Abstract
Adipokines are secreted by adipose tissue and control various physiological systems. Low leptin levels during fasting stimulate feeding, reduce energy expenditure, and modulate neuroendocrine and immune function to conserve energy stores. On the other hand, rising leptin levels in the overfed state prevent weight gain by inhibiting food intake and increasing energy expenditure. These actions are mediated by neuronal circuits in the hypothalamus and brainstem. Leptin also controls glucose and lipid metabolism by targeting enzymes such as AMP-activated protein kinase and stearoyl-coenzyme A desaturase-1 in liver and muscle. Likewise, adiponectin and resistin control energy balance and insulin sensitivity via central and peripheral targets. As highlighted in this review, there are distinct as well as common signaling pathways for adipokines. Understanding adipokine signaling in the brain and other organs will provide insights into the pathogenesis and treatment of obesity, diabetes and various metabolic disorders.
ADIPOKINES
THE WORLDWIDE INCREASE in obesity, diabetes, and related diseases has focused attention on the biology of adipose tissue. Adipose tissue secretes polypeptide hormones, e.g. leptin, adiponectin, and resistin (in rodents), proinflammatory cytokines, complement and coagulation factors, and vasoactive peptides (Table 1) (1,2). Adipose tissue also produces enzymes that control the biosynthesis and activities of steroid hormones. Adipose tissue-derived aromatase and 17β-hydroxysteroid dehydrogenase catalyze the interconversion of sex steroids, whereas 11β-hydroxysteroid dehydrogenase type 1 mediates the conversion of cortisone to cortisol in humans and 11-dehydrocorticosterone to corticosterone in mice (3,4). Collectively, adipose tissue-secreted factors called “adipokines” are involved in energy homeostasis and regulation of glucose and lipid metabolism, immunity, and neuroendocrine systems (Table 1). This review will focus on how leptin, adiponectin, and resistin affect energy homeostasis and glucose and lipid metabolism, and how dysregulation of the central and peripheral actions of these adipokines may underlie the pathogenesis of obesity, diabetes, and lipid disorders.
Table 1.
Actions of Adipokines
| Adipokine | Source and Nutritional Regulation | Energy, Glucose, and Lipid Metabolism |
|---|---|---|
| Leptin | Mainly adipose tissue; low levels in gastric fundus, intestine, and muscle. Obesity: adipose mRNA and protein ↑↑; plasma ↑↑. Fasting: adipose mRNA and protein ↓; plasma ↓. Refeeding: adipose mRNA and protein ↑; plasma ↓ | Inhibits feeding and increases energy expenditure; insulin sensitizer; stimulates fatty acid oxidation |
| Adiponectin | Adipose tissue. Obesity: adipose mRNA and protein ↓; plasma ↓. Fasting: adipose mRNA and protein ↑; plasma ↑. Refeeding: adipose mRNA and protein ↓; plasma ↓ | Insulin sensitizer; stimulates fatty acid oxidation; may increase or decrease adiposity |
| Resistin | Adipose tissue in rodents and macrophages in human. Obesity: rodent adipose mRNA ↓ and protein ↑; plasma ↑. Fasting: rodent adipose mRNA and protein ↓; plasma ↓. Refeeding: rodent adipose mRNA and protein ↑; plasma ↑ | Induces insulin resistance in rodents |
| TNFα | Adipose tissue and immune cells. Obesity: adipose and plasma protein ↑ | Inhibits feeding, induces cachexia, and inhibits insulin sensitivity |
| IL-6 | Adipose tissue, immune cells and muscle. Obesity: adipose and plasma protein ↑ | Inhibits feeding, increases energy expenditure, and induces insulin resistance |
| Adipsin; complement factor D | Adipose tissue. Obesity: adipose mRNA and protein ↓ in rodents; ↑ in humans; adipsin levels are linked to acylation stimulating protein (ASP) | ASP promotes fatty acid and glucose uptake by adipocytes and stimulates insulin secretion |
| Plasminogen-activator inhibitor-1 | Adipose tissue and liver. Obesity: adipose and plasma protein ↑; suppressed by thiazolidinediones | Increases adiposity and insulin resistance in rodents |
| Renin-angiotensin system | Adipose tissue, kidney, and vasculature | Angiotensin II increases adipogenesis and reduces insulin sensitivity |
| Retinol binding protein-4 | Adipose tissue and liver. Obesity: adipose and plasma protein ↑ in rodents; not consistently elevated in humans | Enhances insulin action in rodents |
| Fasting-induced adipose factor; angiopoeitin-like protein 4 | Expressed in adipose tissue, liver, and intestinal epithelium. mRNA and protein ↑ by calorie restriction, PPAR-α, and fenofibrate | Increases triglycerides by inhibiting lipoprotein lipase; stimulates cholesterol synthesis; implicated in obesity and insulin resistance induced by gut bacteria |
| Visfatin; pre-B cell colony-enhancing factor 1; extracellular nicotinamide phosphoribosyltransferase | Adipose tissue, liver, and various tissues. Obesity: adipose and plasma protein ↑ | Not an insulin mimetic as originally proposed |
| Vaspin | Adipose tissue and liver. Obesity: adipose and plasma protein ↑ | Insulin sensitizer in rodents |
↑, Increased; ↓, decreased.
LEPTIN
Leptin is mainly expressed by adipocytes but low levels are produced in the stomach, intestine, mammary epithelium, placenta, skeletal muscle, and possibly the brain (5). The concentrations of leptin in adipose tissue and plasma closely parallel the mass of adipose tissue and adipocyte size and triglyceride content. Thus, leptin increases in obesity and falls with weight loss (5). These changes are dependent on insulin and glucose. Leptin is also higher in women, partly due to higher production by sc adipose tissue, stimulation by estrogens, and inhibition by androgens. Moreover, leptin is increased by chronic glucocorticoid exposure and inflammatory cytokines. In contrast, cold exposure and adrenergic stimulation decrease leptin (1,5).
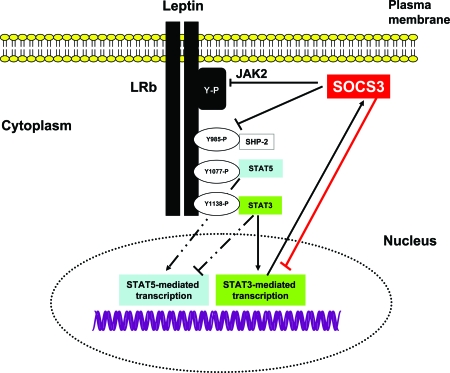
Leptin reaches neuronal targets via the circumventricular organs and a saturable transport mechanism across the blood-brain barrier (1). Five leptin receptor isoforms, LRa–LRe, derived from alternate splicing of lepr mRNA have been identified (1,5,6). The most abundant short leptin receptor, LRa, lacks the cytoplasmic domain necessary for Janus family of tyrosine kinases (JAK)-signal transducer and activator of transcription (STAT) signaling. LRa is abundantly expressed in brain capillary endothelium and peripheral organs and proposed to mediate leptin transport (1). The long leptin receptor, LRb, is restricted to the hypothalamus, brainstem, and key regions of the brain that control feeding, metabolism, and neuroendocrine systems (1,5,6). Binding of leptin to LRb leads to association with JAK2, autophosphorylation of JAK2, phosphorylation of tyrosine residues 985 and 1138 on LRb, and activation of STAT3 (6) (Fig. 1). This cascade of events culminates in translocation of STAT3 to the nucleus and transcription of neuropeptides (6) (Fig. 1). Phosphorylated Tyr985 of LRb binds Src homology 2 (SH2)-containing tyrosine phosphatase-2, which activates ERK. Additionally, Tyr985 binds the suppressor of cytokine signaling (SOCS)3, leading to the termination of LRb signaling (6). Studies have also demonstrated that leptin stimulates the phosphorylation of Tyr1077 on LRb and activates STAT5 (Fig. 1) and ribosomal protein S6 kinase (7). Tyr1138 has a secondary role in the acute phosphorylation of STAT5. Moreover, Tyr1138 and STAT3 attenuate STAT5-dependent transcription (7). Protein-tyrosine phosphatase 1B activity is also stimulated by leptin and inactivates JAK2 and leptin signaling (6,8). Studies have also demonstrated an interaction between the signaling pathways for leptin and insulin signaling in the hypothalamus (9). Both hormones inhibit food intake through activation of insulin receptor substrate-2, MAPK, ERK, Akt, and phosphatidylinositol 3-kinase (9).
Figure 1.
Intracellular Signaling Pathways Regulated by LRb
Binding of leptin to the extracellular domain of LRb activates JAK2 tyrosine kinase leading to autophosphorylation of tyrosine residues on JAK2 and phosphorylation of Tyr 985, Tyr1077, and Tyr1138 on LRb. Phosphorylation of Tyr1138 mediates the activation and nuclear translocation of STAT3, which induces the transcription neuropeptides in the hypothalamus as well as SOCS3, which terminates leptin signaling. Tyr985 also promotes interaction of SOCS3 with LRb-JAK2, thereby attenuating leptin signaling. Tyr1077 plays a dominant role in the transcriptional activation of STAT5, and this action is inhibited by Tyr1138-STAT3. SHP2, Src homology 2-containing tyrosine phosphatase-2.
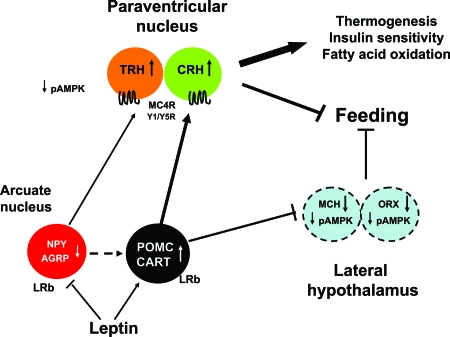
LRb and downstream leptin signaling molecules have been localized in the hypothalamus and brain regions that control energy balance, hormone levels, and glucose metabolism (5). Leptin directly inhibits neurons in the arcuate nucleus of the hypothalamus expressing neuropeptide Y (NPY) and agouti-related protein (AGRP) (5) (Fig. 2). Conversely, leptin induces proopiomelanocortin (POMC), precursor of MSH, and cocaine- and amphetamine-regulated transcript in the arcuate nucleus (Fig. 2). These neurons project to the paraventricular nucleus and perifornical, dorsomedial, and lateral hypothalamic areas to suppress feeding, stimulate thermogenesis, and enhance lipid oxidation and insulin sensitivity in peripheral organs. Neurons in the paraventricular nucleus expressing melanocortin-4 receptor, CRH, TRH, and vasopressin, have been implicated as critical mediators of central leptin action (1,5) (Fig. 2). Leptin also indirectly controls expression of melanin-concentrating hormone and orexins in the lateral hypothalamus as well as mesolimbic dopaminergic circuits (10,11). Deficiency of leptin, LRb, and STAT3 specifically in POMC neurons induces hyperphagia and impairs thermogenesis leading to morbid obesity (5,12,13,14,15). In contrast, the loss of orexigenic peptides, e.g. NPY and melanin-concentrating hormone, attenuates obesity in leptin-deficient Lepob/ob mice (16,17). SOCS3 and PTP1B deficiency ameliorates obesity by enhancing leptin sensitivity (18,19,20,21).
Figure 2.
Leptin Signaling in the Hypothalamus
Leptin binds to LRb on NPY/AGRP and POMC/cocaine- and amphetamine-regulated transcript (CART) neurons in the arcuate nucleus, leading to inhibition of feeding, and stimulation of thermogenesis, fatty acid oxidation, and enhancement of peripheral insulin sensitivity. Leptin-sensitive neurons in the arcuate nucleus project to the paraventricular nucleus to increase CRH and TRH, and lateral hypothalamic area to suppress melanin-concentrating hormone (MCH) and orexins (ORX). Leptin also inhibits AMPK phosphorylation.
Apart from activating JAK-STAT signaling, leptin has rapid effects on neurotransmission and neuropeptide secretion and also modulates neuronal plasticity. Leptin inhibits NPY secretion by the hypothalamus, depolarizes POMC neurons by decreasing the inhibitory tone of γ-aminobutyric acid released from NPY terminals in the arcuate nucleus, and hyperpolarizes NPY neurons (22,23,24). Congenital leptin deficiency has been associated with a decrease in brain size, impaired myelination, and reduction in expression of neuronal and glial proteins in mice (25). Moreover, gray matter defects have been demonstrated in the anterior cingulate gyrus, inferior parietal lobule, and cerebellum in patients with congenital leptin deficiency (26). These abnormalities are partially reversed by leptin treatment (25,26). Leptin stimulates the development of axonal projections from the arcuate nucleus to paraventricular nucleus and has been shown to increase inhibitory synapses and reduce excitatory synapses in the hypothalamus (27,28).
Leptin is a critical signal for alterations in energy stores in adipose tissue. An extreme manifestation of leptin's role as a starvation hormone is seen in patients and mice with congenital leptin deficiency, which develop voracious appetite, morbid obesity, immunosuppression, and hypothalamic hypogonadism (1,5,11). Acquired leptin deficiency due to fasting or lipodystrophy also stimulates feeding and suppresses immunity, sympathetic nervous activity, and sex and thyroid hormones (29,30,31,32,33,34,35). In contrast, the ability of leptin to signal excess energy storage is less robust (1,5,6). A majority of obese individuals have high levels of leptin but do not respond to rising endogenous leptin levels suggesting leptin resistance (1,6). Studies have shown that leptin resistance in obese rodents is associated with impairment of leptin transport across the blood-brain barrier, reduction of leptin-mediated JAK-STAT signaling, and induction of SOCS3 (6,36). Attenuation of leptin sensitivity in the brain leads to excess triglyceride accumulation in adipose tissue as well as muscle, liver, and pancreas (37).
Leptin plays an important role in preventing triglyceride storage outside adipose tissue (37). In lean healthy individuals, leptin is proposed to act indirectly on muscle and liver to stimulate the phosphorylation and activity of a critical energy sensor, AMP-activated protein kinase (AMPK) (38). Activated AMPK phosphorylates acetyl-coenzyme A (CoA) carboxylase (ACC) and malonyl-CoA decarboxylase, resulting in inhibition of ACC and activation of malonyl-CoA decarboxylase. ACC catalyzes the formation of malonyl-CoA, the first step in fatty acid synthesis, and malonyl-CoA inhibits carnitine palmityl transferase 1 (CPT-1), which controls fatty acid transport into mitochondria. Leptin limits accumulation of triglyceride in liver and muscle by activating AMPK, inhibiting ACC, reducing malonyl-CoA, increasing CPT-1 activity, and stimulating fatty acid oxidation (38). Leptin also acts via the brain to inhibit the activity of stearoyl-CoA desaturase-1, an enzyme that catalyzes the synthesis of monounsaturated fatty acids (mainly oleate and palmitoleate) (39). Leptin resistance in obesity promotes extraadipose lipid storage (steatosis) by diminishing AMPK activity, increasing activities of ACC, fatty acid synthase and stearoyl-CoA desaturase 1, and reducing CPT-1 activity in liver and muscle. Steatosis leads to formation of ceramide and various lipid metabolites that impair insulin sensitivity in liver and muscle as well as insulin secretion (37).
Leptin directly regulates insulin sensitivity and pancreatic β-cell function. Deletion of lepr in the brain induces insulin resistance and diabetes, whereas restoration of leptin signaling in the arcuate nucleus decreases insulin and normalizes glucose levels (40). Administration of leptin in the hypothalamus attenuates hepatic insulin resistance and glucose production in rodents on a high-fat diet, partly through activation of melanocortin signaling (41,42). Importantly, deletion of lepr in the pancreas limits islet growth and insulin secretion in diet-induced obese mice, thus providing a link between leptin signaling in islets and obesity-associated diabetes (43).
ADIPONECTIN
Adiponectin is produced exclusively by adipocytes and circulates at high concentrations (μg/ml) in plasma (44). Native adiponectin exists as homotrimers that form low-molecular weight hexamers and high-molecular weight (HMW) complexes. Plasma concentrations of total and HMW adiponectin are higher in women than men, partly due to suppression of adiponectin by testosterone. Unlike leptin, adiponectin is reduced in obesity, increased in response to fasting, and decreased by refeeding (44). Adiponectin deficiency induces insulin resistance, glucose intolerance, and hyperlipidemia and increases susceptibility to vascular injury and atherosclerosis (44,45,46). Adiponectin reverses these abnormalities by stimulating fatty acid oxidation, suppressing gluconeogenesis, and inhibiting inflammation (44,45,46). The levels of HMW adiponectin are highly predictive of insulin sensitivity (47). Insulin-sensitizing thiazolidinediones increase HMW adiponectin in humans and rodents, and mice lacking adiponectin do not respond to thiazolidinedione treatment (48). Thus, adiponectin plays an essential role in mediating the antidiabetic effect of thiazolidinediones.
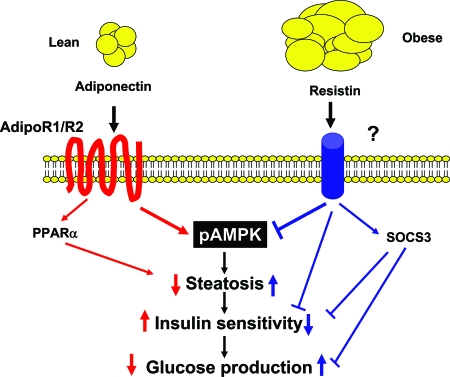
The actions of adiponectin in suppressing gluconeogenesis and enhancing lipid oxidation are related to activation of AMPK and inhibition of ACC in liver and muscle, whereas the antiinflammatory effect of adiponectin is associated with suppression of nuclear factor-κB and vascular adhesion molecules (44). Adiponectin is proposed to signal through two seven-transmembrane domain-containing proteins, AdipoR1 and AdipoR2, which are widely expressed and induce AMPK phosphorylation and activity (44) (Fig. 3). APPL1 (adaptor protein containing pleckstrin homology domain phosphotyrosine binding domain and leucine zipper motif) binds to adiponectin receptors and has been linked to the insulin-sensitizing action of adiponectin in vitro (49). The expression of AdipoR1 and AdipoR2 was found to be diminished in livers of obese mice, and this was related to attenuation of AMPK activity and insulin resistance (50). These defects were reversed by adenovirus-mediated expression of AdipoR1 and -R2. Ablation of AdipoR1 prevented the ability of adiponectin to activate AMPK, whereas AdipoR2 deficiency decreased peroxisome proliferator-activated receptor-α (PPARα) signaling. Deficiency of AdipoR1 and R2 prevented adiponectin binding and induced steatosis, inflammation, oxidative stress, and insulin resistance, demonstrating important roles in glucose and lipid metabolism and immune function (50). AdipoR2 deletion in another study decreased lipid levels and improved insulin sensitivity in diet-induced obese mice, yet diabetes ensued because of pancreatic β-cell failure (51). Furthermore, AdipoR1 and AdipoR2 appeared to have opposing metabolic roles (52). AdipoR1 deficiency increased decreased energy expenditure, increased body fat, and induced insulin resistance. On the other hand, AdipoR2 deficiency led to higher energy expenditure, a leaner phenotype, reduced plasma cholesterol, and improved glucose levels (52).
Figure 3.
Adiponectin and Resistin Signaling in the Liver
High adiponectin levels in lean individuals bind to AdipoR1 and -R2 in the liver, leading to phosphorylation and activation of AMPK and increased PPARα activity. Adiponectin stimulates fatty acid oxidation, prevents steatosis, enhances insulin signaling, and suppresses hepatic glucose production. Resistin is increased in obesity, inhibits AMPK activity, increases SOCS3, and induces insulin resistance.
Adiponectin receptors are widely distributed in the brain, but questions have been raised about a central action because adiponectin did not cross the blood-brain barrier in mice (53,54). Nonetheless, several lines of evidence support the notion that adiponectin affects energy and glucose metabolism by targeting the brain (55). Trimeric and low molecular weight adiponectin are present in cerebrospinal fluid (CSF) in humans and rodents (56,57,58). The concentration of adiponectin in CSF increases after iv injection of adiponectin, suggesting blood-to-brain transport of adiponectin (55,58). We have reported that intracerebroventricular administration of adiponectin potently increased energy expenditure and fatty acid oxidation and reduced body weight (55). Adiponectin and leptin both acted in the brain to stimulate energy expenditure and decrease glucose and lipids, showed similar patterns of signaling by increasing CRH expression and inducing Fos immunoreactivity in the paraventricular nucleus, and were inactive in agouti (Ay/a) mice lacking melanocortin signaling (55).
An antiobesity action of adiponectin is also supported by the ability of systemic administration of adiponectin to decrease body weight and fat via fatty acid oxidation (59,60). However, other studies suggest an opposite effect of adiponectin on energy metabolism (61,62). Transgenic overexpression of adiponectin in wild-type and Lepob/ob mice resulted in obesity (61,62). In Lepob/ob mice, elevation of adiponectin decreased food intake and energy expenditure (62). Remarkably, insulin resistance and inflammation of adipose tissue were attenuated in these extremely obese mice (62). Kubota et al. (58) have reported that peripheral injection of adiponectin increased AMPK activity in the arcuate nucleus via AdipoR1, and this resulted in stimulation of food intake, reduction in energy expenditure, and weight gain. Conversely, hypothalamic AMPK activation was attenuated in adiponectin-deficient mice and was related to reduction of food intake, increased energy expenditure, and lean phenotype. Furthermore, adiponectin concentration in CSF increased after fasting and decreased after refeeding (58). Together, these data suggest that adiponectin acts as a starvation signal (58).
Electrophysiology of adiponectin has been studied in rat brainstem and hypothalamus (63,64). Adiponectin depolarized area postrema (AP) neurons expressing both AdipoR1 and -R2, whereas AP neurons expressing only one subtype of receptor were insensitive (63). In the paraventricular nucleus, adiponectin hyperpolarized oxytocin neurons in contrast to induction of mixed depolarization-hyperpolarization responses in vasopressin neurons (64). Further analysis revealed that adiponectin-responsive oxytocin neurons expressed both AdipoR1 and R2, whereas vasopressin neurons expressed both receptors or one receptor. These results indicate different roles of adiponectin in controlling excitability of neurons in circumventricular areas such as AP that allow free access of large molecules into the brain vs. the paraventricular nucleus, which is protected by a blood-brain barrier (63,64). Further work is needed to elucidate what molecular forms of adiponectin produce specific actions, how adiponectin-mediated electrical activity is coupled to energy balance, and whether AMPK and various cellular mediators are linked to electrical activity of adiponectin.
RESISTIN
Resistin belongs to a family of cystine-rich peptides called resistin-like molecules (65). Resistin is expressed and secreted by adipocytes in rodents and was named for its ability to induce insulin resistance (65). Resistin serum levels increase in diet-induced and genetic models of obesity (65,67), although adipose tissue mRNA levels are reduced (67,68). Multimeric complexes of resistin and resistin-like molecule-β have been identified (66). Each promoter consists of a COOH-terminal disulfide-rich β-sandwich head and an NH2-terminal α-helical tail, and the latter associates to form three-stranded coils, linked by interchain disulfide linkages to form tail-to-tail hexamers. As with leptin, resistin levels are higher in women, fall during fasting, and increase after refeeding (67). These changes are controlled partly by insulin and glucose (68). Very recently, resistin has been linked to incretin hormones and lipoprotein lipase (LPL) activity (69,70). Resistin failed to increase when mice lacking receptors for glucagon-like peptide 1 and gastric inhibitory polypeptide (GIP) were fed a high-fat diet (69). This was associated with resistance to diet-induced obesity and preservation of pancreatic islet function (69). Chronic elevation of GIP levels increased plasma resistin levels in Zucker rats (70). Furthermore, treatment of 3T3-L1 adipocytes with resistin or GIP inhibited activities of AMPK and LPL (70). RNA interference-mediated suppression of resistin attenuated the effect of GIP on AMPK and LPL pathways in 3T3-L1 adipocytes, suggesting resistin acted downstream of GIP (70).
Systemic treatment or transgenic overexpression of resistin in rodents decreases the ability of insulin to suppress hepatic glucose production (71,72). Conversely, ablation of the retn gene or reduction in resistin protein through antisense oligonucleotide treatment improves insulin sensitivity through AMPK activation (73,74). Resistin inhibits adipogenesis, whereas the loss of resistin function increases body weight and fat and enhances insulin sensitivity (75,76). Thus, resistin has significant roles in energy and glucose homeostasis. In agreement, we found that loss of resistin in leptin-deficient Lepob/ob mice increased body weight and fat by decreasing energy expenditure (77). Insulin sensitivity improved in Lepob/ob lacking resistin and was reversed by resistin treatment (77). The resistin receptor is not known but the effect of resistin to induce insulin resistance is associated with attenuation of AMPK phosphorylation and increased SOCS3 expression (72,73,74,77) (Fig. 3). Thus, resistin may act at similar targets as leptin and adiponectin to affect glucose metabolism (Figs. 1–3).
Muse et al. (78) have reported that infusion of either resistin or an active cysteine mutant in the mediobasal hypothalamus stimulated glucose production, whereas antagonism of resistin action in the hypothalamus prevented the ability of plasma resistin to increase glucose production. Central resistin induced insulin resistance in liver, and this was related to induction of TNF-α, IL-6, and SOCS-3 (78). We have extended these findings by showing that intracerebroventricular resistin treatment induces hepatic insulin resistance and inflammatory markers by increasing expression of NPY and AGRP in the hypothalamus (79). The ability of resistin to increase glucose production and TNF-α, IL-6, and SOCS3 was attenuated in NPY-deficient mice as well as pharmacological blockade of NPY-Y1 receptor (79). These findings provide a framework for further investigation into the connection between resistin and inflammation and glucose metabolism.
Human resistin is made and secreted by macrophages (80,81). Plasma resistin levels and single-nucleotide polymorphisms have been linked to obesity and lipid and glucose abnormalities in some studies (82,83,84,85), although others have failed to establish such a relationship (85,86). Resistin has been associated with inflammation and atherosclerosis (88,89). Resistin is strongly related to the levels of soluble TNFα receptor-2, IL-6- and lipoprotein-associated phospholipase A2, and severity of coronary artery calcification (90). The connection between resistin and inflammation was examined by injecting a low dose of lipopolysaccharide in humans (91). Lipopolysaccharide induced fever and increased adipose TNFα and IL-6 levels in parallel with insulin resistance. These effects were associated with increases in resistin and leptin, suggesting a link between inflammation, adipokines, and glucose metabolism (91). We also examined the link between inflammation and adipokines by treating patients with etanercept for 4 wk to neutralize TNFα (92). Etanercept increased the level of total adiponectin but not HMW adiponectin, and increased resistin. Etanercept decreased muscle fat content but did not enhance insulin sensitivity (91,92). Longer studies are needed to establish whether the changes in proinflammatory cytokines and adipokines are indeed linked to glucose metabolism (91,92).
CONCLUSION
This review highlights the effects of adipokines on energy homeostasis. Knowledge of specific signaling pathways will benefit the diagnosis and treatment of diabetes, lipid disorders, and various metabolic diseases related to obesity. As the list of adipokines continues to grow, it has become apparent that factors that control the production of adipokines vary according to the species under study. Adipokines may affect energy homeostasis via hormonal, paracrine, or autocrine mechanisms in the brain and peripheral organs. Future research requires systematic approaches in animal models and especially humans to elucidate the biology of adipokines and how this impacts diseases.
Footnotes
This work was supported by National Institutes of Health Grant PO1-DK49210.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 17, 2008
Abbreviations: ACC, Acetyl-CoA carboxylase; AGRP, agouti-related protein; AMPK, AMP-activated protein kinase; AP, area postrema; CoA, coenzyme A; CPT-1, carnitine palmityl transferase 1; CSF, cerebrospinal fluid; GIP, gastric inhibitory polypeptide; JAK, Janus family of tyrosine kinases; LPL, lipoprotein lipase; LR, leptin receptor; NPY, neuropeptide Y; POMC, proopiomelanocortin; PPAR, peroxisome proliferator-activated receptor; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription.
References
- Badman MK, Flier JS 2007 The adipocyte as an active participant in energy balance and metabolism. Gastroenterology 132:2103–2115 [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS 2003 Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 112:1785–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger C, Luu-The V, Dupont P, Tchernof A 2002 Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm Metab Res 34:737–745 [DOI] [PubMed] [Google Scholar]
- Seckl JR, Morton NM, Chapman KE, Walker BR 2004 Glucocorticoids and 11β hydroxysteroid dehydrogenase in adipose tissue. Recent Prog Horm Res 59:359–393 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Saper CB, Flier JS, Elmquist JK 2000 Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21:263–307 [DOI] [PubMed] [Google Scholar]
- Munzberg H, Myers Jr MG 2005 Molecular and anatomical determinants of central leptin resistance. Nat Neurosci 8:566–570 [DOI] [PubMed] [Google Scholar]
- Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Münzberg H, Myers Jr MG 2007 The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem 282:31019–31027 [DOI] [PubMed] [Google Scholar]
- Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG 2002 PTP1B regulates leptin signal transduction in vivo. Dev Cell 2:489–495 [DOI] [PubMed] [Google Scholar]
- Niswender KD, Baskin DG, Schwartz MW 2004 Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab 15:362–369 [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS 2006 Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51:811–822 [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ 2006 Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51:801–810 [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O'Rahilly S 2002 Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110:1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM 2001 Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108:1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers Jr MG 2003 STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421:856–859 [DOI] [PubMed] [Google Scholar]
- Buettner C, Pocai A, Muse ED, Etgen AM, Myers Jr MG, Rossetti L 2006 Critical role of STAT3 in leptin's metabolic actions. Cell Metab 4:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JC, Hollopeter G, Palmiter RD 1996 Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 274:1704–1707 [DOI] [PubMed] [Google Scholar]
- Segal-Lieberman G, Bradley RL, Kokkotou E, Carlson M, Trombly DJ, Wang X, Bates S, Myers Jr MG, Flier JS, Maratos-Flier E 2003 Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci USA 100:10085–10090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS 2004 Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 10:734–738 [DOI] [PubMed] [Google Scholar]
- Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A 2004 Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10:739–743 [DOI] [PubMed] [Google Scholar]
- Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS 2006 Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab 4:123–132 [DOI] [PubMed] [Google Scholar]
- Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB 2006 Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 12:917–924 [DOI] [PubMed] [Google Scholar]
- Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A 1995 The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377:530–532 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ 2001 Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484 [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Cone RD 2005 Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/agouti-related protein neurons. Endocrinology 146:1043–1047 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Bjorbaek C, Osei S, Flier JS 1999 Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology 140:2755–2762 [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, Wong ML, Licinio J 2005 Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab 90:2851–2854 [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB 2004 Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304:108–110 [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL 2004 Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304:110–115 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS 1996 Role of leptin in the neuroendocrine response to fasting. Nature 382:250–382 [DOI] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI 1998 Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394:897–901 [DOI] [PubMed] [Google Scholar]
- Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS 2003 The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 111:1409–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS 2004 Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351:987–997 [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL 2005 Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115:3579–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A 2002 Leptin-replacement therapy for lipodystrophy. N Engl J Med 346:570–578 [DOI] [PubMed] [Google Scholar]
- Musso C, Cochran E, Javor E, Young J, Depaoli AM, Gorden P 2005 The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism 54:255–263 [DOI] [PubMed] [Google Scholar]
- El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS 2000 Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105:1827–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH 2002 Lipotoxic diseases. Annu Rev Med 53:319–336 [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG 2005 AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25 [DOI] [PubMed] [Google Scholar]
- Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM 2002 Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 297:240–243 [DOI] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua Jr SC, Lowell BB, Elmquist JK 2005 The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab 1:63–72 [DOI] [PubMed] [Google Scholar]
- Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L 2005 Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 54:3182–3189 [DOI] [PubMed] [Google Scholar]
- Gutierrez-Juarez R, Obici S, Rossetti L 2004 Melanocortin-independent effects of leptin on hepatic glucose fluxes. J Biol Chem 279:49704–49715 [DOI] [PubMed] [Google Scholar]
- Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkarni RN 2007 Disruption of leptin receptor expression in the pancreas directly affects β cell growth and function in mice. J Clin Invest 117:2860–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T 2005 Adiponectin and adiponectin receptors. Endocr Rev 26:439–451 [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y 2002 Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8:731–737 [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T 2002 Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277:25863–25866 [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE 2004 Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279:12152–12162 [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE 2006 Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. J Biol Chem 281:2654–2660 [DOI] [PubMed] [Google Scholar]
- Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ 2006 APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol [Erratum (2006) 8:642] 8:516–523 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T 2007 Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13:332–339 [DOI] [PubMed] [Google Scholar]
- Liu Y, Michael MD, Kash S, Bensch WR, Monia BP, Murray SF, Otto KA, Syed SK, Bhanot S, Sloop KW, Sullivan JM, Reifel-Miller A 2007 Deficiency of adiponectin receptor 2 reduces diet-induced insulin resistance but promotes type 2 diabetes. Endocrinology 148:683–692 [DOI] [PubMed] [Google Scholar]
- Bjursell M, Ahnmark A, Bohlooly-Y M, William-Olsson L, Rhedin M, Peng XR, Ploj K, Gerdin AK, Arnerup G, Elmgren A, Berg AL, Oscarsson J, Lindén D 2007 Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 56:583–593 [DOI] [PubMed] [Google Scholar]
- Spranger J, Verma S, Gohring I, Bobbert T, Seifert J, Sindler AL, Pfeiffer A, Hileman SM, Tschop M, Banks WA 2006 Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes 55:141–147 [PubMed] [Google Scholar]
- Pan W, Tu H, Kastin AJ 2006 Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides 27:911–916 [DOI] [PubMed] [Google Scholar]
- Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS 2004 Adiponectin acts in the brain to decrease body weight. Nat Med 10:524–529 [DOI] [PubMed] [Google Scholar]
- Kusminski CM, McTernan PG, Schraw T, Kos K, O'Hare JP, Ahima R, Kumar S, Scherer PE 2007 Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia 50:634–642 [DOI] [PubMed] [Google Scholar]
- Ebinuma H, Miida T, Yamauchi T, Hada Y, Hara K, Kubota N, Kadowaki 2007 Improved ELISA for selective measurement of adiponectin multimers and identification of adiponectin in human cerebrospinal fluid. Clin Chem 53:1541–1544 [DOI] [PubMed] [Google Scholar]
- Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T 2007 Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6:55–68 [DOI] [PubMed] [Google Scholar]
- Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF 2001 Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98:2005–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, Campbell-Thompson M, Crawford J, Shek EW, Scarpace PJ, Zolotukhin S 2003 Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats.Proc Natl Acad Sci USA 100:14217–14222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding YY, Russell RG, Lindemann D, Hartley A, Baker GR, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer PE 2004 A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145:367–383 [DOI] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE 2007 Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117:2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M, Smith PM, Hoyda TD, Duncan M, Ahima RS, Sharkey KA, Ferguson AV 2006 Area postrema neurons are modulated by the adipocyte hormone adiponectin. J Neurosci 26:9695–9702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyda TD, Fry M, Ahima RS, Ferguson AV 2007 Adiponectin selectively inhibits oxytocin neurons of the paraventricular nucleus of the hypothalamus. J Physiol 585:805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA 2001 The hormone resistin links obesity to diabetes. Nature 409:307–312 [DOI] [PubMed] [Google Scholar]
- Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L 2004 Disulfide-dependent multimeric assembly of resistin family hormones. Science 304:1154–1158 [DOI] [PubMed] [Google Scholar]
- Rajala MW, Qi Y, Patel HR, Takahashi N, Banerjee R, Pajvani UB, Sinha MK, Gingerich RL, Scherer PE, Ahima RS 2004 Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes 53:1671–1679 [DOI] [PubMed] [Google Scholar]
- Way JM, Görgün CZ, Tong Q, Uysal KT, Brown KK, Harrington WW, Oliver Jr WR, Willson TM, Kliewer SA, Hotamisligil GS 2001 Adipose tissue resistin expression is severely suppressed in obesity and stimulated by peroxisome proliferator-activated receptor γ agonists. J Biol Chem 276:25651–25653 [DOI] [PubMed] [Google Scholar]
- Hansotia T, Maida A, Flock G, Yamada Y, Tsukiyama K, Seino Y, Drucker DJ 2007 Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest 117:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Nian C, McIntosh CH 2007 Resistin is a key mediator of glucose-dependent insulinotropic polypeptide (GIP) stimulation of lipoprotein lipase (LPL) activity in adipocytes. J Biol Chem 282:34139–34147 [DOI] [PubMed] [Google Scholar]
- Rajala MW, Obici S, Scherer PE, Rossetti L 2003 Adipose-derived resistin and gut-derived resistin-like molecule-β selectively impair insulin action on glucose production. J Clin Invest 111:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Nguyen MT, Miles PD, Imamura T, Usui I, Olefsky JM 2004 Adenovirus-mediated chronic “hyper-resistinemia” leads to in vivo insulin resistance in normal rats. J Clin Invest 114:224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, Steppan CM, Ahima RS, Obici S, Rossetti L, Lazar MA 2004 Regulation of fasted blood glucose by resistin. Science 303:1195–1198 [DOI] [PubMed] [Google Scholar]
- Muse ED, Obici S, Bhanot S, Monia BP, McKay RA, Rajala MW, Scherer PE, Rossetti L 2004 Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest 114:232–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Lee K, Moon YS, Sul HS 2001 A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J Biol Chem 276:11252–11256 [DOI] [PubMed] [Google Scholar]
- Kim KH, Zhao L, Moon Y, Kang C, Sul HS 2004 Dominant inhibitory adipocyte-specific secretory factor (ADSF)/resistin enhances adipogenesis and improves insulin sensitivity. Proc Natl Acad Sci USA 101:6780–6785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Nie Z, Lee YS, Singhal NS, Scherer PE, Lazar MA, Ahima RS 2006 Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes 55:3083–3090 [DOI] [PubMed] [Google Scholar]
- Muse ED, Lam TK, Scherer PE, Rossetti L 2007 Hypothalamic resistin induces hepatic insulin resistance. J Clin Invest 117:1670–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal NS, Lazar MA, Ahima RS 2007 Central resistin induces hepatic insulin resistance via neuropeptide Y. J Neurosci 27:12924–12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, O'Rahilly S 2001 Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-γ action in humans. Diabetes 50:2199–2202 [DOI] [PubMed] [Google Scholar]
- Lazar MA 2007 Resistin- and obesity-associated metabolic diseases. Horm Metab Res 39:710–716 [DOI] [PubMed] [Google Scholar]
- Ochi M, Osawa H, Hirota Y, Hara K, Tabara Y, Tokuyama Y, Shimizu I, Kanatsuka A, Fujii Y, Ohashi J, Miki T, Nakamura N, Kadowaki T, Itakura M, Kasuga M, Makino H 2007 Frequency of the G/G genotype of resistin single nucleotide polymorphism at -420 appears to be increased in younger-onset type 2 diabetes. Diabetes 56:2834–2838 [DOI] [PubMed] [Google Scholar]
- Xu JY, Sham PC, Xu A, Tso AW, Wat NM, Cheng KY, Fong CH, Janus ED, Lam KS 2007 Resistin gene polymorphisms and progression of glycaemia in southern Chinese: a 5-year prospective study. Clin Endocrinol (Oxf) 66:211–217 [DOI] [PubMed] [Google Scholar]
- Menzaghi C, Coco A, Salvemini L, Thompson R, De Cosmo S, Doria A, Trischitta V 2006 Heritability of serum resistin and its genetic correlation with insulin resistance-related features in nondiabetic Caucasians. J Clin Endocrinol Metab 91:2792–2795 [DOI] [PubMed] [Google Scholar]
- Conneely KN, Silander K, Scott LJ, Mohlke KL, Lazaridis KN, Valle TT, Tuomilehto J, Bergman RN, Watanabe RM, Buchanan TA, Collins FS, Boehnke M 2004 Variation in the resistin gene is associated with obesity and insulin-related phenotypes in Finnish subjects. Diabetologia 47:1782–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, Orlova C, Mantzoros CS 2003 Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab 88:4848–4856 [DOI] [PubMed] [Google Scholar]
- Gerber M, Boettner A, Seidel B, Lammert A, Bär J, Schuster E, Thiery J, Kiess W, Kratzsch J 2005 Serum resistin levels of obese and lean children and adolescents: biochemical analysis and clinical relevance. J Clin Endocrinol Metab 90:4503–4509 [DOI] [PubMed] [Google Scholar]
- Osawa H, Tabara Y, Kawamoto R, Ohashi J, Ochi M, Onuma H, Nishida W, Yamada K, Nakura J, Kohara K, Miki T, Makino H 2007 Plasma resistin, associated with single nucleotide polymorphism -420, is correlated with insulin resistance, lower HDL cholesterol, and high-sensitivity C-reactive protein in the Japanese general population. Diabetes Care 30:1501–1506 [DOI] [PubMed] [Google Scholar]
- Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ 2005 Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 111:932–939 [DOI] [PubMed] [Google Scholar]
- Anderson PD, Mehta NN, Wolfe ML, Hinkle CC, Pruscino L, Comiskey LL, Tabita-Martinez J, Sellers KF, Rickels MR, Ahima RS, Reilly MP 2007 Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab 92:2272–2279 [DOI] [PubMed] [Google Scholar]
- Lo J, Bernstein LE, Canavan B, Torriani M, Jackson MB, Ahima RS, Grinspoon SK 2007 Effects of TNF-α neutralization on adipocytokines and skeletal muscle adiposity in the metabolic syndrome. Am J Physiol Endocrinol Metab 293:E102–E109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK 2006 Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med 166:902–908 [DOI] [PMC free article] [PubMed] [Google Scholar]