Abstract
Binding of hormones to their cognate G protein-coupled receptors (GPCRs) induces conformational shifts within the receptor based on evidence from a few hormone-receptor systems. Employing an engineered disulfide bond formation strategy and guided by a previously established model of the PTH-PTH receptor (PTHR)1 bimolecular complex, we set out to document and characterize the nature of agonist-induced changes in this family B GPCR. A mutant PTHR1 was generated which incorporates a Factor Xa cleavage site in the third intracellular loop. Treatment with Factor Xa fragments the receptor. However, if a new disulfide bond was formed before exposure to the enzyme, the fragments remain held together. A set of double cysteine-containing mutants were designed to probe the internal relative movements of transmembrane (TM) helices 2 and TM7. PTH enhanced formation of disulfide bonds in the K240C/F447C and A242C/F447C mutants. For the F238C/F447C mutant, a disulfide bond is formed in the basal state, but is disrupted by interaction with PTH. For the D241C/F447C PTHR1 construct, no disulfide bond formation was observed in either the basal or hormone-bound state. These findings demonstrate that the conformation of PTHR1 is altered from the basal state when PTH is bound. Novel information regarding spatial proximities between TM2 and TM7 of PTHR1 and the nature of relative movements between the two transmembrane regions was revealed. The data confirm and extend the experimentally derived model of the PTH-PTHR1 complex and provide insights at a new level of detail into the early events in PTHR1 activation by PTH.
G PROTEIN-COUPLED RECEPTORS (GPCRs) mediate a multitude of physiological functions by binding specific (cognate) ligands. In response to external stimuli such as light, odorants, proteins, peptides, lipids, and small molecules, GPCRs undergo conformational changes that activate intracellular signaling pathways leading to specific biological functions. The nature of the receptor switch from basal to activated state, triggered by binding of an agonist, is still not well understood for the vast majority of GPCRs. A number of experiments utilizing engineered disulfide bond formation, site-directed spin labeling, fluorescence spectroscopy, solution nuclear magnetic resonance (NMR), fluorescence resonance energy transfer (FRET), and substituted cysteine accessibility (SCAM) approaches have been used to elucidate the early events in activation of the rhodopsin (1,2,3,4,5), β2-adrenergic (β2AR) (6,7,8,9,10,11), TRH type-1 (12), acetylcholine (13), muscarinic (14,15,16,17), and chemotactic cytokine complement factor 5a (18) receptors.
Little is known about the conformational changes that take place upon ligand docking into the human PTH receptor type-1 (PTHR1), a family B GPCR. Previous studies using different approaches have shown that conformational shifts take place within PTHR1 upon association of PTH-(1–34) (19,20). Elucidating structure/conformation/function relations at a higher level of resolution for the PTH-PTHR1 complex is now possible and should provide fundamental insights into the basis of molecular recognition and the early events that lead to signal transduction and expression of PTH biological activity. The extracellular regions of the receptor known to participate in ligand binding, i.e. the N-terminal extracellular domain, extracellular loops (ECLs), and extracellular ends of the transmembrane (TM) helices are spatially remote from the intracellular G proteins and the regions of PTHR1 that interact with G proteins, i.e. intracellular loops (ICLs) and C-terminal intracellular domain. Shifts in conformation of the TMs that connect the extracellular and intracellular regions (21,22) result in conformation changes in the ICLs and the receptor interface with G proteins (12). Therefore, TMs are likely to play a vital role in transition of the receptor from the ground to the activated state. Hence, we undertook an investigation directed at detecting conformational changes within the TM bundle associated with PTHR1 activation.
The extracellular-facing region of TM2 of PTHR1 has been implicated in ligand binding (23), and TM7 has been suggested to interact with TM2 during the ligand binding and activation steps (24,25). The interaction between amino acid residues in these functionally important TMs in the ground and activated states was examined in this study.
We used an engineered disulfide bond formation strategy to detect changes in proximities between amino acid residues of the two TMs. A PTHR1 construct containing a c-myc epitope in the C-terminal tail and Factor Xa (FXa) cleavage sites in ICL3, referred to as XM-PTHR1, was used as a template onto which we inserted the double cysteine substitutions (Fig. 1). XM-PTHR1 was previously shown to have biological activity similar to wild-type (wt)PTHR1 (26). A schema of the experimental design is depicted in Fig. 2. We report here the observation that disulfide bonds form between TM2 and TM7 for some of the double cysteine mutants in the presence of PTH; inhibition of disulfide bond formation by PTH occurs for another double cysteine mutant. Molecular dynamics simulations of the PTH ligand-receptor interaction generated a model that predicts precisely the experimental results we obtained. The experimental approach is quite sensitive to even subtle differences in orientation of Cys sulfhydryl groups at different positions along the TMs in the PTHR1. These observations suggest that the extracellular facing regions of TM2 and TM7 move relative to each other when PTH docks into the receptor.
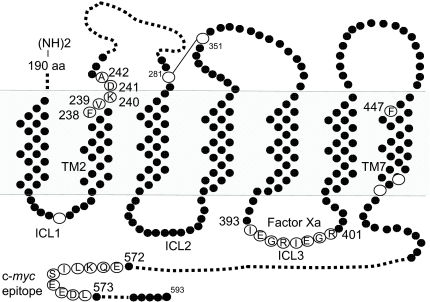
Figure 1.
PTHR1 Template and Sites of Substitution
Shown are amino acid residues A242, D241, K240, V239, F238 in TM2 and F447 in TM7 that were substituted with Cys in the present study. Also shown is the location of two FXa cleavage sites in ICL3. For detection by Western blot, a c-myc tag was inserted between residues 572 and 573 of the C-terminal intracellular domain. The endogenous cysteines are shown as open circles. aa, Amino acids.
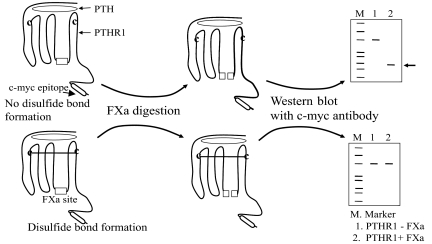
Figure 2.
Schema for Detection of Disulfide Bond Formation between Engineered Cysteines
PTHR1 constructs containing mutant cysteines are incubated with PTH as described in Materials and Methods. If a disulfide bond forms, then digestion with FXa will yield a band of size equal to undigested receptor because the receptor is held together by the disulfide bridge despite cleavage. However, if a disulfide bond is not formed, then a low molecular mass (∼23 kDa) C-terminal fragment (indicated by arrow) containing the c-myc epitope will be detected by Western blot with anti-c-myc antibody.
RESULTS
Construction of Double Cysteine-Containing PTHR1 Mutants
All mutations were introduced into the XM-PTHR1 template. Sites of cysteine substitution (Table 1) were guided by the experimentally derived model of the PTH—PTHR1 biomolecular complex (27). The presence of specific cysteine substitutions was confirmed by DNA sequencing (Tufts core facility, Boston, MA).
Table 1.
Receptors Used in the Study
| Construct | Contains | Mutation Location |
|---|---|---|
| PTHR1 (wt) | wtPTHR1 | None |
| XM-PTHR1 (XM) | FXa cleavage sites, and c-myc epitope sequence in PTHR1 | c-myc tag (EQKLISEEDL) inserted between residues 572 and 573. FXa cleavage sites, (IEGR)2, inserted between residues 393 and 401. |
| F238C/F447C (FF) | F238C, F447C in XM | TM2 and TM7 |
| V239C/F447C (VF) | V239C, F447C in XM | TM2 and TM7 |
| K240C/F447C (KF) | K240C, F447C in XM | TM2 and TM7 |
| D241C/F447C (DF) | D241C, F447C in XM | ECL 1 and TM7 |
| A242C/F447C (AF) | A242C, F447C in XM | ECL 1 and TM7 |
The c-myc epitope, FXa cleavage sites, and cysteine mutations were introduced by in vitro site-directed mutagenesis of single- stranded DNA as explained in Materials and Methods.
Biological Characterization
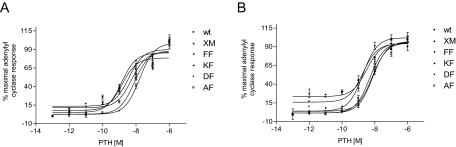
The mutants were tested for biological activity using a PTH-stimulated adenylyl cyclase activity assay. Only one mutant, namely V239C/F447C, lacked PTH-stimulated-adenylyl cyclase activity (Table 2). The EC50 values for all the double cysteine-containing mutants, except V239C/F447C, were comparable to that of XM-PTHR1, i.e. in the nanomolar range (Table 2 and Fig. 3A). In contrast, the ECmax values (expressed as fold increase seen with 1 μm PTH over basal levels) were decreased in the double cysteine PTHR1 mutants compared with wtPTHR1 and XM-PTHR1 (Table 2). The effect of iodine on PTH-stimulated adenylyl cyclase activity also was tested. No significant increase or decrease of the EC50 values for the functional double cysteine-containing mutants was observed with exposure to iodine; EC50 values were comparable to that of XM-PTHR1 (Fig. 3B).
Table 2.
Functional Characterization of PTHR1 and PTHR1 Mutants
| Construct | PTH-Stimulated Adenylyl Cyclase Activity
|
|
|---|---|---|
| EC50 (nm) | ECmax (Fold Increase over Basal) | |
| PTHR1 (wt) | 3.5 ± 1.0 | 31.7 ± 9.2 |
| XM-PTHR1 (XM) | 1.7 ± 0.5 | 35.4 ± 2.6 |
| F238C/F447C (FF) | 3.0 ± 3.8 | 14.6 ± 4.7 |
| V239C/F447C (VF) | No activity | No activity |
| K240C/F447C (KF) | 1.7 ± 0.3 | 12.0 ± 2.7 |
| D241C/F447C (DF) | 2.6 ± 1.4 | 7.4 ± 5.2 |
| A242C/F447C (AF) | 9.6 ± 5.8 | 15.8 ± 12.3 |
PTH-stimulated adenylyl cyclase activity assays were carried out in Cos-7 cells transfected with wtPTHR1 or mutant PTHR1 constructs. PTH was used as agonist (see Materials and Methods). EC50 values were calculated by nonlinear regression analysis of concentration curves using GraphPad Prism software. The data are expressed as mean ± se of three separate experiments, each performed in triplicate. ECmax values are expressed as fold increase in activity observed with 1 μm PTH over basal activity.
Figure 3.
Biological Characterization
Transiently transfected Cos-7 cells were assayed for PTH-stimulated adenylyl cyclase activity as described in Materials and Methods. A, PTH-stimulated adenylyl cyclase response curves for increasing doses of PTH displayed as percent of maximal stimulation. Values are means ± sem of triplicate wells. Similar results were obtained for two additional experiments. B, PTH-stimulated adenylyl cyclase response curves in the presence of oxidizing agent, iodine. Values are percent of maximal stimulation and are means ± sem of triplicate wells. Similar results were obtained for two additional experiments. wt, wtPTHR; XM, XM-PTHR1; FF, F238C/F447C; KF, K240C/F447C; DF, D241C/F447C; AF, A242C/F447C.
Detection of Disulfide Bond Formation
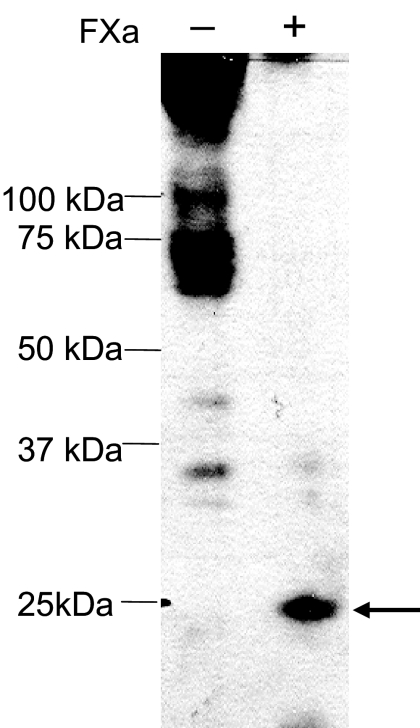
The biologically active double cysteine mutants were tested further for the presence/absence of engineered disulfide bond formation in the ground and activated states. Cysteines sometimes do not form disulfide bonds spontaneously, even when they are sufficiently close to form a bond. Therefore, we used a mild oxidizing agent, iodine, to facilitate disulfide bond formation. We standardized the method for assessing disulfide bond formation by detecting the c-myc label in PTHR1 fragments upon FXa digestion. Because the c-myc epitope is not present endogenously, only PTHR1 or fragments of the receptor are detected by antibodies to the c-myc epitope. Cos-7 cells transfected with control XM-PTHR1 were used to prepare membrane preparations. The membrane preparations were then treated in vitro with or without FXa. Nonreducing gel loading buffer was used. A low molecular mass band of approximately 23 kDa was seen when XM-PTHR1 membrane preparations were digested with FXa (Fig. 4, lane 2). Despite repeated attempts, we were not able to detect a distinct band for the full-length PTHR1. This may be due to the heterogeneity of full-length receptor because of the presence of a number of endogenous disulfide bonds and glycosylation sites. Indeed, we are able to detect a distinct full-length receptor after deglycosylation (supplemental Fig. 1, published as supplemental data on The Endocrine Society's Journals Online web site at http://mend.endojournals.org) providing further evidence in support of the c-myc-based detection method. Because enzymatic degylycosylation requires that the receptor be denatured, we did not attempt it with the double cysteine mutants because it would break any disulfide bond that formed between the engineered cysteines. Instead, for the subsequent studies we focused on monitoring the appearance or disappearance of the low molecular mass band (∼23 kDa) generated upon FXa digestion.
Figure 4.
Treatment of the Template, XM-PTHR1 (Control), with FXa. XM-PTHR1 with No Inserted Cysteines Was Tested
A low molecular mass band (∼23 kDa) was detected when XM-PTHR1 was cleaved with FXa (indicated by arrow). Detection is by Western blot with anti-c-myc antibody.
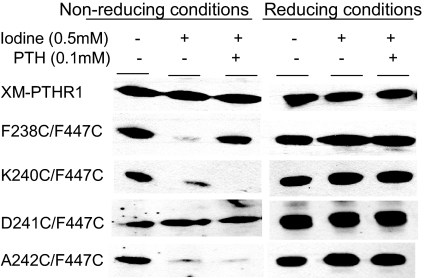
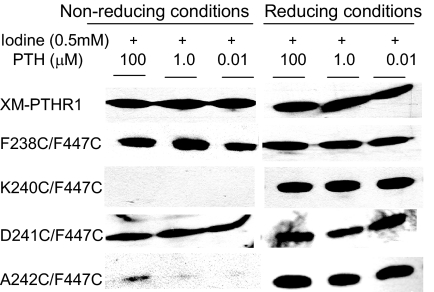
Next we tested the various double cysteine mutants. Digestion of F238C/F447C alone with FXa showed generation of the approximately 23-kDa band (i.e. no disulfide bond present). The band intensity was reduced dramatically upon addition of oxidizing agent alone (Fig. 5). This indicates that the distance between α-carbons in C238 in TM2 and C447 in TM7 are sufficiently proximate in the ground state to form a disulfide bond (<7Å apart), but upon addition of PTH, reappearance of the low molecular mass band was observed (Fig. 5). These data suggest that residues 238 and 447 move apart when hormone binds, resulting in disruption of disulfide bond formation between the two engineered cysteines. We obtained further support for this conclusion when we observed diminished band intensity upon reduction of ligand concentration to 10 nm (Fig. 6). A242C/F447C and K240C/F447C showed qualitatively different results. There was a reduction in the approximately 23-kDa band intensity in the presence of oxidizing agent alone but further reduction in band intensity or even disappearance of the low molecular mass band upon addition of PTH to A242C/F447C and K240C/F447C, respectively (Fig. 5). Therefore, amino acid residues 240 and 242 are close enough to position 447 in the ground state to form a disulfide bond. They move even closer upon addition of PTH. In contrast, no difference in the low molecular mass band intensity was observed with the D241C/F447C mutant upon addition of oxidizing agent alone or along with PTH. Consequently, although only one position removed from A242 and K240, C241is predicted to face away from C447, precluding disulfide bond formation.
Figure 5.
Western Blots of TM2/TM7 Double Cysteine-Containing Mutant PTHR1s Probed with anti-c-myc Antibody
Cos-7 cells transfected with the constructs shown (XM-PTHR1, F237C/F447C, K240C/F447C, D241C/F447C, and A242C/F447C) were used to make membrane preparations. Of these membrane preparations 100 μg were then treated with FXa after treatment with or without oxidizing agent and PTH as indicated on the top rows of the figure. The left panel depicts samples run under nonreducing conditions; the right panel represents same sample sets run under reducing conditions. Only the low molecular mass band of interest (∼23 kDa) is shown.
Figure 6.
Western Blot of Mutant Receptor-Transfected Membrane Preparations
Control (XM-PTHR1) and double cysteine-containing PTHR1 mutants were incubated with different concentrations of PTH in the presence of iodine as oxidizing agent. After SDS-PAGE of samples processed under nonreducing and reducing conditions, anti-c-myc antibody was used to detect the low molecular mass (∼23 kDa) band.
Molecular Modeling
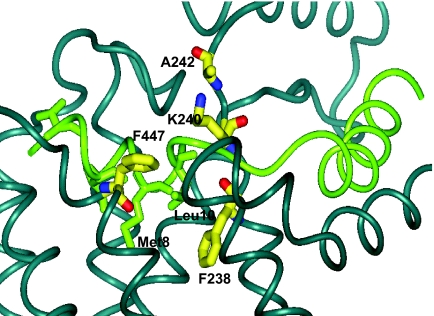
Structural insight into the formation of the disulfide bonds was obtained from a molecular model of the PTH-PTHR1 complex. This model incorporates the experimental findings from NMR-based structural studies of the extracellular domain of PTHR1 and a number of ligand-receptor contact sites defined by photoaffinity labeling. The model predicts, as illustrated in Fig. 7, that the extracellular ends of TM2 and TM7 are in close proximity, with F447 projecting toward TM2. The distances between F447 and 238, 240, or 242 in the model are consistent with the experimental results that demonstrate the feasibility of forming disulfide bonds. The model also suggests that the binding of PTH would interfere with the F447-F238 association: Met-8 and Leu-10 of PTH project down toward the receptor core, in between TM2 and TM7. The modeling results, taken together with the experimental results, lead to the inference that there is a change in proximity between amino acid residues 447 and 238 to accommodate hormone docking into the receptor.
Figure 7.
Molecular Modeling of PTH-PTHR1 Complex
Expanded view of the PTH-PTHR1 complex illustrating the close proximity of the residues in TM2 (238, 240, 242) to TM7 (447). Upon binding of PTH, Met-8 and Leu-10 of the hormone interfere with the interaction between F447 and F238. In contrast, hormone binding does not disrupt, and may even enhance, the interaction between F447 and K240. D241 is not shown because it faces away from the reader in this figure.
DISCUSSION
Changes in the conformation of TM regions of GPCRs are postulated to be the critical early events in activation of receptor upon ligand binding and subsequent signal transduction (15). Little is known, however, about the nature of the conformational changes triggering PTHR1 activation. Previous studies using metal chelating His substitutions showed that both β2AR and PTHR share with rhodopsin general structural features and activation mechanisms. The study showed that all three receptors display ligand-induced movement of the cytoplasmic ends of TM6 away from TM3 (20). Photoaffinity cross-linking and other structural studies have provided detailed information about the nature of the ligand-receptor bimolecular complex, but these photoaffinity cross-linking approaches do not provide insights into the dynamics of receptor activation. In the present study, we probed and detected conformational differences between the ground and activated states at specific amino acid positions within PTHR1 using the engineered disulfide bond formation strategy, which has been successfully applied to a number of families (but not family B) of GPCRs (15,16,18). Previously, other methods such as FRET have been used to study conformational shifts in PTHR (19), and the Zn-chelating His-substitution method has been used to probe TM helical arrangements in the PTHR (20).
We chose to study the conformational shifts of TM2 and TM7 domains relative to each other upon hormone binding and receptor activation. The extracellular side of TM2 has been suggested to interact with the midregion of PTH, especially through amino acid residue 19 (23). We hypothesized that regions in the receptor that are in the vicinity of ligand binding sites may be focal sites or the site of origin of conformational shifts which propagate within the TM bundle, leading to receptor activation. In a study on a family A GPCR, the muscarinic acetylcholine receptor, conformational changes in the vicinity of the ligand binding site were documented (15). Also, a recent study suggests that multiple residues in TM2 may be critically important for receptor function (28). In our study, we also included amino acid residues predicted to be outside the TM2 helix according to the rhodopsin model. All the double cysteine-containing mutants include one cysteine substitution toward the extracellular region of TM2 (within the domain F238–A242) and a constant cysteine substitution at F447 in TM7. We chose to study interaction of TM2 with TM7 because earlier studies using other methodologies indicated that there is interaction between the two TMs that may be involved in activity of the receptor (25). Phe-447 in TM7 was chosen to be substituted with cysteine because molecular modeling suggests that it projects into the heptahelical core toward TM2.
Our study indicates that substitution of Cys for Val at position 239 is not tolerated and results in an inactive receptor. This may be attributed, in part, to the observed homology at this position with similar residues, i.e. Leu or Ile, present in different members of the family B GPCR (29). The functionality of the other mutated receptors was not affected because they all display EC50 values similar to that of XM-PTHR1, but the ECmax values were reduced for all the active Cys-substituted PTHR1 mutants. This may be attributed to the critical role of the extracellular facing regions of TM2 to ligand binding and receptor activation (30). The interaction of ligand with the body of the receptor, including TM2 and TM7, is considered to stabilize the active conformation of the receptor and subsequently trigger intracellular signaling pathways (30,31).
Molecular iodine was used as the oxidizing agent because of its small size (<2.7 Å), which permits access to binding pockets even in the presence of bound agonists/antagonists (32). Iodine promotes disulfide bond formation between cysteines that have Cα to Cα distances less than 7 Å apart. The XM-PTHR1 mutant with no cysteine substitutions was used as a control: the low molecular mass band intensity remained the same in the absence or presence of oxidation agent alone, or together with agonist, PTH. For F238C/F447C, the decreased intensity of the low molecular mass band with iodine treatment was reversed in a dose-dependent manner when PTH was added, indicating disruption of disulfide bond formation. Molecular modeling of the ligand-receptor complex is in agreement with the experimental data. The side chains of Met-8 and Leu-11 of PTH reach into the binding groove. F238 and F447 move apart to accommodate the ligand. Association between the cytoplasmic ends of TM2 and TM7 has been suggested to be responsible for the ligand-free activation of constitutively active receptors containing a substitution at position 223 (24). Our data suggest that the opposite movement occurs at the other end of the helices, i.e. movement of TMs at the extracellular side of the receptor is away from each other. Movement of F238 and F447 away from each other may result in a more open and potentially dynamic structure. According to a recently published article on the crystal structure of the β2AR (33), mutations that result in a more loosely packed, dynamic structure in interacting regions, such as the cytoplasmic ends of TM6 and TM3/TM5/ TM2, shift the receptor equilibrium to a more active state (33). The observed changes in interaction between TM2 and TM7 residues of PTHR1 upon binding of ligand may result in generation of a stable active conformation and subsequent activation of the G protein-associated signaling pathways.
K240C/F447C and A242C/F447C form disulfide bonds between two engineered cysteines in the ground state as well as the activated state of the receptor. According to the molecular model, K240, D241, and A242 are part of a loop just outside TM2 (Fig. 7). Whereas K240 and A242 are pointed in the same direction (toward TM7), D241 faces the opposite direction. Because the side chain of position 241 is pointing away from F447, no disulfide bond formation is observed with cysteine substitutions at 241 and 447.
Our investigation reveals that hormone docking results in F238 moving away from F447 whereas K240 and A242 move toward F447. These movements at the extracellular face of TM2 and TM7 must be transmitted across the TM bundle to the cytoplasmic surface of the receptor, leading to interaction with G proteins. In a recently published article describing crystallization of engineered β2AR (34) with bound inverse agonist, it was suggested that agonist-induced changes in regions required for function of receptor are linked to movements of side chains of amino acid residues through packing interactions. These shifts are then transmitted to the cytoplasmic ends of helices and then to the ICLs and finally to G proteins (34). Although the exact mechanism remains to be elucidated, by using a technique that allows us to monitor changes in proximities of amino acid residues in the receptor in the ground as well as the ligand-bound state, we have demonstrated the dynamic nature of the hormone-receptor system near a ligand binding site of PTHR1.
Binding of ligand to receptor is considered to take place in at least two steps: initial high affinity fast interaction with the N terminus of the receptor followed by a second slower step in which the TM bundle of the receptor, including TM7, is involved (19,31). The second step is considered essential for receptor activation. Therefore, the observations made in this study provide new insights into the early events leading to receptor activation and provide the impetus to undertake similar studies with different TM regions of the receptor. This approach promises to generate a more refined molecular model of the ligand receptor complex. Because of similarity in structure and function, we anticipate that the insights we gain about the PTH-PTHR1 system will be applicable to other, especially family B GPCRs, hormone-GPCR systems.
MATERIALS AND METHODS
Materials
SuperSignal West Pico chemiluminiscent substrate was obtained from Pierce Chemical Co. (Rockford, IL). DMEM, Opti-MEM serum-free medium, penicillin, streptomycin, fetal bovine serum, nitrocellulose membranes (0.2 μm), and PBS, as well as the 5′-phosphorylated oligonucleotides, were obtained from Invitrogen Life Technologies (Carlsbad, CA). FuGENE6 and restriction protease FXa were from Roche Molecular Biochemicals (Indianapolis, IN). Dual-Glo luciferase assay system was from Promega Corp. (Madison, WI). Plasmid maxi and mini prep kits were from QIAGEN (Valencia, CA). Anti-c-myc antibody (clone 4A6) was obtained from Upstate Biotechnology (Lake Placid, NY). Secondary antibody, antimouse horseradish peroxidase antibody, was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Polyacrylamide solution (30%) and molecular weight standards (Precision Plus protein standards, dual color) were from Bio-Rad Laboratories, Inc. (Richmond, CA). GraphPad prism software was from GraphPad software (San Diego, CA). All other reagents and plasticware were obtained from Fisher Scientific (Pittsburgh, PA).
Plasmid Construction
The wtPTHR1 had been cloned into pcDNA 3.1 as previously described (35). The single-stranded DNA of this genomic clone was used as a template to insert the c-myc epitope sequence (EQKLISEEDL) and FXa cleavage sites (IEGR)2 by in vitro mutagenesis using a modification of the Kunkel method (36) as previously described (26). The resulting PTHR1 construct is referred to as XM-PTHR1 throughout this manuscript. The same method was used to create the double cysteine-containing mutants using appropriate oligonucleotide(s). The sites of cysteine substitutions in the PTHR1 are shown in Fig. 1 and Table 1. The authenticity of isolated clones was verified by DNA sequencing using appropriate PTHR1 primers (Tufts University Core Facility, Boston, MA).
Cell Culture and DNA Transfection
Cos-7 cells were used because they do not express endogenous PTHR1. The cells were maintained in DMEM supplemented with 10% FBS and 1× penicillin and streptomycin in a humidified 5% CO2 atmosphere at 37 C. For transient transfections, Cos-7 cells were seeded onto 24-well collagen-coated plates (BD Biocoat Cellware, BD Biosciences, San Jose, CA) at a seeding density of 60,000 cells per well or onto 15-cm2 plates at a seeding density of 1.5 × 106 cells per plate. The cells were transiently transfected the next day using FuGENE 6 transfection reagent as described earlier (37).
Adenylyl Cyclase Activity
Cos-7 cells were seeded and transfected as described above. The cells were cotransfected with 100 ng/well wtPTHR1 or specific mutant receptor and 100 ng/well Cre-luciferase DNA and 10 ng/well Renilla luciferase DNA (acts as internal control for transfection efficiency). The minimal medium (OPTIMEM) was replaced with complete medium 18 h after transfection. PTH-stimulated adenylyl cyclase activity was measured as previously described (37). The EC50 was calculated by nonlinear regression analysis using GraphPad Prism software. Maximum stimulation obtained with 1 μm bovine PTH was used to determine the ECmax and is expressed as fold increase over basal activity.
Membrane Preparations
Cos-7 cells were seeded onto 15-cm2 plates as described above and allowed to grow overnight. After transfection with 5.2 μg DNA (wt or mutant PTHR1 constructs), the cells were allowed to grow overnight in the transfection medium. The following day, fresh complete medium was added to plates, and the cells were grown for another 24 h; the cells were then washed twice with cold Hanks' PBS 48 h after transfection. The cells were scraped into Hanks' PBS and spun at 2000 rpm for 10 min at 4 C. The cell pellet was then resuspended in membrane lysis buffer containing 0.1 mm phenylmethylsulfonylfluoride. Lysis was ensured by subjecting the cell suspension to four freeze-thaw cycles and Dounce homogenization (30 vertical strokes) steps. After centrifugation at 12,000 rpm for 10 min at 4 C, the pellet was discarded. The supernatant was recentrifuged at 40,000 rpm for 1 h at 4 C. The resulting membrane pellet was resuspended in solubilization buffer (10 mm Tris-Cl; pH 7.4, 1 mm EDTA; 0.5% Triton X-100 with freshly added 0.1 m phenylmethylsulfonylfluoride) and protein quantified using Lowry reagents from Bio-Rad according to the manufacturer's protocol. The samples were aliquoted and stored at −70 C until use.
Preparation of Iodine Solution
Stock of iodine solution was prepared as described elsewhere (32): 50 mg of iodine was dissolved in 1ml of ethanol (∼197 mm), and the volume was then adjusted with water and stirred at room temperature (RT) to yield a 5 mm stock solution. Iodine was used at a final concentration of 0.5 mm as an oxidant in the test samples.
Treatment with PTH and/or Oxidizing Agent Followed by FXa Digestion
The membrane preparations were thawed and a 100-μg aliquot was taken for each reaction set. Initially, for testing hydrolysis of the full-length receptor, two controls were prepared. In one, the untreated sample membrane was not subjected to FXa digestion; in the other, FXa was added to the sample (at 1:50 enzyme-protein ratio). The samples were treated with oxidizing agent alone (ground state of receptor) or with PTH along with oxidizing agent (activated state of receptor). In addition, various concentrations of agonist were tested by incubating 100 μm, 1 μm, and 0.01 μm PTH along with iodine with the membrane preparations for 45 min at RT in the dark. After this treatment, FXa was added as specified above, and the samples were incubated at 20 C for 1 h. Then the gel loading buffer was added and samples were incubated for an additional 1 h at 30 C. All the above samples were prepared in duplicate. In one set, nonreducing gel loading buffer was used (3% sodium dodecyl sulfate, 7.5% glycerol, 0.05% Coomassie blue, 37.5 mm Tris-Cl, pH 6.8). In the second set, reducing buffer was used (containing 6% mercaptoethanol). The second set served as a control for authentic FXa digestion and verification of disulfide bond formation.
Western Blot
The appropriately treated membrane preparations were separated on 12% sodium dodecyl sulfate-polyacrylamide gel and electrophoretically transferred onto nitrocellulose membranes (0.2 μm) using the Bio-Rad semidry transfer unit (according to the manufacturer's protocol). Blocking was done with 4% milk powder (MP) in 0.05% TBST (Tris-Cl, NaCl buffer with 0.05% Tween 20) for 45 min at RT. After overnight incubation with anti-c-myc antibody (1:2000 in 0.05% TBST with 2% MP) at 4 C, the blot was washed five times with TBST. Incubation with secondary antibody, antimouse horseradish peroxidase antibody (1:4000 in 0.05% TBST with 1% MP), was carried out for 45 min at RT. After washing five times with 0.05% TBST, the blots were developed with chemiluminiscent substrate according to the manufacturer's protocol.
Molecular Modeling
The molecular model of PTHR1 was built using template forcing to the x-ray structure of rhodopsin and the Discover molecular mechanics program. The structural features of the receptor domains previously studied by NMR (including the juxtamembrane portion of the N-terminal extracellular domain [PTHR1(168-198)], ECL1 [PTHR1(241-285)], and ECL3 [PTHR1(420-450)] were incorporated into the model using the experimentally determined distance restraints from NMR studies (22,38). The model of the receptor was refined by molecular dynamics simulations employing a water/decane/water simulation cell to mimic the hydrophilic/hydrophobic biphasic nature of the membrane and the GROMACs (http://www.gromacs.org) program. The ligand was placed into the model using the contact points previously identified by photoaffinity labeling (37).
Supplementary Material
Footnotes
This work was supported by Grants DK-47940 (to M.R.) and GM-54082 (to D.F.M.) from the National Institutes of Health.
Disclosure Statement: The authors have nothing to disclose.
First Published Online February 7, 2008
Abbreviations: β2AR, β2-Adrenergic receptor; ECL, extracellular loop; FXa, factor Xa; GPCR, G protein-coupled receptor; ICL, intracellular loop; MP, milk powder; NMR, nuclear magnetic resonance; PTHR1, PTH receptor type-1; RT, room temperature; TBST, Tris-Cl, NaCl buffer with 0.05% Tween 20; TM, transmembrane; wt, wild type.
References
- Taniguchi Y, Ikehara T, Kamo N, Yamasaki H, Toyoshima, Y 2007 Dynamics of light-induced conformational changes of the phoborhodopsin/transducer complex formed in the n-dodecyl β-D-maltoside micelle. Biochemistry 46:5349–5357 [DOI] [PubMed] [Google Scholar]
- Klein-Seetharaman J, Yanamala NV, Javeed F, Reeves PJ, Getmanova EV, Loewen MC, Schwalbe H, Khorana HG 2004 Differential dynamics in the G protein-coupled receptor rhodopsin revealed by solution NMR. Proc Natl Acad Sci USA 101:3409–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Seetharaman J, Hwa J, Cai K, Altenbach C, Hubbell WL, Khorana HG 2001 Probing the dark state tertiary structure in the cytoplasmic domain of rhodopsin: proximities between amino acids deduced from spontaneous disulfide bond formation between Cys316 and engineered cysteines in cytoplasmic loop 1. Biochemistry 40:12472–12478 [DOI] [PubMed] [Google Scholar]
- Altenbach C, Cai K, Klein-Seetharaman J, Khorana HG, Hubbell WL 2001 Structure and function in rhodopsin: mapping light-dependent changes in distance between residue 65 in helix TM1 and residues in the sequence 306–319 at the cytoplasmic end of helix TM7 and in helix H8. Biochemistry 40:15483–15492 [DOI] [PubMed] [Google Scholar]
- Hubbell WL, Cafiso DS, Altenbach C 2000 Identifying conformational changes with site-directed spin labeling, Nat Struct Biol 7:735–739 [DOI] [PubMed] [Google Scholar]
- Nakanishi J, Takarada T, Yunoki S, Kikuchi Y, Maeda M 2006 FRET-based monitoring of conformational change of the β2 adrenergic receptor in living cells. Biochem Biophys Res Commun 343:1191–1196 [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Gaietta G, Bunemann M, Adams SR, Oberdorff-Maass S, Behr B, Vilardaga JP, Tsien RY, Ellisman MH, Lohse MJ 2005 A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nat Methods 2:171–176 [DOI] [PubMed] [Google Scholar]
- Cohen B E, Pralle A, Yao X, Swaminath G, Gandhi CS, Jan YN, Kobilka BK, Isacoff EY, Jan LY 2005 A fluorescent probe designed for studying protein conformational change. Proc Natl Acad Sci USA 102:965–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanouni P, Steenhuis JJ, Farrens DL, Kobilka BK 2001 Agonist-induced conformational changes in the G-protein-coupling domain of the β 2 adrenergic receptor. Proc Natl Acad Sci USA 98:5997–6002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka BK 2002 Agonist-induced conformational changes in the β2 adrenergic receptor. J Pept Res 60:317–321 [DOI] [PubMed] [Google Scholar]
- Kobilka BK, Gether U 2002 Use of fluorescence spectroscopy to study conformational changes in the β2-adrenoceptor. Methods Enzymol 343:170–182 [DOI] [PubMed] [Google Scholar]
- Huang W, Osman R, Gershengorn MC 2005 Agonist-induced conformational changes in thyrotropin-releasing hormone receptor type I: disulfide cross-linking and molecular modeling approaches. Biochemistry 44:2419–2431 [DOI] [PubMed] [Google Scholar]
- Lyford LK, Sproul AD, Eddins D, McLaughlin JT, Rosenberg RL 2003 Agonist-induced conformational changes in the extracellular domain of α 7 nicotinic acetylcholine receptors. Mol Pharmacol 64:650–658 [DOI] [PubMed] [Google Scholar]
- Ward SD, Hamdan FF, Bloodworth LM, Wess J 2002 Conformational changes that occur during M3 muscarinic acetylcholine receptor activation probed by the use of an in situ disulfide cross-linking strategy. J Biol Chem 277:2247–2257 [DOI] [PubMed] [Google Scholar]
- Han SJ, Hamdan FF, Kim SK, Jacobson KA, Bloodworth LM, Li B, Wess J 2005 Identification of an agonist-induced conformational change occurring adjacent to the ligand-binding pocket of the M(3) muscarinic acetylcholine receptor. J Biol Chem 280:34849–34858 [DOI] [PubMed] [Google Scholar]
- Han SJ, Hamdan FF, Kim SK, Jacobson KA, Brichta L, Bloodworth LM, Li JH, Wess J 2005 Pronounced conformational changes following agonist activation of the M(3)muscarinic acetylcholine receptor. J Biol Chem 280:24870–24879 [DOI] [PubMed] [Google Scholar]
- Ward SD, Hamdan FF, Bloodworth LM, Siddiqui NA, Li JH, Wess J 2006 Use of an in situ disulfide cross-linking strategy to study the dynamic properties of the cytoplasmic end of transmembrane domain VI of the M3 muscarinic acetylcholine receptor. Biochemistry 45:676–685 [DOI] [PubMed] [Google Scholar]
- Buck E, Bourne H, Wells JA 2004 Site-specific disulfide capture of agonist and antagonist peptides on the C5a receptor. J Biol Chem 280:4009–4012 [DOI] [PubMed] [Google Scholar]
- Castro M, Nikolaev VO, Palm D, Lohse MJ, Vilardaga JP 2005 Turn-on switch in parathyroid hormone receptor by a two-step parathyroid hormone binding mechanism. Proc Natl Acad Sci USA 102:16084–16089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh SP, Vilardaga JP, Baranski TJ, Lichtarge O, Iiri T, Meng EC, Nissenson RA, Bourne HR 1999 Similar structures and shared switch mechanisms of the β2-adrenoceptor and the parathyroid hormone receptor. Zn(II) bridges between helices III and VI block activation. J Biol Chem 274:17033–17041 [DOI] [PubMed] [Google Scholar]
- Gensure RC, Gardella TJ, Juppner H 2005 Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun 328:666–678 [DOI] [PubMed] [Google Scholar]
- Piserchio A, Bisello A, Rosenblatt M, Chorev M, Mierke DF 2000 Characterization of parathyroid hormone/receptor interactions: structure of the first extracellular loop. Biochemistry 39:8153–8160 [DOI] [PubMed] [Google Scholar]
- Gensure RC, Shimizu N, Tsang J, Gardella TJ 2003 Identification of a contact site for residue 19 of parathyroid hormone (PTH) and PTH-related protein analogs in transmembrane domain two of the type 1 PTH receptor. Mol Endocrinol 17:2647–2658 [DOI] [PubMed] [Google Scholar]
- Rolz C, Pellegrini M, Mierke DF 1999 Molecular characterization of the receptor-ligand complex for parathyroid hormone. Biochemistry 38:6397–6405 [DOI] [PubMed] [Google Scholar]
- Gardella TJ, Luck MD, Fan MH, Lee C 1996 Transmembrane residues of the parathyroid hormone (PTH)/PTH-related peptide receptor that specifically affect binding and signaling by agonist ligands. J Biol Chem 271:12820–12825 [DOI] [PubMed] [Google Scholar]
- Thomas BE, Wittelsberger A, Woznica I, Hsieh M-Y., Monaghan P, Lee B-K, Rosenblatt M 2007 Cysteine at position 217 in the intracellular loop 1 plays a critical role in human PTH receptor type 1 membrane translocation and function. J Bone Miner Res 22:609–616 [DOI] [PubMed] [Google Scholar]
- Wittelsberger A, Corich M, Thomas BE, Lee BK, Barazza A, Czodrowski P, Mierke DF, Chorev M, Rosenblatt M 2006 The mid-region of parathyroid hormone (1–34) serves as a functional docking domain in receptor activation. Biochemistry 45:2027–2034 [DOI] [PubMed] [Google Scholar]
- Li, B, Scarselli M, Knudsen CD, Kim SK, Jacobson KA, McMillin SM, Wess J 2007 Rapid identification of functionally critical amino acids in a G protein-coupled receptor. Nat Methods 4:169–174 [DOI] [PubMed] [Google Scholar]
- Harmar AJ 2001 Family-B G-protein-coupled receptors. Genome Biol 2:3013.1–3013.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PR, Bambino T, Nissenson RA 1996 Mutations of neighboring polar residues on the second transmembrane helix disrupt signaling by the parathyroid hormone receptor. Mol Endocrinol 10:132–139 [DOI] [PubMed] [Google Scholar]
- Turner PR, Mefford S, Bambino T, Nissenson RA 1998 Transmembrane residues together with the amino terminus limit the response of the parathyroid hormone (PTH) 2 receptor to PTH-related peptide. J Biol Chem 273:3830–3837 [DOI] [PubMed] [Google Scholar]
- Hamdan FF, Ward SD, Siddiqui NA, Bloodworth LM, Wess J 2002 Use of an in situ disulfide cross-linking strategy to map proximities between amino acid residues in transmembrane domains I and VII of the M3 muscarinic acetylcholine receptor. Biochemistry 41:7647–7658 [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK 2007 Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature 450:383–387 [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science 318:1266–1273 [DOI] [PubMed] [Google Scholar]
- Adams A, Pines M, Nakamoto C, Behar V, Yang QM, Bessalle R, Chorev M, Rosenblatt M, Levine M, Suva L 1995 Probing the biomolecular interactions of parathyroid hormone and the human parathyroid hormone/parathyroid hormone-related protein receptor II. Cloning, characterization, and photoaffinity labeling of the recombinant human receptor. Biochemistry 34:10553–10559 [DOI] [PubMed] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA 1987 Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol 154:367–382 [DOI] [PubMed] [Google Scholar]
- Wittelsberger A, Thomas BE, Mierke DF, Rosenblatt M 2006 Methionine acts as a “magnet” in photoaffinity crosslinking experiments. FEBS Lett 580:1872–1876 [DOI] [PubMed] [Google Scholar]
- Pellegrini C, O'Brien T, Yap J, Jeppsson A, Tazelaar HD, McGregor CG 1998 Systematic evaluation of distribution of transgene expression after adenovirus-mediated gene transfer to the transplanted heart. Transpl Int 11:373–377 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.