprecipitated with nonimmune rabbit serum (ConIgG) and immunoblotted with the anti-phosphotyrosine antibody; a representative blot is shown in the panel labeled control, and the expected position of SHPS-1 is indicated with the black arrow. The positions of molecular weight standards are also shown. B, The extent of Shc phosphorylation was determined by immunoprecipitating one aliquot of the resulting cell lysates with an anti-Shc antibody and then immunoblotting with an anti-phosphotyrosine antibody (p-Tyr) (top panel). Shc association with SHPS-1 was determined by immunoprecipitating a second aliquot of the same lysate with an anti-SHPS-1 antibody and immunoprecipitating with an anti-Shc antibody (middle panel). Equal amounts of lysate were also immunoprecipitated and immunoblotted for total Shc protein (bottom panel). To control for nonspecific precipitation, cell lysate from SMC grown in both 5 and 25 mm glucose were immunoprecipitated with nonimmune rabbit serum (ConIgG) and then immunoblotted with the anti Shc antibody (the arrows indicate the small amount of p42/p52/p66 Shc that is precipitated nonspecifically); a representative blot is shown in the panel labeled control. The positions of molecular weight standards are also shown. C, SHPS-1 association with IAP was determined by immunoprecipitating cell lysates with an anti-SHPS-1 antibody and then immunoblotting with an anti-IAP monoclonal antibody, B6H12 (top panel). Membranes were stripped and reprobed with an anti-SHPS-1 antibody (bottom panel). To control for nonspecific precipitation of IAP, cell lysates from SMC grown in 25 mm glucose were subjected to immunoprecipitation with nonimmune rabbit serum and immunoblotted with B6H12; a representative blot is shown in the panel labeled control, and the expected position of IAP is shown with the black arrow. The positions of the molecular weight standards are also shown. D, Before treatment with IGF-I, SMC grown in 25 mm glucose were treated with the anti-IAP monoclonal antibody B6H12 (5 μg/ml) for 4 h. Shc phosphorylation was determined as described for B. The graphs show the mean result from three independent experiments displayed as arbitrary scanning units (A, B, and D: ***, P < 0.005, and *, P < 0.05 when the increase in response to IGF-I in cells maintained in 25 mm glucose is compared with the response of cells maintained in 5 mm glucose; C: ***, P < 0.005 when the association between IAP and SHPS-1 in 25 mm glucose is compared with that in 5 mm glucose). E, The extent of Shc phosphorylation was determined as for A except cells were treated with PDGF (10 ng/ml) for 5 min before lysis. F, SHPS-1 and Shc phosphorylation was determined as described for B except cells were treated with FGF (25 ng/ml) for 5 min before lysis.
Because we had shown previously that for SHPS-1 to be phosphorylated it must be associated with the extracellular domain of IAP (7), we compared the association of these two proteins in SMC grown in 5 and 25 mm glucose. The results show that there was significantly more IAP (19 ± 7-fold greater, mean ± sd; n = 3; P < 0.01) that was coprecipitated with SHPS-1 when SMC grown in 25 mm glucose were compared with SMC grown in 5 mm glucose (Fig. 1C).
To confirm the importance of the interaction between IAP and SHPS-1 in regulating Shc phosphorylation in response to IGF-I, we treated SMC grown in 25 mm glucose with the anti-IAP antibody, B6H12, that had been shown previously to block SHPS-1 association with IAP and thereby inhibit SHPS-1 phosphorylation (7). In the presence of this antibody, Shc phosphorylation in response to IGF-I was reduced from a 6.7 ± 2.4-fold increase (mean ± sd, n = 3; P < 0.05) in the presence of the control antibody (Fig. 1D) to a 1.08 ± 0.34-fold increase (mean ± sd, n = 3; P value not significant) in the presence of B6H12. This result supports our conclusion that the difference in Shc phosphorylation in response to IGF-I in SMC grown in 5 and 25 mm glucose is due to a change in the amount of IAP that is associated with SHPS-1.
Previous studies have shown that glucose regulates the response of SMC to platelet-derived growth factor (PDGF) (8,9). To determine whether SMC exposed to 25 mm glucose respond to other growth factors in a manner similar to IGF-I, we compared Shc phosphorylation in response to PDGF in SMC grown in 5 and 25 mm glucose. Similar to the response to IGF-I, the increase in Shc phosphorylation in response to PDGF was significantly higher (4.4 ± 2-fold increase, mean ± sd; n = 3; P < 0.05) in SMC grown in 25 mm glucose than those grown in 5 mm glucose (1.01 ± 0.8-fold increase, mean ± sd; n = 3; P < 0.05) (Fig. 1E). We have shown previously, however, that the phosphorylation of SHPS-1 in response to PDGF does not require the association between IAP and SHPS-1 (7). Furthermore, in contrast to the IGF-IR, previous studies have shown that culturing SMC in 25 mm up-regulates the PDGF receptor phosphorylation response (8). Therefore, it seems likely that the difference in Shc phosphorylation in response to PDGF reflects the glucose-regulated difference in PDGF receptor kinase activity. We also examined SHPS-1 and Shc phosphorylation in response to fibroblast growth factor (FGF). After FGF exposure, there was no significant difference in the extent of SHPS-1 or Shc phosphorylation between SMC grown in 5 or 25 mm glucose (Fig. 1F). Taken together, this suggests that although glucose concentrations regulate the response of SMC to various growth factors, only the IGF-I response appears to be altered by glucose-dependent changes in SHPS-1 phosphorylation.
Glucose Concentrations Regulate IAP Association with SHPS-1 by Regulating IAP Cleavage
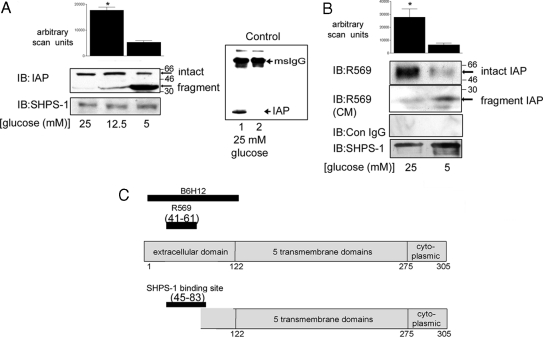
There were no significant differences in the amount of SHPS-1 protein that could be detected in the lysates from SMC grown in 5 or 25 mm glucose (Fig. 2A). However, when IAP was analyzed, there was a significant decrease in the amount of intact IAP in SMC grown in 5 mm glucose compared with SMC grown in 25 mm glucose (3.0 ± 0.2-fold decrease, mean ± sd; n = 3; P < 0.05) (Fig. 2A). This difference in intact IAP appeared to be due to the proteolytic cleavage, because analysis of the lysates from SMC grown in 5 mm glucose by immunoblotting, shown in Fig. 2A, revealed a lower molecular weight form of IAP. The amount of intact IAP detectable when SMC grown in 12.5 mm glucose was equal to the amount of intact IAP detectable when SMC were grown in 25 mm glucose (i.e. 101 ± 4.2%, mean ± sd; n = 3; P value not significant).
Figure 2.
Glucose Regulation of IAP Proteolysis
Cells were grown to confluency in 25, 12.5, or 5 mm glucose before overnight incubation in SFM. After lysis, equal amounts of protein were separated by SDS-PAGE, and IAP was visualized by immunoblotting (IB) with an anti-IAP monoclonal antibody (B6H12). A, To visualize both intact and residual fragment, the anti-IAP monoclonal antibody (B6H12) was used (top panel). Membranes were stripped and reprobed with an anti-SHPS-1 antibody (bottom panel). The graph shows the difference in intact IAP detected with B6H12 in SMC grown in 25 mm glucose and those grown in 5 mm glucose expressed as arbitrary scanning units (mean ± sd, n = 3; *, P < 0.05). To control for nonspecific immunoprecipitation of IAP, cell lysates from SMC grown in 25 mm glucose were immunoprecipitated with either B6H12 (lane 1) or mouse control Ig (Con IgG; lane 2) and immunoblotted with B6H12. To detect IAP, samples were separated without the addition of reducing agents and thus the immunoprecipitating antibody remained intact and was detected by the antimouse horseradish peroxidase secondary antibody, and this is indicated with the arrow at the top of the blot and labeled msIgG. B, To determine whether cleavage resulted in loss of the extracellular domain, the anti-IAP polyclonal antibody (R569) raised using a peptide that contained amino acids 43–61 of IAP as an immunogen was used (top panel). To detect the fragment shed after cleavage, conditioned medium samples were concentrated, and then IAP was visualized using the anti-IAP antibody R569 (second panel). Cell lysate samples were also immunoblotted with nonimmune rabbit serum (Con IgG). The membranes were stripped and reprobed with an anti-SHPS-1 antibody (bottom panel). The graph shows the difference in intact IAP detected with R569 in SMC grown in 25 mm glucose and those grown in 5 mm glucose expressed as arbitrary scanning units (mean ± sd, n = 3; *, P < 0.05). C, Schematic representation of IAP to illustrate the regions recognized by the antibodies used to detect intact and fragmented IAP.
To confirm that the reduction in intact IAP in SMC grown in 5 mm glucose was due to proteolytic cleavage, we used an antibody that was specific for a region of the extracellular domain of IAP between amino acids 43 and 61 (antibody recognition site illustrated in Fig. 2C). Immunoblotting cell lysates from SMC grown in 25 mm glucose with this antibody resulted in the detection of a single band of IAP. However, there was significantly less (4.9 ± 1.4-fold decrease, mean ± sd; n = 3; P < 0.05) intact IAP detected with this antibody in lysates from cells grown in 5 mm glucose (Fig. 2B, top panel). In contrast, immunoblotting of conditioned medium from SMC grown in 5 mm glucose using the same antibody yielded a lower molecular weight IAP immunoreactive band, whereas the conditioned medium from SMC grown in 25 mm glucose did not contain that band (Fig. 2B, middle panel).
These data suggest that cleavage of IAP results in the shedding of a region of its extracellular domain and that the rate of cleavage is altered in high glucose. Because the extracellular domain of IAP is required for its association with SHPS-1 (10,11), these results suggest that the cleavage of IAP accounts for the lack of association between these two proteins when SMC are grown in 5 mm glucose.
Inhibition of Matrix Metalloprotease (MMP)-2 Inhibits IAP Cleavage in Normal Glucose
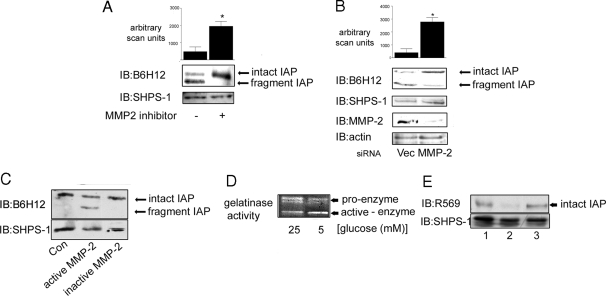
The activity of the MMP family of proteases, in particular MMP-2/9, has been shown to be regulated by glucose (12,13,14,15,16,17). Therefore, we tested whether this family of proteases may be responsible for the cleavage of IAP. We tested the effect of a commercially available inhibitor that is highly specific for MMP-2 (and to a lesser extent MMP-9) (18,19,20). When SMC grown in 5 mm glucose were treated with this inhibitor, there was a significant (4.9 ± 1.6-fold, mean ± sd; n = 3; P < 0.05) increase in the amount of intact IAP, and the IAP fragment was not detected (Fig. 3A).
Figure 3.
MMP-2 Cleaves IAP in 5 mm Glucose
A, Cells were grown to confluency in medium containing 5 mm glucose before the initiation of the experiment then incubated overnight in SFM. The MMP-2/9 inhibitor (3 μg/ml) was added to some cultures for 4 h before terminating the experiment. After lysis and separation by SDS-PAGE, intact and fragmented IAP were visualized with the monoclonal anti-IAP antibody, B6H12 (top panel). Membranes were stripped and reprobed with an anti-SHPS-1 antibody. The graph shows the difference in intact IAP detected with B6H12 in SMC grown in 5 mm in the presence or absence of the MMP-2 inhibitor expressed as arbitrary scanning units (mean ± sd, n = 3; *, P < 0.05). B (upper panel), Cells transfected with the siRNA construct targeting MMP-2 (MMP-2) and the empty vector control (Vec) were grown to confluency in medium containing 5 mm glucose and then incubated overnight in SFM (5 mm) before lysis. IAP was visualized by immunoblotting (IB) with the anti-IAP monoclonal antibody B6H12. The graph shows the difference in intact IAP detected with B6H12 in siRNA MMP-2 SMC compared with empty vector control cells that had been grown in 5 mm glucose expressed as arbitrary scanning units (mean ± sd, n = 3; *, P < 0.05). B (lower panel), SMC that were transfected with either the siRNA-MMP-2 (MMP-2) or the empty vector control construct were screened by immunoblotting for MMP-2 protein levels. To control for loading, membranes were stripped and reprobed for β-actin. C, Membrane extracts prepared from SMC grown in 25 mm glucose were incubated with (+) or without (−) activated MMP-2 for 24 h. Membrane extracts were then separated by SDS-PAGE and IAP visualized by Western immunoblotting with the anti-IAP antibody B6H12. To control for the level of total protein, the blot was stripped and reprobed with an anti SHPS-1 antibody. The results shown are representative of three similar experiments with similar results. D, Conditioned media from SMC grown in 25 and 5 mm glucose were collected, concentrated, and then applied to a gelatin zymography gel. After staining with Coomassie blue, cleared areas were visualized as an indicator of MMP-2 gelatinase activity. The results shown are representative of three independent experiments with similar results. E (top panel), Membrane samples from SMC grown in 25 mm glucose were incubated with conditioned medium from SMC grown in 25 mm (lane 1) or 5 mm (lane 2) glucose or 5 mm glucose SFM from a cell-free culture (lane 3). After 24 h incubation at 37 C, the membrane extracts were separated by SDS-PAGE and immunoblotted with the anti-IAP antibody R569, specific for intact IAP. Reprobing with an anti SHPS-1 antibody demonstrated that there was no difference in the total amount of protein in each lane (bottom panel). The results shown are representative of three independent experiments with similar results.
To confirm the identity of the protease responsible for IAP cleavage, we used RNA interference to decrease the MMP-2 protein level in SMC grown in 5 mm glucose. Cells grown in 5 mm glucose and expressing the small interfering RNA (siRNA)-MMP-2 construct had a significant reduction in the level of detectable MMP-2 compared with cells transduced with the empty vector alone (Fig. 3B, lower panel). When the transduced cells were maintained in 5 mm glucose, there was a significant (7.9 ± 4-fold, mean ± sd; n = 3; P < 0.05) increase in the amount of intact IAP and a corresponding decrease in the amount of fragment when compared with SMC transduced with the empty vector (Fig. 3B, upper panel). Immunoblotting with an anti-SHPS-1 antibody indicated that the same amount of cellular protein was loaded in each lane. Thus, by employing two different methods of inhibiting MMP-2 activity, we were able to prevent IAP cleavage in SMC grown in 5 mm glucose.
To provide further evidence that IAP was a substrate for MMP-2, the ability of recombinant MMP-2 to cleave IAP was determined. Membrane extracts obtained from SMC cultures grown in 25 mm glucose (i.e. with intact IAP) were incubated in the presence or absence of activated or inactive recombinant MMP-2. The ability of the activated MMP-2 to cleave IAP is evident by the 2.1 ± 0.03-fold decrease (mean ± sd; n = 3; P < 0.05) in intact IAP and the appearance of a smaller molecular weight fragment of the same apparent size as that detectable in SMC grown in 5 mm glucose (Fig. 3C). The complete absence of cleavage in the presence of the inactive MMP-2 further supports the conclusion that MMP-2 is the specific protease that cleaves IAP.
To determine whether the difference in MMP-2-mediated cleavage of IAP was due to a difference in the amount of active MMP-2 under the two different conditions, MMP-2 activity was assessed using gelatin zymography. MMP-2 is secreted as a pro-form and must be cleaved from this inactive precursor to the active form of the enzyme (21,22). Gelatin zymography can be used to visualize MMP-2 activity in different samples. Even though gelatinase activity is detected with both the inactive pro-form as well as the active form, the two forms are distinguishable based upon differences in size reflected by a difference in electrophoretic mobility. In the conditioned medium from SMC grown in 5 mm glucose, 83 ± 6.8% of the total amount of MMP-2 activity was detectable in the active form, whereas only 34 ± 6% of the total MMP-2 activity was in the active form in the medium from the SMC grown in 25 mm glucose (mean ± sd; n = 3; P < 0.05) (Fig. 3D). Immunoblotting of cell lysates and conditioned medium (data not shown) showed no significant difference in the amount of MMP-2 protein between SMC cultured in 25 and 5 mm glucose that would account for the difference in activity.
To demonstrate that the difference in the extent of IAP cleavage in the SMC grown in 5 and 25 mm glucose was due to a difference in the amount of active MMP-2 in the conditioned medium obtained from two different cell populations, we harvested conditioned serum-free medium (SFM) from SMC grown in 5 and 25 mm glucose. The two preparations of conditioned medium were then incubated with membrane extracts prepared from SMC grown in 25 mm glucose. Using an anti-IAP antibody that is specific for the detection of intact IAP (R569), significantly less intact IAP (83 ± 16% decrease, mean ± sd; n = 3; P < 0.05) could be detected in the sample that was incubated with the conditioned medium prepared from cultures exposed to 5 mm glucose compared with the conditioned medium from the 25 mm glucose cultures (Fig. 3E). This was not due to a difference in the amount of membrane extract because there was no significant difference in the amount of SHPS-1 that could be detected in both samples. Incubation of membrane extracts with SFM (5 mm glucose) that had not been conditioned by cells did not result in a decrease in the amount of IAP that could be detected.
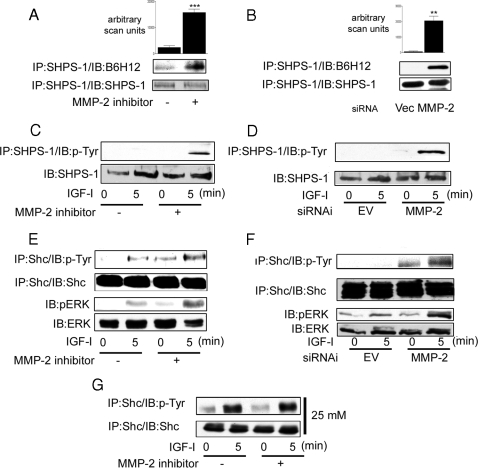
Inhibition of IAP Cleavage Restores Its Association with SHPS-1 and Enhances Downstream Signaling Responses to IGF-I in 5 mm Glucose
If, as we hypothesized, the lack of IAP-SHPS-1 association and therefore lack of signaling in response to IGF-I in SMC grown in 5 mm glucose was due to the cleavage of IAP, then it would be anticipated that inhibition of IAP cleavage would increase the association between IAP and SHPS-1 and enhance IGF-I signaling responses.
In the presence of the MMP-2 inhibitor, there was a significant increase (6.5 ± 1.7-fold increase, mean ± sd; n = 3; P < 0.005) in the amount of IAP associated with SHPS-1 compared with control cultures (Fig. 4, A and B). Similarly, there was a significant increase (27 ± 10-fold, mean ± sd; n = 3; P < 0.05) in IAP-SHPS-1 association in the siRNA MMP-2 cells compared with the empty vector control cultures. This is consistent with the effect of MMP-2 inhibition on IAP association with SHPS-1, in the presence of both the MMP-2 inhibitor and in the siRNA MMP-2 cells (Fig. 4, C and D). The inhibition of MMP-2 activity by both methods was associated with an increase in the basal level of Shc phosphorylation. Furthermore, there was a significant increase in Shc phosphorylation after the addition of IGF-I compared with SMC grown in 5 mm glucose in the absence of MMP-2 inhibition (Fig. 4, E and F, upper panels). As would be predicted from the increase in Shc phosphorylation, IGF-I activation of MAPK, as measured by assessing phosphorylation of threonine 202 and tyrosine 204 of ERK, is also significantly increased in the cells in which MMP-2 protease activity has been suppressed (Fig. 4, E and F, lower panels).
Figure 4.
Inhibition of MMP-2 Activity Enhances IGF-I-Mediated Signaling Events in SMC Grown in 5 mm Glucose
SMC grown to confluency in 5 mm glucose were incubated in SFM overnight. This was followed by a 4-h incubation in the presence of the MMP-2 inhibitor (3 μg/ml) before lysis and immunoprecipitation (IP). SMC expressing the siRNA-MMP-2 (MMP-2) or the empty vector control (Vec or EV) were grown to confluency in 5 mm glucose (or 25 mm glucose where stated). This was followed by overnight incubation in SFM followed by lysis and immunoprecipitation. Cells were treated with IGF-I (100 ng/ml) for the times indicated. A and B, SHPS-1 association with IAP was determined by immunoprecipitating cell lysates with an anti-SHPS-1 antibody and then immunoblotting with an anti-IAP monoclonal antibody B6H12 (top panel). Membranes were stripped and reprobed with an anti-SHPS-1 antibody (bottom panel). The graphs show the difference in IAP association with SHPS-1 expressed as arbitrary scanning units (mean ± sd, n = 3; ***, P < 0.05; **, P < 0.01). C and D, The extent of SHPS-1 phosphorylation was determined by immunoprecipitating with an anti-SHPS-1 antibody and then immunoblotting with an anti-phosphotyrosine antibody (p-Tyr) (top panel). Equivalent amounts of cell lysate were immunoprecipitated and immunoblotted with an anti-SHPS-1 antibody (bottom panel). E–G, The extent of Shc phosphorylation and activation of the MAPK pathway visualized by Western immunoblotting with antibodies that recognize the dual phosphorylated form of ERK1/2 was determined. In G, SMC were grown in 25 mm glucose.
The addition of the MMP-2 inhibitor had a small but nonsignificant effect on Shc phosphorylation in response to IGF-I in SMC grown in 25 mm glucose (Fig. 4G).
Importantly, the increase in signaling in response to IGF-I that occurred when SMC grown in 5 mm glucose that had been treated with the MMP-2 inhibitor resulted in an increase cell proliferation in response to IGF-I compared with the response of cells to IGF-I that were not exposed to the MMP-2/9 inhibitor (Fig. 5A). When the cells were maintained in 5 mm glucose in the presence of the MMP-2 inhibitor, there was a significant (2.3 ± 0.34-fold, mean ± sd; n = 3; P < 0.005) increase in cell proliferation in response to IGF-I. In contrast, in the absence of the inhibitor, there was no increase in response to IGF-I (1.12 ± 0.17-fold increase, n = 3; P value not significant). Cell proliferation in response to IGF-I in SMC grown in 25 mm glucose was also slightly, but not significantly, increased in the presence of the MMP-2 inhibitor compared with the increase in response to IGF-I in the absence of the inhibitor.
Figure 5.
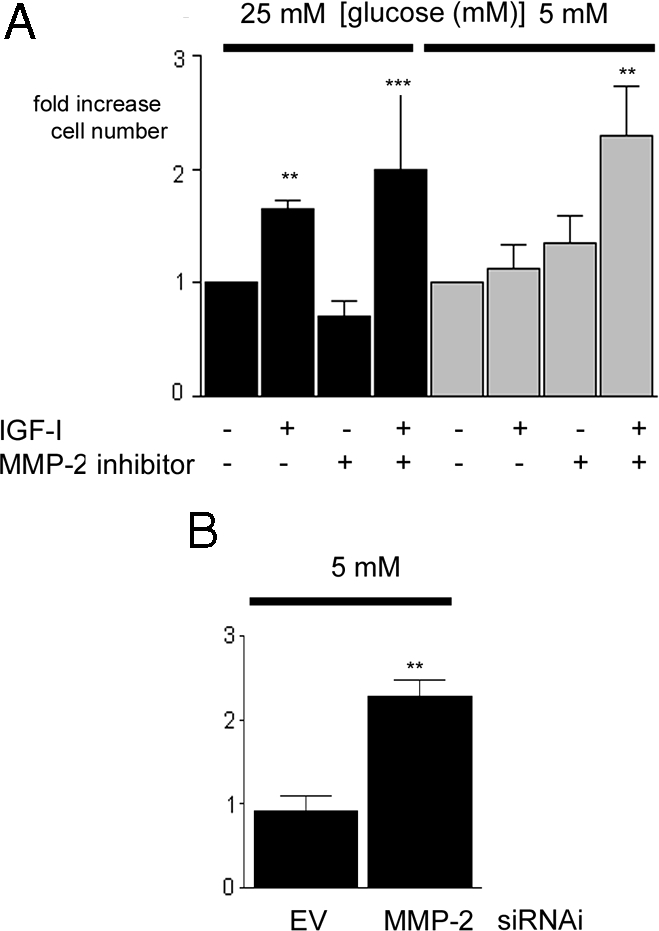
Inhibition of MMP-2 Protease Activity Enhances the Proliferative Response of SMC Maintained in 5 mm Glucose to IGF-I
A, Cells that had been grown in either 25 or 5 mm glucose were plated (2 × 104 cells per well) in 24-well plates for 24 h to allow attachment. After an overnight incubation in SFM, they were then exposed to IGF-I (50 ng/ml) in the presence or absence of the MMP-2 inhibitor (or vehicle control) with 0.2% platelet-poor plasma. After an additional 48 h, cell number was determined by trypan blue staining and counting. ***, P < 0.005 when cell number in response to IGF-I in cells grown in 5 mm glucose in the presence of the MMP-2 inhibitor is compared with the response of cells grown in 5 mm glucose alone; **, P < 0.01 when the response of SMC grown in 25 mm glucose with IGF-I is compared with SMC grown in 25 mm glucose and no IGF-I. B, SMC expressing the siRNA-MMP-2 (MMP-2) or the empty vector control (EV) that had been grown in 5 mm glucose were plated (2 × 104 cells per well) in 24-well plates for 24 h to allow attachment. After an overnight incubation in SFM, they were then exposed to IGF-I (50 ng/ml) in DMEM plus 0.2% platelet-poor plasma. After an additional 48 h, cell number was determined by trypan blue staining and counting. **, P < 0.01 when the response of siRNA-MMP-2 (MMP-2) to IGF-I is compared with the response of the empty vector (EV) control cells (n = 3). There was no significant difference in the basal proliferation between SMC grown in 5 or 25 mm glucose. Cell numbers were 2.3 ± 0.3 × 104 when SMC were grown in high glucose in the absence of IGF-I for 24 h and 2.7 ± 0.4 × 104 when SMC were grown in normal glucose in the absence of IGF-I for 24 h. Hence, the proliferation response is expressed as fold increase over basal.
Similarly, transduction of SMC with the siRNA MMP-2 construct significantly increased their growth response to IGF-I in 5 mm glucose (Fig. 5B). The 2.3 ± 0.17-fold increase in cell proliferation in response to IGF-I elicited in the siRNA MMP-2 cells was significantly higher than the 0.9 ± 0.1-fold increase in cell proliferation elicited by the empty vector control cells, mean ± sd).
DISCUSSION
Our prior studies determined that Shc phosphorylation is required for activation of the MAPK signaling pathway and cell proliferation in response to IGF-I (4) and that this difference is due to a difference in the ability of IGF-I to stimulate Shc phosphorylation (3). Because SMC grown in medium containing physiologically normal levels of glucose have an attenuated Shc phosphorylation response compared with SMC maintained in medium containing high glucose, this difference in Shc phosphorylation appears to be responsible for the attenuated cell growth and migration responses to IGF-I compared with SMC grown in medium with high glucose (3). The major finding in this study is that glucose levels regulate IAP proteolysis and, as a consequence of this, cellular responsiveness to IGF-I. The increase in the amount of intact IAP that is detected when SMC are grown in glucose concentrations of 12 mm or greater results in an increase in association of IAP with SHPS-1. Because IAP binding to SHPS-1 is required for SHPS-1 phosphorylation (7) and, therefore, the subsequent recruitment and phosphorylation of Shc (4), inhibition of IAP cleavage leads to enhanced SHPS-1 phosphorylation, Shc phosphorylation, and downstream signaling responses after exposure to IGF-I.
Our results indicate that the change in the amount of intact IAP that is detected in SMC grown in normal glucose compared with SMC grown in high glucose is due to differences in proteolytic cleavage. Using an antibody prepared with a peptide that contained amino acids 43–61 of IAP, we detected a low molecular weight fragment of IAP in the conditioned medium from SMC grown in normal glucose, but this fragment was not detected in cells exposed to high glucose. Furthermore, this antibody did not detect IAP on the surface of SMC maintained in normal glucose, but it did detect IAP in cells maintained in high glucose. These results led us to conclude that glucose-dependent proteolytic cleavage results in shedding of part of the extracellular domain of IAP and that the residual IAP that remains cell surface associated does not contain this fragment.
The extracellular domain of IAP has been shown to be required for its association with SHPS-1 (7,11). Using a SHPS-1 construct in which the amino-terminal Ig domain of SHPS-1 had been deleted, Seiffert et al. (10) showed that SHPS-1 and IAP did not associate. This led them to conclude that the binding site in IAP was located in its extracellular domain (10). Vernon-Wilson et al. (11) extended those observations to show that the domain on IAP that interacted directly with SHPS-1 was located within its Ig domain, between amino acids 19 and 125. Recently, Subramanian et al. (23) used in vitro mutagenesis to further localize the binding site to a region between R45 and D83 of porcine IAP. Taken together, we conclude that the IAP cleavage site must be contained within the region of IAP that binds to SHPS-1, and therefore cleavage of IAP destroys the SHPS-1 binding site, preventing either the shed fragment or the residual membrane-associated fragment from binding to SHPS-1.
Our results show that the protease responsible for the cleavage of IAP is MMP-2. We can conclude this for a number of reasons. The inhibitor used for these experiments (also referred to as SB-3CT) was designed based upon the mechanism of action of tissue inhibitor of metalloprotease (TIMP)-1 and -2 [which are specific for MMPs, unlike TIMP-3, which also inhibits members of the a disintegrin and metalloprotease (ADAM) family] (18,19,20). Therefore, this inhibitor is highly specific for MMP-2 (18,19,20). The Ki of this inhibitor for MMP-2 is 43-fold lower than its Ki for MMP-9 and 266-fold lower than its Ki for a disintegrin and metalloprotease (ADAM)-17 (24). Therefore, at the concentrations used in this study, it is highly likely that the inhibitor was relatively specific for MMP-2. The conclusion that MMP-2 is the protease that cleaves IAP is supported by the lack of IAP cleavage in cells in which the MMP-2 protein was reduced by RNA interference. Furthermore, incubation of intact IAP with recombinant MMP-2 resulted in significant reduction in the amount of intact IAP and the appearance of an IAP fragment. Taken together, these data lead us to conclude that MMP-2 is the protease most likely responsible for the cleavage of IAP that occurs in 5 mm glucose.
Our data suggest that at least part of the mechanism by which elevated glucose concentrations increase IAP stability is by decreasing the amount of active MMP-2 released into the conditioned medium. There are a number of possible mechanisms by which this could occur. Pro-MMP-2 is activated on the cell surface by a complex interaction between membrane-type 1-MMP, TIMP-2, and MMP-2 (25). It has been shown that high glucose concentrations reduce membrane-type-MMP-1 levels, which results in reduced MMP-2 activation (12,13,14,15,16,17). The amount of MMP-2 activity is also regulated by the concentration of members of the TIMP family of inhibitors. It has been shown that glucose disrupts the balance between TIMP-2 and MMP-2 in diabetic rats (26). Streptozotocin treatment of rats, which results in hyperglycemia, is associated with increased glomerular and tubular levels of TIMP-2 and decreased levels of MMP-2 (26). Similarly, in endothelial (27), mesangial (28), and renal tubule cell cultures, glucose increased TIMP-2 and decreased MMP-2. Additional studies will be required to determine how hyperglycemia regulates the activation of MMP-2 in SMC.
An alternative, or perhaps an additional, mechanism by which glucose levels may regulate IAP cleavage is by regulating the accessibility of the IAP cleavage site to the protease responsible for cleavage. We, and others, have shown that increased glucose concentrations are associated increases in thrombospondin (TS-1) levels (3). TS-1 binds to the extracellular domain of IAP (29), and it enhances IGF-I signaling by enhancing stability of the IAP-SHPS-1 complex (30). Therefore, another possible mechanism by which elevated glucose levels increase the level of intact IAP is by increasing the binding of TS-1 to IAP and thus protecting it from cleavage, either by directly obscuring the cleavage site or by inducing a conformational change that alters the accessibility of the IAP cleavage site to the protease.
The results of this study have important implications in advancing our understanding of how SMC respond to hyperglycemic stress. Diabetic patients have an increased risk of developing atherosclerosis, which is characterized by increases in SMC migration and proliferation. Understanding the mechanism by which hyperglycemia contributes to the enhanced responsiveness of SMC to IGF-I will reveal new targets to prevent the increase in response to IGF-I and thereby potentially delay or inhibit the progression of atherosclerosis in diabetic patients.
IAP, or CD47 as it is also known, is ubiquitously expressed and has been shown to be involved in a wide variety of biological effects. For example, CD47 acts as a marker of self on red blood cells by binding to macrophage SHPS-1, thereby preventing phagocytosis of the red blood cells (31). In the absence of IAP, osteoclast formation is significantly impaired as a result of the loss of the binding between IAP and SHPS-1 (32). Association between IAP and SHPS-1 has also been implicated in the adhesion of leukocytes to endothelial cells during an inflammatory response (33). Recently, Johansen and Brown (34) demonstrated that the interaction between IAP and SHPS-1 was required for SHPS-1 phosphorylation in endothelial cells derived from both the lung and brain of mice. To our knowledge, this study is the first report of the regulation of IAP by proteolysis and subsequent shedding of its extracellular domain resulting in a loss of binding to SHPS-1. Given the ubiquitous nature of IAP expression and the diversity of its roles in regulating cellular activity, this novel mechanism of regulation is likely to have implications in a wide variety of cell systems.
MATERIALS AND METHODS
Human IGF-I was a gift from Genentech (South San Francisco, CA). PDGF and FGF were purchased from USB (Cleveland, OH). Polyvinyl difluoride membrane (Immobilon P) was purchased from Millipore Corp. (Billerica, MA). Cl-Xposure autoradiographic film was obtained from Pierce (Rockford, IL). Fetal bovine serum (FBS), DMEM, penicillin, and streptomycin were purchased from Life Technologies (Grand Island, NY). The monoclonal anti-phosphotyrosine antibody (PY99) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-Shc and phospho-/total ERK1/2 antibodies were purchased from BD Transduction Laboratories (Lexington, KY). The anti-SHPS-1 antibody was purchased from Upstate Cell Signaling Solutions (Charlottesville, VA). The anti-MMP-2 antibody was purchased from AbD Serotec (Raleigh, NC). The MMP-2/9 inhibitor IV (catalog item 444274) was purchased from EMD Biosciences (San Diego, CA). Mouse Ig was purchased from R&D Systems (Minneapolis, MN). All other reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless stated otherwise.
Anti-IAP Antibodies
The anti-IAP monoclonal antibody, B6H12, was purified from a specific cell line derived from a B cell hybridoma as we have described previously (30). The anti-IAP antibody (referred to as R569) was generated by conjugating a peptide homologous to amino acids 41–61 of the extracellular domain of IAP (KGRDIYTFDGALNKSTVPTC) to keyhole limpet hemagglutinin and used for immunization (35). Serum from a nonimmunized rabbit was prepared to use as control antiserum.
Porcine SMC
Porcine SMC were isolated from the porcine aortic explants by a modification of the protocol by Ross (36) as we have described previously (3). After isolation, SMC were maintained in either high-glucose growth medium (HG-GM) [DMEM containing 4500 g/liter (25 mm) glucose plus 10% FBS and penicillin (1000 U/ml) and streptomycin (160 μg/ml) and 1.0 mm sodium pyruvate] or in normal-glucose (NG)-GM [DMEM containing 900 g/liter glucose (5 mm) plus 10% FBS and penicillin and streptomycin]. SMC were fed every 3 d with either HG- or NG-GM and were passed every 7 d in the appropriate medium. All experiments were performed on SMC between passage numbers 4 and 10. We have previously determined that SMC cultured under these two different conditions do not differ significantly in their differentiation status (3). To control for differences in osmolarity, mannitol (19.5 mm) was added to the NG-SFM (3). We have determined previously that the presence or absence of mannitol does not influence the responses of SMC grown in NG (3).
There was no significant decrease in glucose levels between d 1 and 3 of culture (glucose levels at d 3 were 93 ± 6.8% of glucose levels at d 1; P value not significant; n = 4). We have previously determined that overnight incubation in SFM also did not result in a significant decrease in glucose levels (3). We also determined that when SMC were grown in glucose concentrations of 6 and 7 mm, IAP was cleaved to a similar extent as that seen when SMC were grown in 5 mm glucose; however, the presence of 8 mm glucose was sufficient to protect IAP from cleavage (data not shown). Furthermore, incubation of SMC grown in 25 mm glucose to SFM containing 5 mm glucose for 2 h was sufficient to result in significant IAP cleavage (data not shown).
Generation of pLenti-U6 siRNA-MMP-2 Construct to Silence MMP-2 Gene Expression and pLenti-U6 Empty Vector Control
The siRNA MMP-2 plasmid was generated using BLOCK-iT U6 RNAi system (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The region of MMP-2 that was targeted corresponded to bp 1025–1050 of the MMP-2 sequence. Two single-stranded oligonucleotides corresponding to the sequence 5′-CACCGCGACAAGAAGTACGGCTTCTCGAAAGAAGCCGTACTTCTTGTCGC-3′ (sense) and 3′-CGCTGTTCTTCATGCCGAAGAAGAGCTTTCTTCGGCATGAAGAACAGCGAAAA-5′ (antisense) were prepared and annealed to generate a double-stranded oligonucleotide that was cloned into the pENTR/U6 vector. Integrity of the construct was confirmed by DNA sequencing and then excised from pEntr/U6 and ligated into pLentiU6 Gateway using the LR clonase reaction according to the manufacturer's instructions (Invitrogen).
Generation of Virus Stocks and Establishment of SMC Expressing the pLenti siRNA MMP-2 and Empty Vector Control Construct
293FT cells (Invitrogen) were prepared for generation of virus stocks of either the siRNA-MMP-2 or empty vector construct as we have described previously (6). The viral complexes were precipitated, and SMC (maintained in 5 mm glucose) were transduced as we have described (6). The degree of reduction of MMP-2 protein in cells transduced with the siRNA-MMP-2 construct compared with the empty vector alone was determined by immunoblotting cell lysates (Fig. 3) using the anti-MMP-2 antibody (1:500). The transduction was performed on two separate occasions, and similar results were obtained after both transductions.
Cell Proliferation
Cell proliferation assays using either nontransduced cells (maintained in either 5 or 25 mm glucose) or SMC transduced with either the siRNA MMP-2 or empty vector constructs (maintained in 5 mm glucose) were performed as we have described previously (37). SMC were plated at 2 × 104 cells per well in each well of a 24-well plate in either HG-SFM or NG-SFM plus 2% FBS. Cells were allowed to attach overnight before the medium was replaced with HG-SFM or NG-SFM, and 24 h later, the SFM was replaced with SFM plus 0.2% platelet-poor plasma with or without the addition of IGF-I (50 ng/ml). The MMP-2/9 inhibitor was added at a concentration of 3 μg/ml immediately before the addition of IGF-I. Cell number was determined after trypsinization, trypan blue staining, and counting (38). The MMP-2/9 inhibitor was reconstituted in dimethylsulfoxide (DMSO); the addition of an equal amount of DMSO alone had no effect (data not shown).
Cell Lysis, Immunoprecipitation, and Western Immunoblotting
SMC were plated in 10-cm dishes in HG- or NG-GM and grown to confluency for 7 d with the medium being changed after 3 d. In one experiment, SMC grown in 25 mm glucose were switched to medium containing 12.5 mm glucose at the time of feeding. On d 7, the growth medium was removed and the confluent monolayers were rinsed three times with HG- or NG-SFM (or in SFM containing 12.5 mm glucose) and incubated overnight (16–17 h). The MMP-2 inhibitor was added (3 μg/ml) for 4 h before the addition of IGF-I (100 ng/ml). The MMP-2 inhibitor was reconstituted in DMSO; addition of an equal amount of DMSO alone had no effect on IAP cleavage (data not shown).
The anti-IAP antibody (B6H12) or control Ig were added (5 μg/ml) 4 h before the addition of IGF-I (100 ng/ml). For the direct detection of IAP, SHPS-1, MMP-2, and β-actin, cells were lysed in a modified RIPA buffer. After centrifugation, equal amounts of cellular protein were mixed with nonreducing gel loading buffer, heated to 70 C for 10 min, and separated by SDS-PAGE (8%). Immunoprecipitation studies were performed as previously described (30). After SDS-PAGE, proteins were visualized by immunoblotting as we have previously described (30). For immunoblotting, the antibodies were used at concentrations between 1:500 and 1:1000.
To control for nonspecificity, nonimmune rabbit serum or mouse Ig was used for either immunoprecipitating or immunoblotting. Results are shown as separate figures because longer film exposures were required for the control blots due to a very weak signal as a result of almost no cross-reactivity or nonspecificity.
To detect the fragment of IAP in the conditioned medium samples, SMC grown to confluency in NG- and HG-GM were incubated overnight in SFM with the appropriate level of glucose. A 500-μl aliquot of conditioned medium was then collected and concentrated (Amicon Inc., Beverley, MA). Nonreducing Laemmli sample buffer was used to remove the proteins from the filters before separation by SDS-PAGE (8%) and immunoblotting with the anti-IAP antibody R569.
Analysis of MMP-2 Gelatinase Activity by Zymography
SMC were grown to confluency (in both HG and NG) and then incubated overnight in SFM. Fresh SFM was added the following day, and after 15 min incubation at 37 C, the conditioned medium was collected and concentrated 10-fold using 10K NMWL membrane centrifugal filters (Millipore). Concentrated conditioned medium was then diluted 1:1 with nonreducing Laemmli buffer, and proteins were separated using a 10% zymography (gelatin) SDS-PAGE system (Invitrogen). Gels were first incubated in renaturing buffer (2.5% Triton X-100 in water) for 30 min and then incubated in developing buffer (50 mm Tris base; 50 mm Tris-HCl; 0.2 m NaCl; 5 mm CaCl2; and 0.02% Nonidet P-40, pH 7.5) overnight at 37 C (with solution being refreshed after the first 30 min). Gels were then stained with a 0.5% Coomassie Blue solution (in 40% methanol and 7% acetic acid). Finally, gels were destained using a 40% methanol and 7% acetic acid solution and then incubated in a solution of 5% glycerol in water before drying.
In Vitro Cleavage of IAP
Membrane extracts were prepared from confluent 10-cm plates of SMC grown in HG-GM as we have described previously (39). Briefly, after washing with PBS, cells were detached from the dish using cell dissociation solution. Cells from several plates were pooled, and cell pellets were washed with PBS. The cell pellet was then resuspended in PBS (to achieve a concentration of 6 × 106 cells/ml), and several 1-ml aliquots were prepared before freezing at −80 C. Cells were then lysed by thawing at room temperature and then sonicating for 2 min. Samples were centrifuged at 14,000 rpm for 10 min to pellet the membrane fraction. Membrane extracts were then used for protease assays using either recombinant MMP-2 or conditioned medium obtained from SMC cultures.
In Vitro Cleavage with Recombinant MMP-2
MMP-2 (R&D Systems) was activated with 4-aminophenylmercuric acetate (at a final concentration of 1 mm) according to the manufacturer's instructions immediately before use; then, 100 ng of either inactive or activated MMP-2 was added to each aliquot of membrane extract (derived from 1 × 106 cells) in a total volume of 100 μl TCNB (50 mm Tris; 10 mm CaCl2; 150 mm NaCl; and 0.05% Nonidet P-40, pH 7.5), and 100μl TCNB (with no MMP-2) was added to a second aliquot of membrane extract. The samples were then incubated for 18 h at 37 C with gentle shaking. At the end of the incubation period, the membrane extracts were centrifuged at 14,000 × g for 10 min. The membrane extracts were then resuspended in 2× Laemmli buffer and heated for 10 min at 70 C before separation by SDS-PAGE. IAP was visualized by Western immunoblotting as described above.
In Vitro Cleavage with Conditioned Medium from SMC Grown in HG- and NG-GM
SMC grown in HG-GM and NG-GM were incubated overnight in either HG- or NG-SFM. The following day, the SFM was refreshed and then collected 15 min later. Then, 100 μl HG-SFM or NG-SFM conditioned medium was added to separate aliquots of the membrane extracts prepared as described above. In addition, one aliquot of membrane extract was incubated with cell-free NG-SFM. After a 1-h incubation at 37 C with gentle shaking, the membrane extracts were pelleted by centrifugation at 14,000 × g for 10 min.
The membrane extracts were then resuspended in 2× Laemmli buffer and heated for 10 min at 70 C before separation by SDS-PAGE. IAP was visualized by Western immunoblotting as described above.
Statistical Analysis
Chemiluminescent images were scanned using a DuoScan T1200 (AGFA, Brussels, Belgium), and band intensities of the scanned images were analyzed using NIH Image, version 1.61. The Student's t test was used to compare differences between treatments. The results shown are expressed as the mean ± sd and are representative of at least three separate experiments.
Acknowledgments
We thank Drs. Jane Badley-Clarke and Walker H. Busby for preparation of reagents.
Footnotes
This work was supported by National Institutes of Health Grant HL56850 to D.R.C. and an American Heart Association Mid Atlantic Affiliate Beginning Grant in Aid (0465462U) to L.A.M.
Disclosure Statement: The authors have nothing to declare.
First Published Online February 21, 2008
Abbreviations: DMSO, Dimethylsulfoxide; FGF, fibroblast growth factor; HG-GM, high-glucose growth medium; IAP, integrin-associated protein; IGF-IR, IGF-I receptor; MMP, matrix metalloprotease; NG, normal-glucose; PDGF, platelet-derived growth factor; SFM, serum-free medium; SH2, Src homology 2; SHP, SH2-domain-containing phosphatase; SHPS-1, SHP substrate-1; siRNA, small interfering RNA; SMC, smooth muscle cells; TIMP, tissue inhibitor of metalloprotease; TS-1, thrombospondin.
References
- Hardie DG 2004 The AMP-activated protein kinase pathway: new players upstream and downstream. J Cell Sci 117:5479–5487 [DOI] [PubMed] [Google Scholar]
- Gulati P, Thomas G 2007 Nutrient sensing in the mTOR/S6K1 signalling pathway. Biochem Soc Trans 35:236–238 [DOI] [PubMed] [Google Scholar]
- Maile LA, Capps BE, Ling Y, Xi G, Clemmons DR 2007 Hyperglycemia alters the responsiveness of smooth muscle cells to insulin-like growth factor-I. Endocrinology 148:2435–2443 [DOI] [PubMed] [Google Scholar]
- Ling Y, Maile LA, Lieskovska J, Badley-Clarke J, Clemmons DR 2005 Role of SHPS-1 in the regulation of insulin-like growth factor I-stimulated Shc and mitogen-activated protein kinase activation in vascular smooth muscle cells. Mol Biol Cell 16:3353–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maile LA, Clemmons DR 2002 Regulation of insulin-like growth factor I receptor dephosphorylation by SHPS-1 and the tyrosine phosphatase SHP-2. J Biol Chem 277:8955–8960 [DOI] [PubMed] [Google Scholar]
- Lieskovska J, Ling Y, Badley-Clarke J, Clemmons DR 2006 The role of Src kinase in insulin-like growth factor-dependent mitogenic signaling in vascular smooth muscle cells. J Biol Chem 281:25041–25053 [DOI] [PubMed] [Google Scholar]
- Maile LA, Badley-Clarke J, Clemmons DR 2003 The association between integrin-associated protein and SHPS-1 regulates insulin-like growth factor-I receptor signaling in vascular smooth muscle cells. Mol Biol Cell 14:3519–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M, Allen WE, Silversides JA, Trimble ER 2003 Glucose-induced phosphatidylinositol 3-kinase and mitogen-activated protein kinase-dependent upregulation of the platelet-derived growth factor-β receptor potentiates vascular smooth muscle cell chemotaxis. Diabetes 52:519–526 [DOI] [PubMed] [Google Scholar]
- Campbell M, Allen WE, Sawyer C, Vanhaesebroeck B, Trimble ER 2004 Glucose-potentiated chemotaxis in human vascular smooth muscle is dependent on cross-talk between the PI3K and MAPK signaling pathways. Circ Res 95:380–388 [DOI] [PubMed] [Google Scholar]
- Seiffert M, Cant C, Chen Z, Rappold I, Brugger W, Kanz L, Brown EJ, Ullrich A, Buhring HJ 1999 Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood 94:3633–3643 [PubMed] [Google Scholar]
- Vernon-Wilson EF, Kee WJ, Willis AC, Barclay AN, Simmons DL, Brown MH 2000 CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPα1. Eur J Immunol 30:2130–2137 [DOI] [PubMed] [Google Scholar]
- McLennan SV, Wang XY, Moreno V, Yue DK, Twigg SM 2004 Connective tissue growth factor mediates high glucose effects on matrix degradation through tissue inhibitor of matrix metalloproteinase type 1: implications for diabetic nephropathy. Endocrinology 145:5646–5655 [DOI] [PubMed] [Google Scholar]
- McLennan SV, Martell SK, Yue DK 2002 Effects of mesangium glycation on matrix metalloproteinase activities: possible role in diabetic nephropathy. Diabetes 51:2612–2618 [DOI] [PubMed] [Google Scholar]
- McLennan SV, Kelly DJ, Cox AJ, Cao Z, Lyons JG, Yue DK, Gilbert RE 2002 Decreased matrix degradation in diabetic nephropathy: effects of ACE inhibition on the expression and activities of matrix metalloproteinases. Diabetologia 45:268–275 [DOI] [PubMed] [Google Scholar]
- McLennan SV, Fisher E, Martell SY, Death AK, Williams PF, Lyons JG, Yue DK 2000 Effects of glucose on matrix metalloproteinase and plasmin activities in mesangial cells: possible role in diabetic nephropathy. Kidney Int Suppl 77:S81–S87 [DOI] [PubMed] [Google Scholar]
- McLennan SV, Martell SY, Yue DK 2000 High glucose concentration inhibits the expression of membrane type metalloproteinase by mesangial cells: possible role in mesangium accumulation. Diabetologia 43:642–648 [DOI] [PubMed] [Google Scholar]
- McLennan SV, Yue DK, Turtle JR 1998 Effect of glucose on matrix metalloproteinase activity in mesangial cells. Nephron 79:293–298 [DOI] [PubMed] [Google Scholar]
- Kleifeld O, Kotra LP, Gervasi DC, Brown S, Bernardo MM, Fridman R, Mobashery S, Sagi I 2001 X-ray absorption studies of human matrix metalloproteinase-2 (MMP-2) bound to a highly selective mechanism-based inhibitor. Comparison with the latent and active forms of the enzyme. J Biol Chem 276:17125–17131 [DOI] [PubMed] [Google Scholar]
- Ikejiri M, Bernardo MM, Meroueh SO, Brown S, Chang M, Fridman R, Mobashery S 2005 Design, synthesis, and evaluation of a mechanism-based inhibitor for gelatinase A. J Org Chem 70:5709–5712 [DOI] [PubMed] [Google Scholar]
- Ikejiri M, Bernardo MM, Bonfil RD, Toth M, Chang M, Fridman R, Mobashery S 2005 Potent mechanism-based inhibitors for matrix metalloproteinases. J Biol Chem 280:33992–34002 [DOI] [PubMed] [Google Scholar]
- Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M 1994 A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370:61–65 [DOI] [PubMed] [Google Scholar]
- Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI 1995 Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem 270:5331–5338 [DOI] [PubMed] [Google Scholar]
- Subramanian S, Boder ET, Discher DE 2007 Phylogenetic divergence of CD47 interactions with human signal regulatory protein α reveals locus of species specificity. Implications for the binding site. J Biol Chem 282:1805–1818 [DOI] [PubMed] [Google Scholar]
- Solomon A, Rosenblum G, Gonzales PE, Leonard JD, Mobashery S, Milla ME, Sagi I 2004 Pronounced diversity in electronic and chemical properties between the catalytic zinc sites of tumor necrosis factor-α-converting enzyme and matrix metalloproteinases despite their high structural similarity. J Biol Chem 279:31646–31654 [DOI] [PubMed] [Google Scholar]
- Nagase H 1998 Cell surface activation of progelatinase A (proMMP-2) and cell migration. Cell Res 8:179–186 [DOI] [PubMed] [Google Scholar]
- Han SY, Jee YH, Han KH, Kang YS, Kim HK, Han JY, Kim YS, Cha DR 2006 An imbalance between matrix metalloproteinase-2 and tissue inhibitor of matrix metalloproteinase-2 contributes to the development of early diabetic nephropathy. Nephrol Dial Transplant 21:2406–2416 [DOI] [PubMed] [Google Scholar]
- Singh R, Song RH, Alavi N, Pegoraro AA, Singh AK, Leehey DJ 2001 High glucose decreases matrix metalloproteinase-2 activity in rat mesangial cells via transforming growth factor-β1. Exp Nephrol 9:249–257 [DOI] [PubMed] [Google Scholar]
- McGinn S, Poronnik P, Gallery ED, Pollock CA 2004 The effects of high glucose and atorvastatin on endothelial cell matrix production. Diabet Med 21:1102–1107 [DOI] [PubMed] [Google Scholar]
- Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA 1996 Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem 271:21–24 [DOI] [PubMed] [Google Scholar]
- Maile LA, Clemmons DR 2003 Integrin-associated protein binding domain of thrombospondin-1 enhances insulin-like growth factor-I receptor signaling in vascular smooth muscle cells. Circ Res 93:925–931 [DOI] [PubMed] [Google Scholar]
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP 2000 Role of CD47 as a marker of self on red blood cells. Science 288:2051–2054 [DOI] [PubMed] [Google Scholar]
- Lundberg P, Koskinen C, Baldock PA, Lothgren H, Stenberg A, Lerner UH, Oldenborg PA 2007 Osteoclast formation is strongly reduced both in vivo and in vitro in the absence of CD47/SIRPα-interaction. Biochem Biophys Res Commun 352:444–448 [DOI] [PubMed] [Google Scholar]
- Cooper D, Lindberg FP, Gamble JR, Brown EJ, Vadas MA 1995 Transendothelial migration of neutrophils involves integrin-associated protein (CD47). Proc Natl Acad Sci USA 92:3978–3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen ML, Brown EJ 2007 Dual regulation of SIRPα phosphorylation by integrins and CD47. J Biol Chem 282:24219–24230 [DOI] [PubMed] [Google Scholar]
- Maile LA, Busby WH, Sitko K, Capps BE, Sergent T, Badley-Clarke J, Clemmons DR 2006 IGF-I signaling in smooth muscle cells is regulated by ligand binding to the 177CYDMKTTC184 sequence of the β3 subunit of αVβ3. Mol Endocrinol 20:881–892 [DOI] [PubMed] [Google Scholar]
- Ross R 1971 The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol 50:172–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam TJ, Busby Jr WH, Rees C, Clemmons DR 2000 Thrombospondin and osteopontin bind to insulin-like growth factor (IGF)-binding protein-5 leading to an alteration in IGF-I-stimulated cell growth. Endocrinology 141:1100–1106 [DOI] [PubMed] [Google Scholar]
- Maile LA, Busby WH, Sitko K, Capps BE, Sergent T, Badley-Clarke J, Ling Y, Clemmons DR 2005 The heparin-binding domain of vitronectin is the region that is required to enhance insulin-like growth factor-I signaling. Mol Endocrinol 20:405–411 [DOI] [PubMed] [Google Scholar]
- Ling Y, Maile LA, Clemmons DR 2003 Tyrosine phosphorylation of the β3-subunit of the αVβ3 integrin is required for membrane association of the tyrosine phosphatase SHP-2 and its further recruitment to the insulin-like growth factor I receptor. Mol Endocrinol 17:1824–1833 [DOI] [PubMed] [Google Scholar]