Abstract
Receptor activator of nuclear factor-κB ligand (RANKL) is a TNF-like factor that is both produced by osteoblasts, mesenchymal cells, and activated T cells and required for osteoclast maturation and survival. The gene is up-regulated by the two primary calcemic hormones, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] and PTH. Previous studies have indicated that five enhancer regions located significantly upstream of the mouse Rankl transcriptional start site mediate up-regulation by 1,25(OH)2D3 and PTH. The most distal of these, termed mRLD5, is highly conserved in the human gene at −96 kb where it was also shown to be functionally active. Four additional mouse Rankl upstream enhancers are also highly conserved in the human gene at −20, −25, −75, and −87 kb. In the present studies, we characterized the activity of these regions, explored their capacity to mediate the actions of 1,25(OH)2D3, and identified the vitamin D response elements contained within the two most proximal segments. Interestingly, whereas the most distal of the five enhancers is the dominant mediator of 1,25(OH)2D3 activity in the mouse Rankl gene, that role in the human gene is manifested by the most proximal element at −20 kb. Importantly, activity at this region in response to 1,25(OH)2D3 was associated with a significant increase in histone acetylation as well as the enhanced recruitment of RNA polymerase II. Both likely reflect the primary role of this enhancer in human RANKL gene expression. Our studies confirm the complex nature of RANKL regulation and indicate that although the five enhancers are evolutionarily conserved across several species, their relative contributions to RANKL expression in response to 1,25(OH)2D3 may be different.
BONE REMODELING IS an intricate process that requires the activity of both bone-forming osteoblasts and bone-resorbing osteoclasts (1). Osteoclasts are derived from the monocyte-macrophage lineage and require a series of growth factors, cytokines, and other steroidal components for their proper development (2,3). Many of these regulatory factors are produced by cells in close proximity to osteoclasts, including stromal cells, B and T cells, and osteoblasts. It is now well established that osteoblasts, as well as several other cell types, express receptor activator of nuclear factor (NF)-κB ligand (RANKL), a factor that is both necessary and sufficient for proper osteoclast maturation and function (4). Binding of RANKL to its receptor RANK on osteoclast precursors triggers activation of multiple signaling cascades, including those of the inhibitors of IκB kinase-α/β, the MAPKs, Src, and Akt pathways (5). The direct transcription factor targets of these signaling pathways include c-fos, NF-κB, NF of activated T cells c1, and others, all of which initiate the programs of precursor growth arrest and subsequent osteoclast differentiation, fusion, activation, and survival (2,6). Evidence for the essential role of RANKL in osteoclast differentiation is supported by the inability of mice lacking either RANKL or RANK to produce osteoclasts, resulting in an osteopetrotic phenotype (7,8).
RANKL expression is stimulated by a number of factors, most notably the calcemic hormones 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3] (9) and PTH (10,11). In addition, RANKL can also be induced by glucocorticoids, cytokines, and certain prostaglandins (12,13,14,15,16,17). Although the presence of these compounds at physiologically normal levels is necessary for proper RANKL expression, overexpression of RANKL can result in excess bone resorption, osteoporosis, rheumatoid arthritis, and osteoarthritis as well as bone diseases associated with multiple myeloma, diabetic neuropathy, metastatic cancer, and other skeletal abnormalities (18,19). An understanding of how RANKL expression is regulated is therefore vital to the development of treatments for bone disease.
1,25-(OH)2D3 regulates gene expression by binding to its receptor [vitamin D receptor (VDR)], resulting in the association of VDR with retinoid X receptor (RXR) and the binding of the heterodimer to specific DNA sequences, termed vitamin D response elements (VDREs) located in the regulatory regions of target genes (20). The VDR-RXR heterodimer then recruits additional protein complexes that modulate transcription (21,22,23). Previous work in our laboratory using chromatin immunoprecipitation (ChIP) techniques led to the identification of five evolutionarily conserved enhancer regions located at −16, −22, −60, −69, and −76 kb upstream of the mouse Rankl transcriptional start site (TSS) (24). An assessment of the transcriptional activity of each of these enhancer regions in transfection assays revealed that only the most distal, located at −76 kb, was capable of mediating response to 1,25-(OH)2D3 (24). Despite this, all of the regions contained binding sites for the VDR and its partner RXR, as assessed by ChIP analysis, and mediated the recruitment of RNA polymerase II (RNA pol II) to the Rankl TSS. Interestingly, RNA pol II was also recruited to the individual enhancers, suggesting potential roles for these regions as recruitment centers for the general transcriptional machinery. These findings suggest that RANKL induction by 1,25-(OH)2D3 (9) and in later studies by PTH (11) and cytokines such as oncostatin M (15) is likely to be much more complex than originally envisioned.
In the present studies, we identified the regulatory regions in the human RANKL gene that correspond to those found in the mouse and characterized the relative enhancer properties of each. Interestingly, two regions in addition to the most distal at −96 kb were found to mediate the activity of 1,25-(OH)2D3 and to contain functional VDREs. The most proximal of these at −20 kb, unlike in the mouse, appears to play a dominant role in human RANKL gene expression. Our current studies confirm and extend earlier work done on the mouse gene and provide further insight into the mechanism by which 1,25-(OH)2D3 regulates expression of the human RANKL gene.
RESULTS
Mouse RANKL Distal Enhancers Are Conserved in the Human Locus
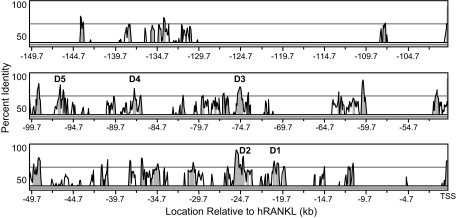
Our previous studies revealed the presence of five distal enhancers (mRLD1–mRLD5) in the upstream region of the mouse Rankl gene (24). Although each manifested unique regulatory features, the most remote, located approximately 76 kb upstream of the RANKL TSS and designated mRLD5, was capable of mediating the actions of 1,25-(OH)2D3 and PTH as well as the gp130-activating cytokine oncostatin M and also contained binding sites for the VDR/RXR heterodimer, cAMP response element-binding protein and Runx2. Importantly, this specific segment of DNA was highly conserved in the human RANKL gene at a site approximately 96 kb upstream of the gene's TSS. Thus, it was not surprising that this region retained regulatory properties similar to those observed in the mouse gene. We predicted that this region was likely responsible for the up-regulation of RANKL by 1,25-(OH)2D3 that is documented in the human osteoblastic MG63 cell line in supplemental Fig. 1 (published as supplemental data on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Interestingly, a 10-way species alignment using the UCSC genome browser also revealed the presence of additional segments of high conservation upstream of the RANKL locus, including regions located in the human gene at −20, −25, −75, and −87 kb that correspond directly to those in mouse mRLD1–mRLD4. Figure 1 depicts a VISTA plot (25) alignment of 150 kb of sequence located immediately upstream of the transcriptional start sites of the human and mouse RANKL genes. This figure highlights not only the multiple regions of conservation that exist between the two species but also the locations of the conserved regions RLD1–RLD5 that we had shown previously by ChIP and ChIP-chip analyses to contain binding sites for the VDR (24). Each of these designated regions contains a block of at least 300 bp that are more than 70% identical between human and mouse.
Figure 1.
Conservation Analysis of the Extended RANKL Locus
150 kb immediately upstream of the mouse and human RANKL TSS were analyzed using the VISTA server (http://genome.lbl.gov/vista/index.shtml), and peaks of conserved sequence were identified. Regions bound by VDR in response to 1,25-(OH)2D3 are labeled D1–D5. Shaded peaks represent greater than 70% identity.
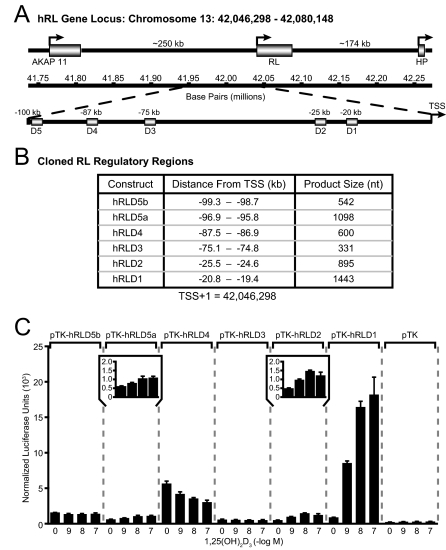
The Human RANKL Enhancers hRLD1, hRLD2, and hRLD5 Are Transcriptionally Active in the Context of a Heterologous Promoter
The similarity in functional activity between the mouse and human RLD5 regions (24) together with the high degree of conservation noted at mouse RLD1–RLD4 prompted us to examine these other segments of DNA from the human gene for their capacity to mediate transcriptional activation by 1,25-(OH)2D3. We therefore amplified DNA fragments that corresponded to human RLD1–RLD4, cloned them into a thymidine kinase (TK) promoter-luciferase reporter plasmid, and assessed their transcriptional activity in response to 1,25-(OH)2D3 after introduction into human osteoblastic MG63 cells. The positions and individual characteristics of these fragments are documented in Fig. 2, A and B. Figure 2C depicts the results of this experiment, which demonstrate that whereas hRLD5a was capable of mediating a modest response to 1,25-(OH)2D3 (see inset), as observed in our earlier studies, hRLD2 (see inset) and especially hRLD1 were strikingly sensitive to 1,25-(OH)2D3. Thus, a 2- to 5-fold induction was observed when the hRLD2 or hRLD5 regions were examined, and an over 20-fold induction was obtained when hRLD1 was present. Neither hRLD3 nor hRLD4 were able to confer any degree of sensitivity to 1,25-(OH)2D3, an observation consistent with that seen in the mouse RLD3 and RLD4 enhancer regions. These findings demonstrate that the five conserved regions in the human gene that correspond to mouse RLD1–RLD5 display both similarities as well as differences in their ability to mediate a transcriptional response to 1,25-(OH)2D3 in MG63 cells. They also suggest the possibility that the regulatory region at RLD1 in the human gene may play a dominant role in human RANKL gene regulation.
Figure 2.
The Transcriptional Response of RANKL to 1,25-(OH)2D3 Is Mediated by the hRLD5a, hRLD2, and hRLD1 Enhancer Regions
A, Schematic diagram of the location of RANKL within human chromosome 13 as well as RANKL enhancer regions upstream of the TSS; B, list of conserved human RANKL enhancer regions cloned into the pTK reporter vector and used in transient transfection assays; C, 1,25-(OH)2D3 induces transcriptional activity of the hRLD5, hRLD2, and hRLD1 enhancer regions of the human RANKL gene. MG63 cells were transfected with pTK reporter vector, pTK-hRLD5b, pTK-hRLD5a, pTK-hRLD4, pTK-hRLD3, pTK-hRLD2, or pTK-hRLD1 (250 ng) as well as pcDNA-hVDR (50 ng) and pCH110-βgal expression vectors (50 ng). The cells were treated with vehicle or the indicated concentration of 1,25-(OH)2D3 for 24 h. Luciferase activity was normalized to β-galactosidase values and is displayed as the average of a triplicate set of transfections ± sem. The insets for pTK-hRLD5a and pTK-hRLD2 provide an enhanced view of each plasmid's activity. This result is representative of at least three independent experiments.
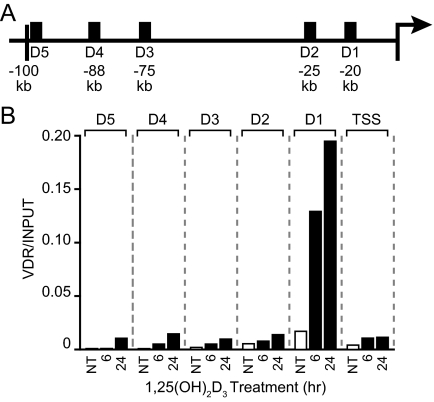
The VDR Localizes Strongly to the hRLD1 Region of the Human RANKL Gene
The striking transcriptional activity of hRLD2 and especially hRLD1 led us to explore the ability of 1,25-(OH)2D3 to induce VDR binding to these regions. MG63 cells were treated for specific periods of time with a maximal stimulating dose of 1,25-(OH)2D3 and then isolated, fixed, and subjected to ChIP analysis using antibodies to VDR or IgG. Co-immunoprecipitated DNA was then analyzed by quantitative PCR using the primer sets whose locations are documented in Fig. 3A. Surprisingly, although localization of the VDR to the human RANKL gene was strikingly evident at hRLD1, as seen in Fig. 3B, it was only modestly detected at hRLD2–hRLD5 and at the TSS. These results together with the transcriptional studies in Fig. 2 suggest that hRLD1 may play a more dominant role in RANKL induction by 1,25-(OH)2D3 than previously anticipated. They also suggest that hRLD3 and hRLD4 may also participate in the activation of human RANKL expression by 1,25-(OH)2D3 despite their inability to mediate such activity after transfection into MG63 cells. Also of relevance, previous studies have suggested the presence of a consensus VDRE located immediately downstream of the mouse Rankl TSS at +33 (26). Although this sequence is not fully conserved in the human gene, it may well be responsible for the modest VDR binding activity detected at the human RANKL TSS.
Figure 3.
1,25-(OH)2D3 Induces VDR Binding to the Human RANKL Enhancers
A, Schematic diagram of the locations of the ChIP primer regions (black boxes); B, treatment with 1,25-(OH)2D3 induces VDR binding to the RANKL enhancer regions in a time-dependent manner. MG63 cells were treated with either vehicle or 10−7 m 1,25-(OH)2D3 for the indicated times, and ChIP assay was performed as described in Materials and Methods using antibodies specific for VDR as well as a nonspecific IgG. Nonimmunoprecipitated samples were taken as input controls. The resulting purified DNA was analyzed by real-time PCR. PCR products were quantitated using an independent standard curve and normalized to the corresponding input value for each sample. NT, No treatment.
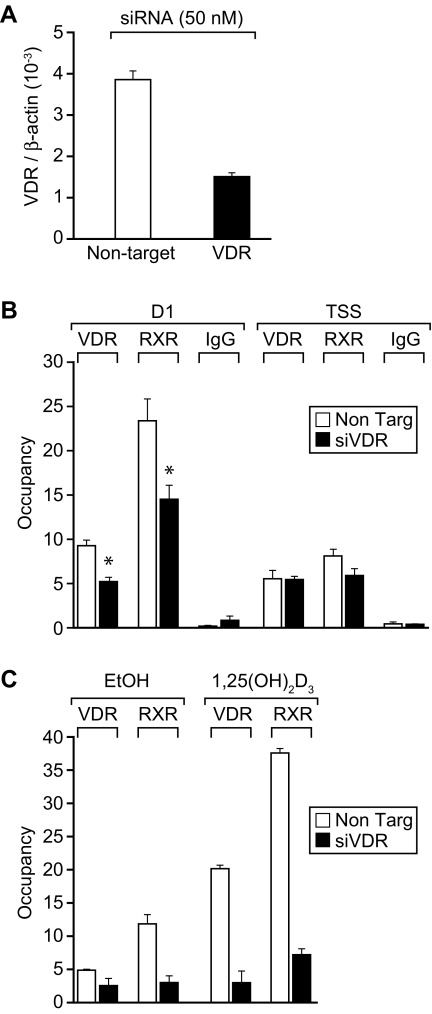
VDR Binds to hRLD1 in the Absence of Exogenously Added 1,25-(OH)2D3
Interestingly, the level of VDR detected at the hRLD1 region in the absence of added 1,25-(OH)2D3 was higher than that observed at hRLD2–hRLD5 or at the TSS, suggesting the possibility that VDR might occupy this site in a ligand-independent manner. To evaluate this possibility, we treated MG63 cells with either nontargeting or previously validated hVDR small interfering RNA (siRNA) (27) for 48 h and then subjected the cells to ChIP analysis using antibodies to VDR, RXR, or IgG. The ability of the VDR siRNA to suppress VDR mRNA levels is documented in Fig. 4A. The results in Fig. 4B reveal that VDR siRNA treatment does produce a decrease in the level of occupancy of both VDR and RXR at the hRLD1 enhancer but not at the TSS. Interestingly, this reduction in unliganded VDR at the hRLD1 enhancer also caused an approximate 50% reduction in the basal level of human RANKL mRNA expression in MG63 cells as well (data not shown). Finally, this reduction in VDR expression also resulted, as seen in Fig. 4C, in a striking decrease in both VDR and RXR binding at the hRLD1 region after 1,25-(OH)2D3 treatment. These results indicate that modest levels of both VDR and RXR are indeed bound to hRLD1 in a ligand-independent fashion. Collectively, our observations support the conclusion that hRLD1 and perhaps hRLD2 play dominant roles in the up-regulation of human RANKL expression by 1,25-(OH)2D3 in MG63 cells.
Figure 4.
Effects of VDR siRNA on VDR and RXR Binding to the Human RANKL Gene
A, VDR siRNA suppresses VDR mRNA levels. MG63 cells were treated with either nontargeting (white bars) or VDR siRNA (black bars) for 48 h (50 nm) and the isolated total RNA evaluated for VDR and β-actin mRNA levels using reverse transcription and real-time PCR. B, VDR binds the hRLD1 region in the absence of 1,25-(OH)2D3 treatment. MG63 cells were transfected with either nontargeting (white bars) or hVDR siRNA (black bars) (50 nm) for 48 h; ChIP analysis was then carried out as described previously using antibodies specific for VDR, RXR, and nonspecific IgG. Quantitative real-time PCR was used to evaluate the binding of VDR and RXR at the hRLD1 enhancer region as well as the TSS. Results are displayed as the average of a triplicate set of PCR ± sem. *, P < 0.05 in comparison with samples transfected with nontargeting siRNA. These results are representative of at least three independent experiments. C, VDR siRNA treatment blunts the ability of 1,25-(OH)2D3 to promote VDR and RXR binding to hRLD1. MG63 cells were transfected with either nontargeting (white bars) or hVDR siRNA (black bars) (50 nm) for 48 h, treated for an additional 24 h with either vehicle or 1,25-(OH)2D3 (10−7 m), and then subjected to ChIP analysis and evaluated as described in B.
The hRLD1 Region Mediates Glucocorticoid Induction of the Human RANKL Gene
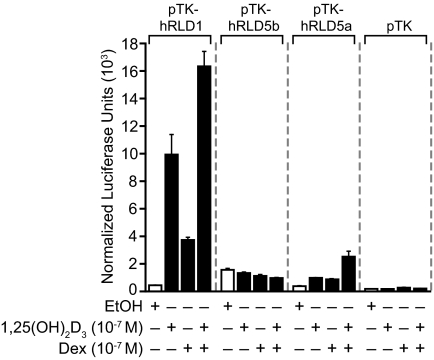
Glucocorticoids are known to induce RANKL gene expression in both mouse and human cells (12,17). Our earlier studies suggest that this regulation is mediated, at least in part, via undefined cis elements located within the RLD5 region of both the mouse and human RANKL genes. The striking ability of the hRLD1 region in the human gene to mediate a response to 1,25-(OH)2D3, however, prompted us to examine further whether this region could also mediate the transcriptional activity of glucocorticoids. MG63 cells were therefore transfected with control vector, glucocorticoid-insensitive hRLD5b, glucocorticoid-sensitive hRLD5a, or cloned hRLD1 segments; the cells were treated with vehicle, 1,25-(OH)2D3, dexamethasone (Dex), or the combination; and transcriptional activity measured 24 h later. As can be seen in Fig. 5, the hRLD1 region of the human gene conferred significant 1,25-(OH)2D3- and glucocorticoid-inducible response to the TK promoter, activities that were additive when both hormones were administered simultaneously. These activities were much more robust than those mediated by the hRLD5 enhancer. These observations provide additional evidence for the hypothesis that the hRLD1 region of the human RANKL gene may represent a dominant regulatory enhancer for human RANKL gene expression.
Figure 5.
The hRLD5a and hRLD1 Enhancer Regions Mediate Transcriptional Response to Dex
MG63 cells were transfected with pTK, pTK-hRLD1, pTK-hRLD5b, or pTK-hRLD5a (250 ng) as well as pcDNA-hVDR and pCH110-βgal expression vectors (50 ng). Cells were treated with ethanol (EtOH) vehicle, 1,25-(OH)2D3, Dex, or 1,25-(OH)2D3 and Dex (all at 10−7 m) for 24 h and assayed for luciferase and β-gal activity. Luciferase activity was normalized to β-gal values and is displayed as the average of a triplicate set of transfections ± sem. These results are representative of two independent experiments.
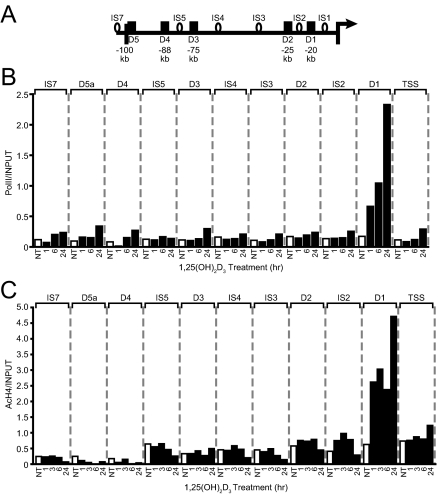
RNA Pol II Is Strongly Recruited to the hRLD1 Enhancer
RNA pol II is recruited to both enhancer regions as well as transcriptional start sites in many genes (28,29,30,31,32). In the mouse Rankl gene, this recruitment can be measured independently at mRLD1–mRLD5 in response to either 1,25-(OH)2D3 or protein kinase A activators such as forskolin (24,33). We therefore asked whether 1,25-(OH)2D3 could induce the recruitment of RNA pol II to the human RANKL TSS and whether the potential recruitment of this factor could also be measured independently at the conserved enhancer regions. MG63 cells were treated with 1,25-(OH)2D3 for periods up to 24 h, the cells subjected to ChIP analysis using antibodies to RNA pol II, and the precipitated DNA evaluated using primer sets whose locations are documented in Fig. 6A. Primers focused upon several regions located between hRLD1 and hRLD5 (IS1–IS5) were used to more precisely gauge the nature of the potential increases noted at the five enhancer regions. RNA pol II was recruited in a time-dependent manner to the RANKL TSS as well as to hRLD1, as documented in Fig. 6B. Although small increases (<2-fold) were also evident at several regions located upstream of the RANKL TSS, including those at the hRLD5, hRLD4, and hRLD3 and at the intervening regions IS7 and IS3, the inducible levels were exceedingly modest compared with the 20-fold increases routinely observed at hRLD1. A kinetic correlation between VDR binding and the recruitment of RNA pol II was also evident. This observation affirms the direct role of this regulatory region in RANKL gene expression and suggests further that VDR DNA binding may provide the direct impetus for the entry of RNA pol II at the hRLD1 enhancer.
Figure 6.
1,25-(OH)2D3 Induces RNA Pol II Recruitment and H4 Acetylation at the hRLD1 Region
A, Schematic diagram of the locations of the ChIP primer regions (black boxes and open circles). B, RNA polymerase II is recruited to the hRLD1 region in response to treatment with 1,25-(OH)2D3. MG63 cells were treated with ethanol vehicle or 10−7 m 1,25-(OH)2D3 for the indicated time and subjected to ChIP analysis as described previously using an RNA pol II-specific antibody (pol II). Samples were quantitated using an independent standard curve and were normalized to the corresponding input value for each sample. These results are representative of at least three similar experiments. C, H4 is acetylated at the hRLD1 region in response to treatment with 1,25-(OH)2D3. MG63 cells were treated with 10−7 m 1,25-(OH)2D3 for the indicated time and subjected to ChIP analysis as described previously using a tetra-acetylated H4-specific antibody (AcH4). Immunoprecipitated DNA was quantitated by real-time PCR using an independent standard curve and was normalized to the corresponding input value for each sample. These results are representative of at least three similar experiments. NT, No treatment.
1,25-(OH)2D3 Induces Striking Histone 4 (H4) Acetylation at the hRLD1 Enhancer
Previous studies have suggested that in addition to its ability to promote RNA pol II recruitment, 1,25-(OH)2D3 and its receptor are also able to induce chromatin modifications that play a role in facilitating changes in the transcriptional output of a number of target genes (22,24,34,35). This is particularly true at the mouse Rankl gene, where 1,25-(OH)2D3 was observed to induce broad changes in H4 acetylation extending from the Rankl TSS upstream 100 kb (24). To explore whether 1,25-(OH)2D3 can also induce this modification to the human gene, we treated MG63 cells with 1,25-(OH)2D3 for periods ranging from 0–24 h, subjected the cells to ChIP analysis using antibodies to tetra-acetylated H4, and measured the level of acetylated H4 activity at the sites indicated using the primers whose locations are documented in Fig. 6A. We used the intervening primer sets (IS1–IS5) to obtain a clearer picture of any increases in H4 acetylation that might emerge. As observed in Fig. 6C, 1,25-(OH)2D3 induced significant changes in H4 acetylation only at hRLD1. Thus, although basal levels of H4 acetylation were observed in the upstream regions of the RANKL gene, including acetylation at hRLD2, hRLD3, hRLD4, and hRLD5, H4 acetylation was not up-regulated by 1,25-(OH)2D3 in these regions. This finding suggests that changes in chromatin structure as well as the recruitment of RNA pol II at hRLD1 may represent events that are integral to the induction of RANKL by 1,25-(OH)2D3. The presence of the VDR at this site suggests again the possibility that the VDR may also play a direct role in the recruitment of coregulators that are responsible for orchestrating these molecular events at hRLD1.
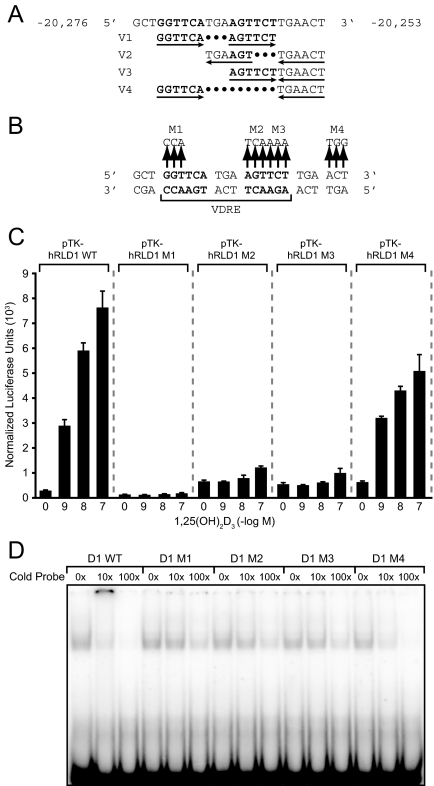
Mapping the VDRE in the hRLD1 Region
Mapping experiments led to the identification of a unique regulatory sequence that mediated the activity of 1,25-(OH)2D3 in the mouse RLD5 region of the Rankl gene (24). This sequence was highly conserved and was subsequently shown to be active in the human RANKL gene as well. The ability of 1,25-(OH)2D3 to induce transcription via the hRLD1 and hRLD2 enhancers suggests that VDREs are also present in these regions. To identify candidate sequences, we carried out an in silico analysis using the CONSITE program (36), which revealed a potential VDRE in the hRLD1 region comprised of a series of overlapping and inverted nuclear receptor half-sites. Four potential sequence combinations related to VDREs were identified, as indicated in Fig. 7A. To determine whether this candidate region was responsible for the activity observed in the hRLD1 region, we created a series of four triplet mutations in each of the putative receptor half-sites within the context of the hRLD1 fragment, as shown in Fig. 7B, transfected these modified hRLD1 constructs into the MG63 cell line, and assessed their sensitivity to increasing concentrations of 1,25-(OH)2D3 24 h later. As can be seen in Fig. 7C, although the native hRLD1 region was capable of mediating a typical dose response to 1,25-(OH)2D3, this activity was completely abrogated by the introduction of mutations at M1, M2, and M3. The activity in response to 1,25-(OH)2D3 observed with the M4 mutant appeared largely intact, although slightly blunted. This result indicates that this complex sequence mediates the activity of 1,25-(OH)2D3 in the hRLD1 region and that the most likely VDRE within this extended sequence comprised of two hexameric half-sites is that identified as version 1. It is possible, however, that the inverted repeats represented by the element in version 3 may also contribute to this activity. To confirm these findings, we employed EMSA. Purified recombinant human VDR and RXR were incubated with labeled wild-type probe and increasing concentrations of unlabeled wild-type, M1, M2, M3, or M4 duplex sequences and the complexes then separated on a nondenaturing gel. The results in Fig. 7D indicate that whereas the VDR/RXR heterodimer bound to the hRLD1 VDRE as a single complex, only the wild-type and the M4 mutant hRLD1 sequences were able to successfully compete with the labeled probe. Modest amounts of competition at 100× excess duplex do suggest, however, that some lower-affinity VDR/RXR binding may be possible at other combinatorial half-sites. These results confirm the location and general nature of the VDRE located in the hRLD1 region of the RANKL gene. Interestingly, when cloned as a single copy upstream of the TK promoter, the hRLD1 VDRE was not capable of mediating a response to 1,25-(OH)2D3 (data not shown). This was not unexpected and reinforces the validity of our overall approach, which involved examining the activity of larger DNA fragments rather than those directly related to the VDREs themselves. Our results suggest that the environment of the VDRE at hRLD1 is critical to its dominant activity in response to 1,25-(OH)2D3.
Figure 7.
Identification of a Functional VDRE within the hRLD1 Region
A, Wild-type DNA sequence identified in the hRLD1 region using the CONSITE transcription factor binding algorithm (http://mordor.cgb.ki.se/cgi-bin/CONSITE/consite) is indicated. Four potential VDREs are shown. B, Location of mutations introduced into the putative VDRE-containing regions of hRLD1. Mutations were introduced into the wild-type sequence as indicated and designated M1–M4. C, Mutations in the hRLD1 VDRE abrogate transcriptional response to treatment with 1,25-(OH)2D3. MG63 cells were transfected with pTK-hRLD1 reporter vectors (250 ng) containing either the putative wild type VDRE (WT) or one in which various portions of the putative VDRE had been mutated (M1–M4). Cells were also transfected with pcDNA-hVDR and pCH110-βgal expression vectors (50 ng), treated with the indicated concentration of 1,25-(OH)2D3 for 24 h, and assayed for luciferase and β-galactosidase activity. Luciferase activity was normalized to β-galactosidase values and is displayed as the average of a triplicate set of transfections ± sem. These results are representative of at least two separate experiments. D, EMSA. The wild-type probe described in A was end labeled with [γ-32P]dATP and incubated with 10 ng VDR, 5 ng RXR, 2 × 10−6 m 1,25-(OH)2D3, 150 mm KCl, and 0, 10-, or 100-fold molar excess of the indicated unlabeled probe. The resulting complexes were separated on 6% nondenaturing polyacrylamide gels, dried, and imaged by autoradiography. These results are representative of at least two separate experiments.
Mapping the VDRE in the RANKL hRLD2 Region
The hRLD2 region of the RANKL locus also contains a putative VDRE, as determined by CONSITE program analysis. This element contains three direct hexanucleotide repeats each separated by 3 bp, as documented in supplemental Fig. 2A. We employed a similar strategy as was outlined for the hRLD1 region by introducing a triplet mutation in each of the three putative half-sites in the context of the hRLD2 DNA fragment (see supplemental Fig. 2A) and assessed the activities of these three mutant hRLD2 constructs after transfection into MG63 cells. The results in supplemental Fig. 2B demonstrate that only mutations in the two proximal (downstream) half-sites fully compromised transcriptional response to 1,25-(OH)2D3. Importantly, the results obtained by EMSA fully confirm this conclusion. Thus, although VDR and RXR bound directly to the native hRLD2 sequence, only the unlabeled wild-type sequence and the sequence containing M1 were capable of competing for VDR/RXR binding. These experiments define the cis acting element that mediates 1,25-(OH)2D3 response in the hRLD2 region of the RANKL gene. As with hRLD1, a single copy of the isolated hRLD2 VDRE was incapable of mediating response to 1,25-(OH)2D3 in the context of the TK promoter (data not shown).
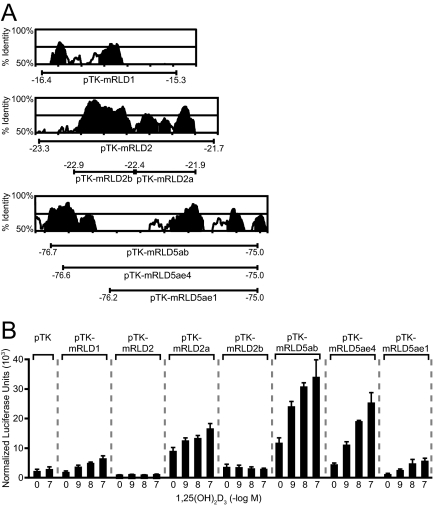
VDREs in the Human RANKL Gene Are Conserved and Functional in the Mouse Gene
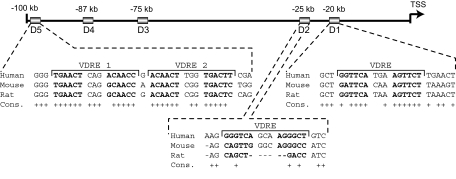
Our earlier studies of the mouse RANKL gene suggested that whereas the VDR bound directly to mRLD1 and mRLD2, the isolated DNA fragments were not capable of mediating a transcriptional response to 1,25-(OH)2D3 (24). Closer inspection of those data, however, revealed that whereas the mRLD1 fragment manifested a very low basal activity, 1,25-(OH)2D3 was able to induce a modest but measurable increase in activity. mRLD2, on the other hand, was fully inactive. Prompted by the results of our human RANKL studies, we initiated additional studies to probe further the activity of the mouse RLD1 and RLD2 regions. These regions are also highly conserved, as documented in Fig. 8A, and contain equally conserved VDRE DNA sequences as well. Although we evaluated the mRLD1 region as cloned previously, the mRLD2 region was subcloned into two fragments to explore the possibility that a suppressive element might be present and prevent activation by 1,25-(OH)2D3. Subclone mRLD2a contained a potential VDRE that was similar to that identified in the human RLD2 region. Each of these constructs as well as several control mouse RLD5 constructs were introduced into mouse ST2 cells and the activity of 1,25-(OH)2D3 assessed 24 h later. As can be seen in Fig. 8B, mRLD1 was indeed sensitive to 1,25-(OH)2D3 and mediated a level of induction not unlike that of the mRLD5 controls. Interestingly, although the mouse RLD2 region remained unresponsive to 1,25-(OH)2D3, as previously reported (24), the hormone was able to induce transcriptional response via the VDRE-containing mRLD2a but not the mRLD2b subclone. These results therefore suggest that the VDREs in the RLD1, RLD2, and RLD5 regions of both the mouse and human RANKL genes are not only conserved but functional as well. They also suggest that further examination of the RLD3 and RLD4 regions using this strategy may be in order. Figure 9 summarizes the locations, conservation, and sequences of the regulatory elements located in RLD1, RLD2, and RLD5 of both the mouse and human RANKL genes.
Figure 8.
The Human RANKL Transcriptional Response to 1,25-(OH)2D3 through the hRLD1 and hRLD2 Enhancer Regions Is Conserved in Mouse
A, Conservation analysis of the hRLD1, hRLD2, and hRLD5 regions. Peaks of conserved human and mouse sequence identified using the VISTA server (http://genome.lbl.gov/vista/index.shtml) are illustrated in the individual boxes. Shaded peaks represent greater than 70% identity. The mouse DNA subfragments whose coordinates are indicated by the bars below the conservation peaks were cloned into the pTK reporter vector and used for transfection analysis. B, 1,25-(OH)2D3 induces transcription via the mRLD1, mRLD2a, and mRLD5 enhancers located in the mouse Rankl gene. ST2 cells were transfected with pTK reporter vector or pTK-mRLD1, pTK-mRLD2, pTK-mRLD2a, pTK-mRLD2b, pTK-mRLD5ae4, pTK-mRLD5ae1, or pTK-mRLD5ab (250 ng) as well as pcDNA-hVDR and pCH110-βgal expression vectors (50 ng). The cells were treated with ethanol vehicle or the indicated concentration of 1,25-(OH)2D3 for 24 h and subjected to luciferase and β-galactosidase activity assays. Luciferase activity was normalized to β-galactosidase values and is displayed as the average of a triplicate set of transfections ± sem.
Figure 9.
Schematic Diagram of the Locations of Four Functional VDREs within the Human RANKL Locus
The sequences of functional VDREs located in the hRLD5, hRLD2, and hRLD1 enhancer regions are listed with + indicating identical bases in human, mouse, and rat. Sequence alignment was performed using the UCSC genome browser (http://genome.ucsc.edu/).
DISCUSSION
RANKL is now widely accepted as a key regulator of bone remodeling and has been shown to be essential for proper osteoclast development, function, and survival (4,8,37,38). Accordingly, loss-of-function mutations in RANKL lead to osteopetrosis in humans, and gain-of-function mutants of its receptor RANK lead to accelerated bone loss and both familial expansile osteolysis and expansile skeletal hypophosphatasia (38,39,40). These features of RANKL and RANK have been recapitulated in mouse models wherein the loss of either Rankl or Rank results in a complete lack of osteoclast production and a striking osteopetrotic phenotype (4,7,8). Interestingly, in addition to its well-established role in bone remodeling, RANKL has also been implicated in atherosclerosis and calcification of arteries and more recently in the regulation of immune response in inflamed skin (41,42,43). Thus, an understanding of the regulatory mechanisms controlling RANKL expression is key to the development of novel treatments for a number of human diseases.
Our previous work identified five important regulatory regions in the mouse Rankl gene that were responsible for mediating 1,25-(OH)2D3- and PTH-induced transcription of Rankl in the ST2 stromal cell line (24). Preliminary work suggested that the most distal of these regulatory regions, termed mRLD5, was highly conserved in the human gene and that this region was functionally active as well. Deletion of this regulatory segment in the mouse genome also resulted in a substantial loss of 1,25-(OH)2D3 and PTH regulatory capabilities and a modest increase in bone mineral density (11,44). In the present studies, we explored four additional regions within the human RANKL gene for their ability to mediate the RANKL-inducing activity of 1,25-(OH)2D3. These additional regions in the human gene were located 20 (hRLD1), 25 (hRLD2), 75 (hRLD3), and 87 (hRLD4) kb upstream of the RANKL TSS and identified based upon their high degree of conservation within the mRLD1–mRLD4 regions of the mouse gene. As assessed by ChIP, 1,25-(OH)2D3 promoted strong binding of the VDR to hRLD1 and weak binding to hRLD2-hRLD5 and to the TSS; the latter activities were similar to that seen previously at the hRLD5 region (24). VDR activity at the TSS may reflect earlier research that supports the presence of a VDRE at +33 in the mouse gene, although in our hands, this general region failed to mediate significant 1,25-(OH)2D3 response even in the context of its natural Rankl promoter. Importantly, the binding of the receptor to hRLD1 correlated kinetically with the recruitment of RNA pol II. Importantly, VDR binding also correlated generally with the capacity of these regions to mediate transcriptional responses to 1,25-(OH)2D3 when cloned upstream of the TK promoter and transfected into MG63 cells. Perhaps most important, the validity of the VDR localization studies determined by ChIP and through the transfection studies was confirmed as accomplished previously for hRLD5 (24) through the discovery of specific, functionally active VDREs in both the RLD1 and RLD2 regions of the human and mouse genes. Collectively, these studies strengthen our original observation that multiple enhancer elements located at significant distances upstream of the mouse Rankl gene play important regulatory roles in gene expression and indicate a similar overall organization with regard to the human RANKL gene.
The VDREs located in the human RANKL gene at hRLD1, hRLD2, and hRLD5, although structurally typical of classic VDR/RXR binding sites, are all unique. In the hRLD5 region, two immediately adjacent VDREs comprise the functional element; in hRLD1 and hRLD2, however, the VDREs are comprised of single elements. Additional half-sites are present in the near vicinity of the hRLD1 and hRLD2 VDREs, however, suggesting that additional complexity at these sites is possible. As with many VDRE sequences, none are capable of mediating activation when isolated directly from their immediate native sequence environment, providing indirect evidence for the presence of additional cis acting elements within hRLD1 and hRLD2 capable of facilitating 1,25-(OH)2D3 response. The recovery of 1,25-(OH)2D3 response in the hRLD2 region of the mouse gene through further fragmentation and subcloning also highlights the reverse: that negative regulatory contributions/elements within a local environment can also lead to the abrogation of inducible activity. Whether this is also the case for RLD3 and RLD4 in both the mouse and human genes will require additional examination. It is worth noting that our approach for examining the transcriptional capabilities of these enhancers entails substantial dissection of the hRANKL gene. This is due entirely to the nature of the regulatory elements themselves, which are both multiple and located at substantial distances from the hRANKL TSS. Full validation of this approach will require specific and cumulative site-directed mutagenesis in the context of the natural gene within MG63 or other human osteoblastic cell lines or examination of this locus in transgenic mice (11,44). Regardless, the identification of specific binding sites for the VDR at RLD1 and RLD2, together with our previous work at RLD5 provides strong evidence that these enhancers function as direct mediators of 1,25-(OH)2D3 response.
Surprisingly, although the five enhancers of the RANKL genes were generally conserved in function across the two species, several striking differences in their properties were observed. For example, although mRLD5 appeared to be the most active in mediating the 1,25-(OH)2D3 response in the mouse gene, that feature in the human gene was manifested by hRLD1. Thus, hRLD1 in the human gene was both a primary target for VDR and fully capable of mediating a striking transcriptional response when cloned into a luciferase reporter. Interestingly, although each of the five RANKL enhancers in the mouse gene functioned as recruitment centers for RNA pol II, this activity was restricted in the human gene to the hRLD1 region. Finally, although the effects of 1,25-(OH)2D3 on H4 acetylation in the mouse gene were extensive, ranging from the proximal promoter to over 100 kb upstream, enhanced acetylation of H4 in response to 1,25-(OH)2D3 in the human gene occurred only at the hRLD1 region. These results suggest that although similar in some respects, substantial differences in the general mechanisms of activation between human and mouse RANKL are apparent. It is possible, however, that some of these observed differences may be due to dissimilarities in the cellular background in which these activation processes were evaluated. Thus, although the mouse gene was routinely examined in a mouse osteoblastic stromal cell line, regulation of the human gene was explored in a human osteoblastic cell line derived from an osteosarcoma. In that vein, although both mouse and human RANKL constructs display significant induction by protein kinase A inducers such as cAMP and forskolin in the mouse ST2 cell line, neither are responsive to these agents in the MG63 cell line (data not shown). More studies will be necessary to determine whether the differences that we have observed here between the mouse and human RANKL genes are due to the genes themselves or to specific properties of the cells in which they are being examined. It is entirely possible, however, that a combination of both is responsible.
The above results highlight a central role for the hRLD1 enhancer in the regulation of human RANKL by 1,25-(OH)2D3. This region together with hRLD5 also appears to mediate the actions of glucocorticoids on RANKL induction. Because the activities are additive in nature, it seems likely that the two mechanisms are not directly linked. Although not shown, additional studies (Nerenz, R. D., and J. W. Pike, unpublished results) support the ability of the mouse RLD1 segment to mediate a glucocorticoid response as well. Thus, both RLD1 and RLD5 in the mouse and human genes retain important similarities. Current studies are focused on defining additional features of Rankl regulation in the mouse gene. Thus, it will be interesting to determine how many of these features can be extended to the human gene and at what sites and in what capacity. Finally, we also observed and confirmed that small amounts of both VDR and RXR could be found associated with the hRLD1 region of the human RANKL gene in the absence of added ligand. Although this has been suggested in past studies (45,46,47,48), our ability to negatively influence these putative VDR- and RXR-generated signals by reducing VDR expression using VDR siRNA provides significant proof, we believe, for the presence of VDR and RXR at VDREs in the absence of ligand. Whether the VDR bound to this site in the absence of ligand manifests a unique functional activity will need to be examined.
In conclusion, the present work confirms our previous discovery of five regulatory regions responsible for mediating the transcriptional response of mouse Rankl to 1,25-(OH)2D3 and provides insight into regulation of the human gene. As in the mouse, the bulk of these regions bind the VDR by ChIP analysis and mediate transcriptional activity in response to 1,25-(OH)2D3 after transfection into osteoblastic cells. In contrast to the regulation of the mouse Rankl gene, however, the human gene seems to be primarily modulated by the most proximal D1 region. Current studies are focused upon delineating additional features of this region of the human gene and defining its specific role in the regulation of RANKL gene expression.
MATERIALS AND METHODS
Reagents
General biochemicals were obtained from Fisher Scientific (Pittsburgh, PA) and Sigma Chemical Co. (St. Louis, MO). 1,25-(OH)2D3 was obtained from Solvay (da Weesp, The Netherlands) and Tetrionics, Inc. (Madison, WI). DMEM and MEM-α were obtained from Invitrogen Corp. (Carlsbad, CA). Oligonucleotide primers were obtained from IDT (Coralville, IA). Anti-VDR (Sc-1008) and anti-RXR (Sc-774) antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-acetyl H4 antibody (06-866) was obtained from Upstate (Charlottesville, VA), and anti-RNA pol II antibody (8WG16) was obtained from Covance Research Products (Dover, PA). TransIT-siQUEST was obtained from Mirus Bio Corp. (Madison, WI). Lipofectamine Plus was obtained from Invitrogen Corp. (Carlsbad, CA). [γ-32P]dATP was obtained from NEN Life Science Products, Inc. (Boston, MA). Dex (D4902) and anti-rat IgG (R5128) were purchased from Sigma.
Cell Culture
Mouse ST2 osteoblastic cells were cultured in MEM-α, and human osteosarcoma MG63 cells were grown in DMEM supplemented with 1% nonessential amino acids. Each medium was supplemented with 10% fetal bovine serum (FBS) obtained from HyClone (Logan, UT), 100 U/ml penicillin, and 100 μg/ml streptomycin. All ligands were added in ethanol at a final vehicle concentration of 0.1%.
RNA Isolation and Analysis
Total RNA was isolated from cells using TRI Reagent obtained from MRC (Cincinnati, OH). Isolated RNA was reverse transcribed using the SuperScript III RNase H reverse transcriptase kit from Invitrogen and then subjected to PCR analysis using qPCR methods. Primers used include those for the human β-actin gene (hβ-actin) (forward, TTAGTTGCGTTACACCCTTTC; reverse, GTCACCTTCACCGTTCCAGTT), hCYP24A1 (forward, CTTTGCTTCCTTTTCCCAGAAT; reverse, CGCCGTAGATGTCACCAGTC), and hRANKL (forward, CCCATAAAGTGAGTCTGTCC; reverse, CAATACTTGGTGCTTCCTCC).
ChIP Assay
ChIP assays were performed as previously described (49). Primer sets used for amplifying human RANKL gene regions of interest are available upon request.
Quantitative PCR Analysis
Real-time PCR was carried out on an Applied Biosystems 7500 Fast Real-Time PCR System using Sybr Green master mix from Applied Biosystems (Foster City, CA) according to standard PCR protocol. Standard curves were obtained by diluting PCR products made by traditional PCR methods. ChIP data for specific proteins were normalized to input data for corresponding samples.
Plasmids
Full-length hVDR and hRXRα were cloned into the pET-29b vector obtained from Novagen (Darmstadt, Germany) and expressed with C-terminal His6 tags. The pCH110-β-galactosidase (pCH110-βgal) reporter plasmid and the pcDNA-hVDR were previously described (50). The parent TK luciferase (luc) vector pTK-luc was used in subsequent cloning efforts. pTK-hRLD1 (−20.8 to −19.4), pTK-hRLD2 (−25.5 to −24.8), pTK-hRLD3 (−75.1 to −74.8), and pTK-hRLD4 (−87.5 to −86.9) were amplified using primers that contained HindIII/BamHI restriction ends and then cloned into the corresponding sites within the pTK-luc vector. Sequences of cloning primers are available upon request. Mutations in the hRLD1 and hRLD2 VDRE half-sites were created using the QuikChange mutagenesis kit from Stratagene (San Diego, CA). All plasmid constructs were sequenced to verify successful cloning.
Transfection Assays
Human MG63 cells or mouse ST2 cells were seeded into 24-well plates at appropriate densities and cultured in DMEM or MEM-α, respectively, containing 10% FBS. Cells were transfected 24 h later with Lipofectamine Plus in serum- and antibiotic-free medium. Individual wells were transfected with 250 ng of a luciferase reporter vector, 50 ng pCH110-βgal, and 50 ng pcDNA-hVDR (which was routinely transfected with all luciferase vectors unless otherwise indicated). After transfection, the cells were cultured for 24 h with or without 1,25-(OH)2D3 in a medium supplemented with 10% FBS. Cells were then harvested, and the lysates were assayed for luciferase and β-galactosidase activities as previously described (50). Luciferase activity was normalized to β-galactosidase values in all cases.
siRNA Suppression Studies
MG63 cells were seeded into 10-cm plates and transfected with either nontargeting siRNA (D-001206-13; Dharmacon, Lafayette, CO) or human VDR-specific siRNA (Dharmacon D-003448-01) at a final concentration of 50 nm for 48 h using Mirus TransIT-siQUEST transfection reagent. Treatment with VDR siRNA reduced the level of VDR mRNA by approximately 50%. ChIP assay was carried out as described above using antibodies against VDR, RXR, or a nonspecific IgG. Immunoprecipitated DNA was purified and analyzed using quantitative PCR.
Protein Purification
Human VDR and RXRα proteins were produced using the bacterial expression vectors pET-hVDR and pET-hRXRα in BL21(DE3) codon plus RIL cells obtained from Stratagene. Soluble full-length hVDR and hRXRα proteins were purified to homogeneity using sequential Ni-nitriloacetic acid and SP-Sepharose column chromatography (50). Two forms of RXRα were present due to redundant start sites.
DNA Bandshift Analysis
The duplex oligonucleotide probes comprised of the hRL-D1 VDRE, D1M1 VDRE, D1M2 VDRE, D1M3 VDRE, D1M4 VDRE, hRL-D2 VDRE, D2M1 VDRE, D2M2 VDRE, and D2M3 VDRE were end labeled using [γ-32P]dATP. Probes were incubated at room temperature with the indicated concentrations of hVDR and hRXRα in 10 mm HEPES (pH 7.4), 1 mm EDTA, 5 mm MgCl2, 10% glycerol, 0.5 mm dithiothreitol, 0.7 mm phenylmethylsulfonyl fluoride, 150 mm KCl, and 0.45 μg poly-dIdC in the presence of 1,25-(OH)2D3 for 30 min. Complexes were resolved on nondenaturing 6% polyacrylamide gels, dried, and then visualized using autoradiography. Previous studies have validated the resulting shifted complex as being comprised of a trimeric VDR/RXR/VDRE complex (24).
Supplementary Material
Acknowledgments
We thank members of the Pike laboratory for their helpful discussions and additional contributions to this work.
Footnotes
This work was supported by grants to J.W.P. from the National Institutes of Health (DK-74993) and from the Robert Draper Technical Innovation Fund at the University of Wisconsin.
Disclosure Statement: The authors state that they have nothing to declare.
First Published Online January 17, 2008
Abbreviations: ChIP, Chromatin immunoprecipitation; Dex, dexamethasone; FBS, fetal bovine serum; H4, histone 4; NF, nuclear factor; 1,25-(OH)2D3, 1,25-dihydroxyvitamin D3; RANKL, receptor activator of nuclear factor-κB ligand; RNA pol II, RNA polymerase II; RXR, retinoid X receptor; siRNA, small interfering RNA; TK, thymidine kinase; TSS, transcriptional start site; VDR, vitamin D receptor; VDRE, vitamin D response element.
References
- Manolagas SC 2000 Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137 [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL 2000 Bone resorption by osteoclasts. Science 289:1504–1508 [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ 1999 Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20:345–357 [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ 1998 Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176 [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Ross FP 2003 Genetic regulation of osteoclast development and function. Nat Rev Genet 4:638–649 [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T 2002 Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901 [DOI] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM 1999 OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323 [DOI] [PubMed] [Google Scholar]
- Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ 1999 Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 96:3540–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Ueno Y, Fujii K, Shinki T 2003 Vitamin D and bone. J Cell Biochem 88:259–266 [DOI] [PubMed] [Google Scholar]
- Kondo H, Guo J, Bringhurst FR 2002 Cyclic adenosine monophosphate/protein kinase A mediates parathyroid hormone/parathyroid hormone-related protein receptor regulation of osteoclastogenesis and expression of RANKL and osteoprotegerin mRNAs by marrow stromal cells. J Bone Miner Res 17:1667–1679 [DOI] [PubMed] [Google Scholar]
- Fu Q, Manolagas SC, O'Brien CA 2006 Parathyroid hormone controls receptor activator of NF-κB ligand gene expression via a distant transcriptional enhancer. Mol Cell Biol 26:6453–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer LC, Gori F, Riggs BL, Lacey DL, Dunstan CR, Spelsberg TC, Khosla S 1999 Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology 140:4382–4389 [DOI] [PubMed] [Google Scholar]
- Wani MR, Fuller K, Kim NS, Choi Y, Chambers T 1999 Prostaglandin E2 cooperates with TRANCE in osteoclast induction from hemopoietic precursors: synergistic activation of differentiation, cell spreading, and fusion. Endocrinology 140:1927–1935 [DOI] [PubMed] [Google Scholar]
- Karst M, Gorny G, Galvin RJ, Oursler MJ 2004 Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-β regulation of osteoclast differentiation. J Cell Physiol 200:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist P, Persson E, Conaway HH, Lerner UH 2002 IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-κB ligand, osteoprotegerin, and receptor activator of NF-κB in mouse calvariae. J Immunol 169:3353–3362 [DOI] [PubMed] [Google Scholar]
- Romas E, Udagawa N, Zhou H, Tamura T, Saito M, Taga T, Hilton DJ, Suda T, Ng KW, Martin TJ 1996 The role of gp130-mediated signals in osteoclast development: regulation of interleukin 11 production by osteoblasts and distribution of its receptor in bone marrow cultures. J Exp Med 183:2581–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivagurunathan S, Muir MM, Brennan TC, Seale JP, Mason RS 2005 Influence of glucocorticoids on human osteoclast generation and activity. J Bone Miner Res 20:390–398 [DOI] [PubMed] [Google Scholar]
- Raisz LG 2005 Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115:3318–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL 2000 The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 15:2–12 [DOI] [PubMed] [Google Scholar]
- Pike JW, Shevde NK 2005 The vitamin D receptor. In: Feldman D, Pike JW, Glorieux FH, eds. Vitamin D. 2nd ed. San Diego: Elsevier; 167–191 [Google Scholar]
- DeLuca HF 2004 Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80:1689S–1696S [DOI] [PubMed] [Google Scholar]
- Rachez C, Freedman LP 2000 Mechanisms of gene regulation by vitamin D3 receptor: a network of coactivator interactions. Gene 246:9–21 [DOI] [PubMed] [Google Scholar]
- Freedman LP, Reszka AA 2005 Vitamin D receptor cofactors: function, regulation, and selectivity. In: Feldman D, Pike JW, Glorieux FH, eds. Vitamin D. 2nd ed. San Diego: Elsevier; 263–274 [Google Scholar]
- Kim S, Yamazaki M, Zella LA, Shevde NK, Pike JW 2006 Activation of receptor activator of NF-κB ligand gene expression by 1,25-dihydroxyvitamin D3 is mediated through multiple long-range enhancers. Mol Cell Biol 26:6469–6486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I 2004 VISTA: computational tools for comparative genomics. Nucleic Acids Res 32:W273–W279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabe Y, Yamada J, Uga H, Yamaguchi Y, Wada T, Handa H 2005 NF-Y is essential for the recruitment of RNA polymerase II and inducible transcription of several CCAAT box-containing genes. Mol Cell Biol 25:512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW 2006 The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol 20:1447–1461 [DOI] [PubMed] [Google Scholar]
- Szutorisz H, Dillon N, Tora L 2005 The role of enhancers as centres for general transcription factor recruitment. Trends Biochem Sci 30:593–599 [DOI] [PubMed] [Google Scholar]
- Liu Y, Xia X, Fondell JD, Yen PM 2006 Thyroid hormone-regulated target genes have distinct patterns of coactivator recruitment and histone acetylation. Mol Endocrinol 20:483–490 [DOI] [PubMed] [Google Scholar]
- Zella LA, Kim S, Shevde NK, Pike JW 2006 Enhancers located within two introns of the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D3. Mol Endocrinol 20:1231–1247 [DOI] [PubMed] [Google Scholar]
- Fretz JA, Zella LA, Kim S, Shevde NK, Pike JW 2006 1,25-Dihydroxyvitamin D3 regulates the expression of low-density lipoprotein receptor-related protein 5 via deoxyribonucleic acid sequence elements located downstream of the start site of transcription. Mol Endocrinol 20:2215–2230 [DOI] [PubMed] [Google Scholar]
- Chiba N, Suldan Z, Freedman LP, Parvin JD 2000 Binding of liganded vitamin D receptor to the vitamin D receptor interacting protein coactivator complex induces interaction with RNA polymerase II holoenzyme. J Biol Chem 275:10719–10722 [DOI] [PubMed] [Google Scholar]
- Kim S, Yamazaki M, Shevde NK, Pike JW 2007 Transcriptional control of receptor activator of nuclear factor-κB ligand by the protein kinase A activator forskolin and the transmembrane glycoprotein 130-activating cytokine, oncostatin M, is exerted through multiple distal enhancers. Mol Endocrinol 21:197–214 [DOI] [PubMed] [Google Scholar]
- Emerson BM 2002 Specificity of gene regulation. Cell 109:267–270 [DOI] [PubMed] [Google Scholar]
- Narlikar GJ, Fan HY, Kingston RE 2002 Cooperation between complexes that regulate chromatin structure and transcription. Cell 108:475–487 [DOI] [PubMed] [Google Scholar]
- Sandelin A, Wasserman WW, Lenhard B 2004 ConSite: web-based prediction of regulatory elements using cross-species comparison. Nucleic Acids Res 32:W249–W252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimi E, Akiyama S, Tsurukai T, Okahashi N, Kobayashi K, Udagawa N, Nishihara T, Takahashi N, Suda T 1999 Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J Immunol 163:434–442 [PubMed] [Google Scholar]
- Sobacchi C, Frattini A, Guerrini MM, Abinun M, Pangrazio A, Susani L, Bredius R, Mancini G, Cant A, Bishop N, Grabowski P, Del Fattore A, Messina C, Errigo G, Coxon FP, Scott DI, Teti A, Rogers MJ, Vezzoni P, Villa A, Helfrich MH 2007 Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet 39:960–962 [DOI] [PubMed] [Google Scholar]
- Lucas GJ, Daroszewska A, Ralston SH 2006 Contribution of genetic factors to the pathogenesis of Paget's disease of bone and related disorders. J Bone Miner Res 21(Suppl 2):P31–P37 [DOI] [PubMed] [Google Scholar]
- Whyte MP 2006 Paget's disease of bone and genetic disorders of RANKL/OPG/RANK/NF-κB signaling. Ann NY Acad Sci 1068:143–164 [DOI] [PubMed] [Google Scholar]
- Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, Schwarz T, Penninger JM, Beissert S 2006 Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med 12:1372–1379 [DOI] [PubMed] [Google Scholar]
- Tintut Y, Demer L 2006 Role of osteoprotegerin and its ligands and competing receptors in atherosclerotic calcification. J Investig Med 54:395–401 [DOI] [PubMed] [Google Scholar]
- Kiechl S, Werner P, Knoflach M, Furtner M, Willeit J, Schett G 2006 The osteoprotegerin/RANK/RANKL system: a bone key to vascular disease. Expert Rev Cardiovasc Ther 4:801–811 [DOI] [PubMed] [Google Scholar]
- Galli C, Zella LA, Fretz JA, Fu Q, Pike JW, Weinstein RS, Manolagas SC, O'Brien CA 2007 Targeted deletion of a distant transcriptional enhancer of the RANKL gene reduces bone remodeling and increases bone mass. Endocrinology 149:146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheskis B, Freedman LP 1994 Ligand modulates the conversion of DNA-bound vitamin D3 receptor (VDR) homodimers into VDR-retinoid X receptor heterodimers. Mol Cell Biol 14:3329–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross TK, Darwish HM, Moss VE, DeLuca HF 1993 Vitamin D-influenced gene expression via a ligand-independent, receptor-DNA complex intermediate. Proc Natl Acad Sci USA 90:9257–9260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo AA, Paredes R, Garcia VM, Flynn G, Johnson CS, Trump DL, Onate SA 2007 Altered VDR-mediated transcriptional activity in prostate cancer stroma. J Steroid Biochem Mol Biol 103:731–736 [DOI] [PubMed] [Google Scholar]
- Griffin MD, Dong X, Kumar R 2007 Vitamin D receptor-mediated suppression of RelB in antigen presenting cells: a paradigm for ligand-augmented negative transcriptional regulation. Arch Biochem Biophys 460:218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Shevde NK, Pike JW 2005 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J Bone Miner Res 20:305–317 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Shevde NK, Warrier A, Plum LA, DeLuca HF, Pike JW 2003 2-Methylene-19-nor-(20S)-1,25-dihydroxyvitamin D3 potently stimulates gene-specific DNA binding of the vitamin D receptor in osteoblasts. J Biol Chem 278:31756–31765 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.