Abstract
4-Methylimidazole (4MI) is used in the manufacture of pharmaceuticals, photographic chemicals, dyes and pigments, cleaning and agricultural chemicals, and rubber. It has been identified as a by-product of fermentation in foods and has been detected in mainstream and side stream tobacco smoke. 4MI was studied because of its high potential for human exposure. Groups of 50 male and 50 female F344/N rats were fed diets containing 0-, 625-, 1,250-, or 2,500-ppm 4MI (males) or 0-, 1,250-, 2,500-, or 5,000-ppm 4MI (females) for 106 weeks. Based on the food consumption the calculated average daily doses were approximately 30, 55, or 115 mg 4MI/kg body weight to males and 60, 120, or 250 mg 4MI/kg to females. Survival of all exposed groups of males and females was similar to that of the control groups. Mean body weights of males in the 1,250- and 2,500-ppm groups and females in the 2,500- and 5,000-ppm groups were less than those of the control groups throughout the study. Feed consumption by 5,000-ppm females was less than that by the controls. Clonic seizures, excitability, hyperactivity, and impaired gait were observed primarily in 2,500- and 5,000-ppm females. The incidence of mononuclear cell leukemia in the 5,000-ppm females was significantly greater than that in the controls. The incidences of hepatic histiocytosis, chronic inflammation, and focal fatty change were significantly increased in all exposed groups of male and female rats. The incidences of hepatocellular eosinophilic and mixed cell foci were significantly increased in 2,500-ppm males and 5,000-ppm females.
Groups of 50 male and 50 female B6C3F1 mice were fed diets containing 0-, 312-, 625-, or 1,250-ppm 4MI for 106 weeks. Based on the food consumption the calculated average daily doses were approximately 40, 80, or 170 mg 4MI/kg body weight to males and females. Survival of all exposed groups of males and females was similar to that of the control groups. Mean body weights of males and females in the 1,250-ppm groups and that in the 312- and 625-ppm females were less than those of the control groups. Feed consumption by exposed groups of male and female mice was similar to that by the controls. The incidences of alveolar/bronchiolar adenoma in all exposed groups of females, alveolar/bronchiolar carcinoma in 1,250-ppm males, and alveolar/bronchiolar adenoma or carcinoma (combined) in 1,250-ppm males and 625- and 1,250-ppm females were significantly greater than those in the control groups. The incidence of alveolar epithelial hyperplasia was significantly increased in the 1,250-ppm females.
4MI is carcinogenic inducing alveolar/bronchiolar adenoma and carcinoma in male and female mice. 4MI may also induce mononuclear cell leukemia in female rats.
Keywords: 4-Methylimidazole, Toxicity, Carcinogenicity, Rats, Mice
INTRODUCTION
4-Methylimidazole (4MI) is formed by interaction of ammonia with reducing sugars, and has been identified in ammoniated hay forage for livestock animals (Morgan and Edwards 1986a; Perdok and Leng 1987; Ray et al. 1984). Ammoniation of carbohydrate-containing material including hay to increase non-protein nitrogen content in feeds is a common practice in farms. The compound has also been identified in milk from cows fed ammoniated forage (Morgan and Edwards 1986b; Perdok and Leng 1987), in several food products including caramel coloring, soy sauce, Worcestershire sauce, wine, ammoniated molasses, and caramel-colored syrups (Huang et al. 1983; Mattyasovszky and Jeszenszky 1985; Wong and Bernhard 1988; Yoshikawa and Fujiwara 1981), and in mainstream and side-stream cigarette smoke (Sakuma et al. 1984; Moree-Testa et al. 1984).
4MI is produced commercially by cyclocondensation of aldehyde and ammonia with methylglyoxal. Production figures for the compound are not available. 4MI is used as a chemical intermediate, starting material, or component in the manufacture of pharmaceuticals, photographic, and agricultural, and photothermographic chemicals, dyes and pigments, agricultural chemicals, and rubber. It has been investigated for use as a starting material in the synthesis of cardiovascular stimulants, epoxy resin, anticholesteremics, neurotransmitter antagonists, disinfectants, antiprotozoal antiseptic agents, and as an aromatase-inhibiting antineoplastic agent. The chemical is also used as a component in imidazole-phenoxyalkanal oven cleaners, crosslinking agent for epoxy resin hardeners, corrosion inhibitor for cooling water in heat-exchange apparatus, component of absorbent to remove acid gases from hydrocarbon or synthesis gas, and starting material for inks and paper dyes (Chemical Dynamics Corp. 1989; Chemical Economics Handbook 1995).
Animals exposed to 4MI exhibited convulsant activity including restlessness, bellowing, frothing at the mouth, and paralysis (Wiggins 1956). Convulsant activity has been observed in cattle fed ammoniated molasses (Morgan and Edwards 1986a; Nishie et al. 1969) and in calves nursing from cows fed ammoniated hay (Fairbrother et al. 1987; Perdok and Leng 1987; Weiss et al. 1986). Ewes fed ammoniated hay showed facial twitching and general body tremors initially, followed by opisthotonus and convulsion (Weiss et al. 1986). In goats and heifers 4MI administered intravenously induced coughing, salivation, urination, defecation, and convulsions or clonic seizure (Nielsen et al. 1993). Neurologic effects similar to those in cattle have been observed in mice administered 4MI (Nishie et al. 1969; 1970). No published human toxicity data of 4MI were found in the literature. No standards or guidelines have been set for exposures to 4MI in the U.S. (Chappel and Howell 1992).
The National Toxicology Program (NTP, 2006) reported that 4MI was not mutagenic in the S. typhimurium mutation assay when tested in strains TA97, TA98, TA100, and TA1535, with and without hamster or rat liver metabolic-activation enzymes. No increases in the frequencies of micronucleated erythrocytes were seen in the bone marrow of male rats or mice treated with 4MI by intraperitoneal injection, or in peripheral blood samples from male and female mice administered the compound in dosed feed for 14 weeks.
Toxicity and carcinogenesis studies of 4MI were conducted because of widespread human exposure and lack of toxicity data. Dosed feed was chosen as the route of exposure because ingestion is the most common route by which humans are exposed. Fifteen-day and 14-week toxicity studies of 4MI in F344/N rats and B6C3F1 mice have been reported (Chan et al. 2006; NTP, 2004a). The present paper reports the 2-year dosed-feed studies of 4MI in F344/N rats and B6C3F1 mice.
MATERIALS AND METHODS
Chemical
4MI (CAS Reg No: 822-36-6) was obtained from Sigma Chemical Company (St. Louis, MO). The chemical was identified as 4MI by infrared, ultraviolet/visible, and proton and carbon-13 nuclear magnetic resonance spectroscopy and melting point determination. The purity determined by elemental analyses, functional-group titration, gas chromatography, and high-performance liquid chromatography was greater than 99%. The stability of the bulk chemical as determined by gas chromatography was at least 14 days at 60° C. The bulk chemical was stored at 5° C in Teflon-sealed containers, protected from light and moisture. The dose formulations were prepared every 2 weeks by mixing 4MI with feed. Homogeneity was confirmed using HPLC; and the dose formulations were stable for at least 36 days. Details of the analyses were reported (NTP, 2006).
Study Design
Groups of 50 male F344/N rats were fed diets containing 0-, 625-, 1250-, or 2,500-ppm 4MI, and groups of 50 female rats were fed diets containing 0-, 1,250-, 2,500-or 5,000-ppm 4MI for 106 weeks. Groups of 50 male and 50 female B6C3F1 mice were fed diets containing 0-, 312-, 625-, or 1,250-ppm 4MI for 106 weeks. The animals (6 weeks old) were purchased from the Taconic Farms, Inc, Germantown, NY. The top dose levels were selected for male and female rats and mice because at these dose levels the survival, body weight, hematology, clinical chemistry, and histopathology findings in the 14-week studies showed no differences from those of controls (NTP, 2004; Chan et al., 2006). The animals were housed three male rats per cage, male mice individually, and female rats and mice five per cage. The animals were kept in a humidity- (50% ±15%), temperature- (72°±3°), and light- (12-hours cycle) controlled room.
The animals were observed twice daily. Body weights were recorded weekly for the first 13 weeks, and every 4 weeks thereafter, and at the end of the studies. Complete necropsies were performed at 106 weeks, and tissues were fixed in 10% neutral buffered formalin, stained with hematoxylin and eosin (H & E), and examined microscopically.
Statistical Evaluation
The probability of survival was estimated by the product-limit procedure of Kaplan and Meier (1958). Statistical analyses for possible dose-related effects on survival used Cox’s (1972) method for testing two groups for equality and Tarone’s (1975) life table test to identify dose-related trends. The poly-k test (Bailer and Portier 1988; Piegorsch and Bailer 1997; Portier and Bailer 1989) was used to assess the prevalence of neoplasm and nonneoplastic lesions prevalence. Continuity-corrected Poly-3 tests were used in the analysis of lesion incidence, and reported P values are one sided. Average severity values were analyzed for significance with the Mann-Whitney U test (Hollander and Wolfe 1973).
RESULTS
Rat
Survival of all exposed groups of male and female rats was similar to that of the control groups. Mean body weights of males in the 1,250- and 2,500-ppm groups and females in the 2,500- and 5,000-ppm groups were less than those of the control groups (95%, 87%, 81%, 65%, respectively), at the end of the study.
Feed consumption by exposed groups of males was generally similar to that by the controls. However, feed consumption by 5,000-ppm females was less than that by the controls. Based on the food consumption the calculated average daily doses were approximately 30, 55, or 115 mg 4MI/kg body weight to males and 60, 120, or 250 mg 4MI/kg to females.
Clonic seizures, excitability, hyperactivity, and impaired gait were observed in 5,000-pm females; some of these clinical findings were also observed in the lower exposed groups at greater frequencies than in the controls (Table 1). However, there were no histopathology lesions observed in the brains of animals exposed to 4MI.
Table 1.
Neurological Clinical Findings in Female Rats in the 2-Year Feed Study of 4-Methylimidazolea.
| 0 ppm | 1,250 ppm | 2,500 ppm | 5,000 ppm | |
|---|---|---|---|---|
| Clonic Seizures | 0/50a | 0/50 | 21/50 | 36/50 |
| Excitability | 0/50 | 2/50 | 9/50 | 50/50 |
| Hyperactivity | 0/50 | 0/50 | 0/50 | 5/50 |
| Impaired Gait | 0/50 | 0/50 | 4/50 | 49/50 |
Number of rats with clinical finding per number of rats in the exposure group
The incidence of mononuclear cell leukemia in 5,000-ppm females was significantly greater than that in the controls, and the incidence slightly exceeded the historical range in feed study controls given NTP-2000 diet (Table 2).
Table 2.
Incidences of Mononuclear Cell Leukemia in Rats in the 2-Year Feed Study of 4-Methylimidazole.
| Males | 0 ppm | 625 ppm | 1,250 ppm | 2,500 ppm |
|---|---|---|---|---|
| Mononuclear Cell Leukemiaa | 15/50b (30%) | 18/50 (36%) | 22/50 (44%) | 20/50 (40%) |
| Females | 0 ppm | 1,250 ppm | 2,500 ppm | 5,000 ppm |
| Mononuclear Cell Leukemiac | 9/50 (18%) | 7/50 (14%) | 16/50 (32%) | 20/50d (40%) |
Historical incidence for 2-year feed study controls given NTP-2000 diet (mean ± standard deviation): 246/510 (46.8% ± 13.0%); range, 30%-68%
Number of animals with neoplasm per number of animals necropsied
Historical incidence: 121/510 (23.8% ± 9.1%); range, 12%-38%
P < 0.05
The incidences of histiocytosis were significantly greater in all exposed groups of male and female rats than in the control groups, and the severities increased with increasing exposure concentrations (Table 3). This lesion was characterized by focal to multifocal clusters of enlarged histiocytes with prominent foamy cytoplasm often containing vacuoles and/or cleft-like spaces. The contents of the cleft-like spaces could not be determined. Occasional syncytial cells were present. In control animals the histiocytes occurred primarily as scattered individual cells and rarely as clusters. The cytoplasm of these cells was foamy, and cleft-like spaces were absent. Incidences of chronic inflammation of the liver in all exposed groups of rats were significantly greater than those in the controls (Table 3). Microscopically, there were small, focal accumulations of macrophages with granular cytoplasm surrounded by variable numbers of lymphoid cells within the hepatic parenchyma. There were significant increases occurring in the incidences of hepatocytic focal fatty change in the 1,250- and 2,500-ppm males and all exposed groups of females, and the severities increased with increasing exposure concentration (Table 3). In the 2,500-ppm males and 5,000-ppm females, the incidence of esosinophilic and mixed cell foci (mixture of eosinophilic and clear cells in which no one cell type exceeds 80%) were significantly increased. No treatment-related increase in clear cell foci were noted in the males treated groups. In females, there was a significant increase in the incidence of clear cell focus in the 625-ppm group. In general, these foci consisted of enlarged hepatocytes with altered tinctorial characteristics of the cytoplasm that usually did not compress the surrounding parenchyma. Occasionally, larger foci were multilobular, contained biliary structures, and compressed the adjacent liver, but there was no loss of normal hepatic architecture.
Table 3.
Incidences of Nonneoplastic Lesions of the Liver in Rats in the 2-Year Feed Study of 4-Methylimidazole.
| Males | 0 ppm | 625 ppm | 1,250 ppm | 2,500 ppm |
|---|---|---|---|---|
| Number Examined Microscopically | 50 | 50 | 50 | 50 |
| Histiocytosisa | 38 (1.1)b | 45* (1.4) | 50** (1.9) | 50** (2.3) |
| Inflammation, Chronic | 18 (1.1) | 32** (1.2) | 31** (1.3) | 36** (1.3) |
| Hepatocyte, Fatty Change, Focal | 21 (1.5) | 24 (1.8) | 37** (1.9) | 33** (2.5) |
| Eosinophilic Focus | 4 | 3 | 7 | 12* |
| Mixed Cell Focus | 5 | 7 | 11 | 27** |
| Females | 0 ppm | 1,250 ppm | 2,500 ppm | 5,000 ppm |
| Number Examined Microscopically | 50 | 50 | 48 | 50 |
| Histiocytosis | 40 (1.0) | 50** (1.3) | 48** (1.9) | 50** (2.5) |
| Inflammation, Chronic | 17 (1.2) | 28* (1.5) | 34** (1.8) | 35** (1.7) |
| Hepatocyte, Fatty Change, Focal | 16 (1.2) | 29** (1.6) | 29** (2.0) | 32** (2.2) |
| Clear Cell Focus | 20 | 32** | 23 | 27 |
| Eosinophilic Focus | 1 | 2 | 5 | 11** |
| Mixed Cell Focus | 10 | 7 | 6 | 18* |
Number of animals with lesion
Average severity grade of lesions in affected animals: 1=minimal, 2=mild, 3=moderate, 4=marked
Significantly different (P < 0.05) from the control group by the Poly-3 test
P < 0.01
There were statistically significant increased incidences of nonneoplastic lesions occurring in the thyroid gland, prostate gland, and pituitary gland (pars distalis) of exposed groups of male rats and in the lung, heart, pancreas, and thyroid gland of exposed groups of female rats (Table 4). In the prostate gland of 2,500-ppm males, the incidence of mild chronic inflammation was significantly increased. Interstitial and intraluminal infiltrates of small numbers of mononuclear cells characterized these lesions. In the pituitary gland (pars distalis) the incidences of focal hypertrophy in 1,250- and 2,500-ppm males were significantly greater than those in the controls. This cellular alteration was characterized by small foci of enlarged, lightly eosinophilic cells with round vesicular nuclei. In the thyroid gland of 2,500-ppm males, the incidence of follicle cyst was significantly greater than that in the controls. A few dilated follicles lined by flattened cuboidal epithelium and distended by normal colloid characterized this lesion. The incidence of follicle mineralization was significantly increased in the thyroid gland of 5,000-ppm females. This lesion consisted of one to two small basophilic (mineralized) structures within the follicular lumen. In the lung, significantly increased incidences of focal chronic inflammation occurred in all exposed groups of females. Histologically, this lesion was of minimal severity and characterized by very small, focal, subpleural accumulations of macrophages and/or mixed inflammatory cells within alveoli. In the heart, the incidences of cardiomyopathy of generally minimal severity were significantly increased in all exposed groups of females. This lesion was characterized by small focal accumulations of mononuclear cells and occasional degenerative myocardial fibers. In all exposed groups of females, there were significantly increased incidences of minimal pancreatic focal acinar atrophy occurred. Microscopically, there was a slight reduction appeared in the number of acini, which were small and lined by flattened epithelial cells with a loss of acidophilic granules.
Table 4.
Increased Incidences of Selected Nonneoplastic Lesions in Rats in the 2-Year Feed Study of 4-mMethylimidazole
| Male | 0 ppm | 625 ppm | 1,250 ppm | 2,500 ppm |
|---|---|---|---|---|
| Thyroid Glanda | 47 | 46 | 48 | 44 |
| Follicle Cystb | 0 | 3 | 1 | 5* |
| Prostate Gland | 50 | 50 | 50 | 49 |
| Inflammation, Chronic | 27 (1.7)c | 24 (2.0) | 28 (1.6) | 36* (2.0) |
| Pituitary Gland (Pars Distalis) | 49 | 49 | 48 | 48 |
| Hypertrophy, Focal | 8 (1.8) | 9 (1.6) | 20** (1.8) | 22** (2.0) |
| Female | 0 ppm | 1,250 ppm | 2,500 ppm | 5,000 ppm |
| Lung | 50 | 50 | 50 | 50 |
| Inflammation, Chronic, Focal | 25 (1.1) | 40** (1.2) | 39** (1.0) | 43** (1.2) |
| Heart | 50 | 50 | 48 | 50 |
| Cardiomyopathy | 30 (1.3) | 43** (1.5) | 38** (1.8) | 44** (1.9) |
| Pancreas | 49 | 49 | 47 | 47 |
| Acinus, Atrophy, Focal | 13 (1.3) | 22* (1.7) | 26** (1.8) | 30** (1.7) |
| Thyroid gland | 47 | 44 | 40 | 45 |
| Follicle Mineralization | 2 (1.0) | 7 (1.0) | 6 (1.0) | 19** (1.0) |
Number of animals with tissue examined microscopically
Number of animals with lesion
Average severity grade of lesions in affected animals: 1=minimal, 2=mild, 3=moderate, 4=marked
Significantly different (P < 0.05) from the control group by the Poly-3 test
P < 0.01
Statistically significant decreases occurred in the incidences of neoplasm of the adrenal medulla and pituitary gland (pars distalis) in exposed groups of males and in the pituitary gland (pars distalis), clitoral gland, mammary gland, and uterus in exposed groups of females. These incidences in the exposed groups were either below the historical control ranges or at the lower end of the historical control ranges in feed study controls given NTP-2000 diet. In the pituitary gland (pars distalis), the incidences of adenoma in 2,500-ppm males (16/49 vs. 7/48) and 1,250- and 5,000-ppm females (29/48 vs. 19/50 and 9/50) were significantly less than those in the controls. In the adrenal medulla, significantly decreased incidences of benign, complex, or malignant pheochromocytoma (combined) occurred in 1,250- and 2,500-ppm males (10/50 vs. 3/50 and 3/50). In all exposed groups of females, the incidences of clitoral gland adenoma, mammary gland fibroadenoma, and uterine stromal polyp were significantly less than those in the control group.
Mouse
Survival of all exposed groups of male and female mice was similar to that of the control groups. Mean body weights of males and females in the 1,250-ppm groups and that of the 312- and 625-ppm groups females were less than those in the control groups. Feed consumption by exposed groups of males and females was similar to that by the controls. Based on the food consumption the calculated average daily doses were approximately 40, 80, or 170 mg 4MI/kg body weight to males and females. No clinical findings in exposed groups of male or female mice were considered related to chemical exposure.
The incidences of alveolar/bronchiolar adenoma in all exposed groups of females, alveolar/bronchiolar carcinoma in 1,250-ppm males, and alveolar/bronchiolar adenoma or carcinoma (combined) in 1,250-ppm males and 625- and 1,250-ppm females were significantly greater than those in the control groups (Table 5). Adenomas were focal, generally well demarcated nodular lesions that usually compressed the surrounding parenchyma. Adenomas were composed of increased numbers of large cuboidal to polygonal epithelial cells that were arranged in abnormal patterns, most commonly as papillary structures that distorted the alveolar architecture. Carcinomas were generally larger (up to 1 centimeter in diameter) than adenomas and composed of cuboidal to columnar, and mildly to markedly pleomorphic epithelial cells. These neoplastic cells were often densely packed and formed multiple layers that showed a tendency toward solid growth. Most of the carcinomas were discrete masses that compressed the adjacent parenchyma, although some were locally invasive into the parenchyma and airways. The incidence of alveolar epithelium hyperplasia in 1,250-ppm females was significantly greater than that in the controls. This lesion was considered a morphologic continuum to adenoma and was characterized by increased numbers of cuboidal epithelial cells that lined alveoli; however, the septa architecture was well maintained. The incidence of histiocytic cellular infiltration, a lesion often associated with lung neoplasms, was significantly greater than that in the controls in 1,250-ppm females and slightly increased in 1,250-ppm males. The infiltration was characterized by small numbers of histiocytes scattered within alveolar luminae adjacent to many of the adenomas and carcinomas.
Table 5.
Incidences of Neoplasms and Nonneoplastic Lesions of the Lung in Mice in the 2-Year Feed Study of 4-Methylimidazole
| 0 ppm | 312 ppm | 625 ppm | 1,250 ppm | |
|---|---|---|---|---|
| Male | ||||
| Alveolar Epithelium, Hyperplasia | 7/50a (2.0)b | 3/50 (1.0) | 150 (2.0) | 9/50 (1.9) |
| Infiltration Cellular, Histiocyte | 5/50 (2.2) | 6/50 (1.7) | 5/50 (1.8) | 11/50 (1.7) |
| Alveolar/bronchiolar Adenoma (includes multiple) c | 8/50 (16%) | 11/50 (22%) | 13/50 (26%) | 15/50 (30%) |
| Alveolar/bronchiolar Carcinoma (includes multiple) d | 2/50 (4%) | 4/50 (8%) | 4/50 (8%) | 8/50 (16%) |
| Alveolar/bronchiolar Adenoma or Carcinoma (combined) e | 9/50 (18%) | 13/50 (26%) | 16/50 (32%) | 22/50** (44%) |
| Female | ||||
| Alveolar Epithelium, Hyperplasia | 3/50 (1.7) | 2/50 (2.5) | 3/50 (1.7) | 11/50* (1.9) |
| Infiltration Cellular, Histiocyte | 1/50 (1.0) | 5/50 (1.4) | 1/50 (1.0) | 8/50* (2.0) |
| Alveolar/bronchiolar Adenoma (includes multiple) f | 0/50 (0%) | 8/50 (16%) | 16/50 (32%) | 8/50 (16%) |
| Alveolar/bronchiolar Carcinoma (includes multiple) g | 3/50 (6%) | 0/50 (0%) | 2/50 (4%) | 7/50 (14%) |
| Alveolar/bronchiolar Adenoma or Carcinoma (combined) h | 3/50 (6%) | 8/50 (16%) | 17/50** (34%) | 14/50** (28%) |
Number of animals with lesion per number of animals with lung examined microscopically
Average severity grade of lesions in affected animals: 1=minimal, 2=mild, 3=moderate, 4=marked
Historical incidence for 2-year feed study controls given NTP-2000 diet (mean ± standard deviation): 75/510 (15.8% ± 6.3%); range, 9%-28%
Historical incidence: 40/510 (7.8% ± 3.8%); range, 4%-14%
Historical incidence: 108/510 (22.2% ± 6.3%); range, 14%-32%
Historical incidence: 19/509 (3.7% ± 3.8%); range, 0%-10%
Historical incidence: 16/509 (2.9% ± 2.5%); range, 0%-6%
Historical incidence: 35/509 (6.6% ± 4.2%); range, 0%-12%
Significantly different (P < 0.05) from the control group by the Poly-3 test
P < 0.01
The incidence of thyroid follicular cyst in 1,250-ppm females was significantly greater than that in the controls (0-ppm: 20/50; 312-ppm: 22/49; 625-ppm: 29/50; 1,250-ppm: 30/48). Histologically, the affected follicles were variably dilated, and filled with pale staining colloid, and often lined by flattened epithelial cells. This change ranged in severity from minimal to moderate. Minimal lesions affected a single follicle, while moderate lesions affected a cluster of adjacent follicles. In larger, more severe lesions, the walls of the follicles appeared to have ruptured and formed highly irregular cystic structures. These changes were usually focal but could be multifocal and occasionally bilateral in more severe cases.
Significant positive trend occurred in the incidences of mammary gland hyperplasia in females (16/50, 10/50, 14/49, 24/49; P=0.013); however, none of the dosed groups differed significantly from the control group. Microscopically, hyperplasia was usually a minimal change that was characterized by an increase in the number of mammary gland ducts with a slight increase in the degree of duct cellularity. Small cuboidal epithelial cells lined unaffected mammary ducts, while affected ducts were lined by increased numbers of larger cuboidal cells.
DISCUSSION
The highest exposure concentrations selected for the 2-year studies were 2,500-ppm for male rats, 5,000-ppm for female rats, and 1,250-ppm for male and female mice. These same exposure concentrations and species combinations in the 14-week toxicity studies exerted minimal effects on survival, hematology, clinical chemistry, organ weights, and histopathology; the final body weights of these animals relative to controls were: 95% (male rats); 94% (female rats); 93% (male mice); and 88% (female mice) (Chan et al. 2006; NTP 2004). These exposure concentrations, however, markedly reduced body-weight gains in rats and mice during the course of the 2-year studies; the effects on body weight were seen as early as week 14 in the rat study. Feed consumption was lower only in 5,000-ppm female rats. The neurobehavioral effects observed in 5,000-ppm female rats probably influenced their food intake. The body weight effects observed in rats and mice were likely attributable to the toxicity of 4MI at the highest exposure concentrations. MacKenzie et al. (1992) reported that male and female F344/N rats given caramel color IV (which contained 110 mg 4MI per kilogram body weight) in drinking water at 10 g/kg for 2 years had significantly lower body weights, but no accompanying histopathology. Tierney (1979) reported that B6C3F1 mice given caramel color IV at up to 63 g/kg body weight per day in drinking water for 4 weeks also had significantly reduced body weight gains. In these studies, the body weight effects were attributed to reduced fluid intake. The reduced body weight gain observed in the present 2-year studies may be partly due to reduced water intake; however, fluid intake was not measured in the current studies.
During the 2-year study period, female rats in the 2,500- and 5,000-ppm groups showed numerous clinical findings associated with 4MI administration, whereas male rats did not. These treatment-related clinical findings included clonic seizures, unusual stance or gait, and excessive activity manifested as either hyperactivity or excitability. No microscopic lesions were observed in nervous tissues that correlated with the observed behavioral effects. The reason why female F344/N rats are more sensitive to the neurobehavioral effects of 4MI than male F344/N rats is unclear. The neurobehavioral effects displayed by female rats are consistent with those observed in farm animals fed ammoniated hay (Wiggins 1956; Nishie et al. 1970; Morgan and Edwards 1986a, 1986b; Nielsen et al. 1993; Weiss et al. 1986; Perdok and Leng 1987). In male albino mice, a single dose of 4MI induced tremors, restlessness, running, sialorrhea, opisthotonus, Straub tail, and tonic extensor seizure (Nishie et al. 1969). The median convulsant dose (CD50) of 4MI estimated for male albino mice by the authors was 155 mg/kg intraperitoneally and 360 mg/kg orally. In the current 2-year dosed feed study, the 1,250-ppm groups of male and female B6C3F1 mice received 4MI equivalent to 170 mg/kg body weight per day and exhibited no convulsions. In the 14-week toxicity studies of 4MI in feed (Chan et al., 2006), the highest exposure concentration groups of male and female mice (10,000-ppm) received estimated doses of 1,840 and 3,180 mg/kg per day, respectively, and exhibited no convulsions. It is probable that the dosed feed route of administration delivered much less 4MI at any time point compared to the bolus gavage effect shown in a previous study of Nishie et al. (1969).
Mononuclear cell leukemia in F344/N rats constitutes a common background lesion, and the control rate in the current study was similar to that in historical controls; the increased incidence of leukemia in 5,000-ppm female rats slightly exceeded the historical range in feed study controls and, therefore, may be attributed to the effects of 4MI.
The liver was a target organ for 4MI toxicity in rats. Increases in the incidences and severities of several nonneoplastic hepatic lesions occurred in both sexes; these included histiocytosis, chronic inflammation, hepatocytic focal fatty change, and eosinophilic and mixed cell foci of hepatocytes. Cytoplasmic vacuolization of hepatocytes was also observed in male and female rats in the 14-week toxicity study (Chan et al. 2006; NTP 2004a). These lesions may be related to altered lipid metabolism and hepatic injury. The hepatic effects were consistent with increases in activities of serum alanine aminotransferase, sorbitol dehydrogenase, and alkaline phosphatase activities, as well as concentrations of bile acid reported in the 14-week toxicity study (Chan et al., 2006; NTP, 2004a).
Incidences of thyroid gland follicle cyst in 2,500-ppm male rats and thyroid gland follicle mineralization in 5,000-ppm female rats were increased. In addition, the increased incidence of thyroid gland follicular cyst in 1,250-ppm female mice was statistically significant. These cysts are commonly found in aging mice, but the increased incidences may be related to 4MI exposure.
In the 14-week toxicity studies (Chan et al., 2006; NTP 2004a), the 4MI treated male and female rats and mice exhibited no specific changes in serum triiodothyronine (T3), total thyroxine (T4), or thyroid stimulating hormone (TSH) levels that could be attributed to 4MI exposure. There were also no changes observed in thyroid gland histopathology at terminal sacrifice in the 14-week toxicity studies compared with controls. In the 2-year dosed feed studies of 2-methylimidazole (2MI) in rats and mice, increased incidences of thyroid gland follicular cell neoplasm was observed (NTP, 2004b). The thyroid gland carcinogenesis induced by 2MI was probably due to increased UDP-glucuronyl transferase metabolism of T4, which in turn stimulated TSH synthesis and release, leading to neoplastic development in the thyroid gland. Sanders et al. (1998) reported that 2MI enhanced whereas 4MI inhibited hepatic UDP-glucuronyl transferase activity. The inhibitory effects of 4MI on UDP-glucuronyl transferase probably did not affect serum T4 and TSH levels, and as a result exerted no stimulatory effect on TSH synthesis and the thyroid gland. Thus, no neoplastic changes were observed in the thyroid gland.
The incidence of prostate gland inflammation in 2,500-ppm male rats was significantly increased. Increased incidences of chronic focal lung inflammation, heart cardiomyopathy, and focal pancreatic acinus atrophy were also observed in all exposed groups of female rats. The cause of these increases was not clear.
Dose-related decreases in the incidences of benign, complex, or malignant adrenal medulla pheochromocytoma (combined) in male rats, pituitary gland adenoma in the pars distalis in both male and female rats, and clitoral gland adenoma, mammary gland fibroadenoma, and uterine stromal polyp in female rats were probably related to loss of body weight resulting from exposure concentration-related body weight loss. However, using the equations in Haseman et al. (1997) to predict the number of neoplasms that can be explained by body weight (Haseman et al. 1997), there appears a greater decrease in pituitary gland neoplasms in 5,000-ppm female rats than can be attributed to body weight differences alone. Likewise, there is a greater decrease in mammary gland neoplasms in all exposed groups of female rats than can be attributed to body weight differences alone. The effect of 4MI in reducing the incidences of these lesions could not be identified.
The incidences of alveolar/bronchiolar adenoma or carcinoma (combined) were significantly increased in 1,250-ppm male and 625- and 1,250-ppm female mice; these increases were exposure concentration-related. The incidence of lung alveolar epithelial hyperplasia was significantly increased in 1,250-ppm female mice compared with controls. Hyperplasia of the alveolar epithelium is thought to be a precursor to neoplastic development. Interestingly, 4MI had no effect on the respiratory epithelium in the 14-week toxicity study at concentrations as high as 10,000-ppm (NTP, 2004a). Clara cells in the terminal bronchiolar epithelium constitute a cell type from which alveolar/bronchiolar neoplasms are thought to arise. This cytochrome P450-containing cell type is capable of xenobiotic metabolism, whereas other cell types in the lung have little or no cytochrome P450. 4MI is structurally similar to 2- and 3-methylfuran. Both alkyl furans are metabolized in the Clara cell to reactive species, which may account for their pulmonary toxicity. Oxidative metabolism of heterocycles like 4MI has been reviewed by Dalvie et al. (2002). They suggested that oxidative metabolism of imidazoles would lead to at least two reactive intermediates, an epoxide and dicarbonyl compound, and pyruvaldehyde. The general scheme can be adapted to 4MI (Figure 1) (Dalvie et al. 2002). Whether these intermediates are formed or are responsible for the development of alveolar/bronchiolar neoplasms is not known at this time. It should be pointed out that it is unlikely that an alkylating intermediate is involved in mouse lung carcinogenesis in view of the genetic toxicity-study findings that 4MI is not mutagenic in Salmonella typhimurium and does not induce micronuclei in mouse peripheral blood erythrocytes or rat and mouse bone marrow cells. The mechanism of action of 4MI in mouse lung tumorigenesis is not clear.
Figure 1.
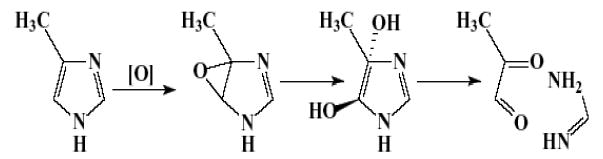
Potential Reactive Intermediates from Metabolism of 4-Methylimidazole (adapted from Dalvie et al., 2002).
Under the condition of the present studies 4MI induced alveolar/bronchiolar adenoma and carcinoma in male and female mice. 4MI also induced clonic seizures and mononuclear cell leukemia in female rats, and hepatic histiocytosis, hepatocellular eosinophilic and mixed cell foci in male and female rats. The related compound methimazole was reported to induce toxic changes in the olfactory epithelium of rats and mice (Bergman and Brittebo 1999; Genter et al. 1995; Jeffry et al. 2006). The nasal cavities of mice and rats exposed to 4MI were checked histologically, but no changes were noted. No other treatment-related changes were noted in any of the organs examined.
Acknowledgments
The authors are grateful to the DIR, NIEHS for support and to Ms. JoAnne Johnson, Dr. Matthew Stout, Dr. Charles Alden, and Dr. Raj Chhabra for critical review of the manuscript.
Contributor Information
P.C. Chan, Toxicology Operation Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina 27709
G. D Hills, Integrated Laboratory Systems, P.O. Box 13501, Research Triangle Park, North Carolina 27709.
G.E. Kissling, Biostatistics Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina 27709
A Nyska, Tel Aviv University, Tel Aviv and Toxicologic Pathologist, Timrat Israel.
References
- Bailer AJ, Portier CJ. Effects of treatment-induced mortality and tumor-induced mortality on tests for carcinogenicity in small samples. Biometrics. 1988;44:417–431. [PubMed] [Google Scholar]
- Bergman U, Brittebo EB. Methimazole toxicity in rodents: covalent binding in the olfactory mucosa and detection of glial fibrillary acidic protein in the olfactory bulb. Toxicol Appl Pharmacol. 1999;155:190–200. doi: 10.1006/taap.1998.8590. [DOI] [PubMed] [Google Scholar]
- Chan P, Mahler J, Travlos G, Nyska A, Wenk M. Induction of thyroid lesions in 14-week toxicity studies of 2 and 4-methylimidazole in Fischer 344/N rats and B6C3F1 mice. Arch Toxicol. 2006;80:168–180. doi: 10.1007/s00204-005-0018-4. [DOI] [PubMed] [Google Scholar]
- Chappel CI, Howell JC. Caramel colours — a historical introduction. Food Chem Toxicol. 1992;30:351–357. doi: 10.1016/0278-6915(92)90060-x. [DOI] [PubMed] [Google Scholar]
- Chemical Economic Handbook. 1995. Online. Dialog. 359. Bibliographic database. [Google Scholar]
- Chemical Dynamics, Corp. Catalog/Handbook of Biochemicals, Organic Chemicals and Inorganic Chemicals. Chemical Dynamics, Corp.; South Plainfield, NJ: 1989. The 1989-90 Chemalog. [Google Scholar]
- Cox DR. Regression models and life-tables. J R Stat Soc. 1972;B34:187–220. [Google Scholar]
- Dalvie DK, Kalgutkar AS, Khojasteh-Bakht SC, Obach RS, O’Donnell JP. Biotransformation reactions of five-membered aromatic heterocyclic rings. Chem Res Toxicol. 2002;15:269–299. doi: 10.1021/tx015574b. [DOI] [PubMed] [Google Scholar]
- Fairbrother TE, Kerr LA, Essig HW. Effects of 4-methylimiazole in young calves. Vet Hum Toxicol. 1987;29:312–315. [PubMed] [Google Scholar]
- Genter MB, Deamer NJ, Blake BL, Wesley DS, Levi PE. Olfactory toxicity of methimazole: dose-response and structure-activity studies and characterization of flavin-containing monooxygenase activity in the Long-Evans rat olfactory mucosa. Toxicol Pathol. 1995;23:477–486. doi: 10.1177/019262339502300404. [DOI] [PubMed] [Google Scholar]
- Haseman JK, Young E, Eustis SL, Hailey JR. Body weight-tumor incidence correlations in long-term rodent carcinogenicity studies. Toxicol Pathol. 1997;25:256–263. doi: 10.1177/019262339702500302. [DOI] [PubMed] [Google Scholar]
- Hollander M, Wolfe DA. Nonparametric Statistical Methods. John Wiley and Sons; New York: 1973. pp. 120–123. [Google Scholar]
- Huang Y, Zhang S, Yang R. Survey of communal industrial production of caramel by the ammonium process. Tiaowei Fushipin Keji. 1983;3:11–12. Abstr. [Google Scholar]
- Jeffry AM, Iatropoulos MJ, Williams GM. Nasal cytotoxic and carcinogenic activities of systemically distributed organic chemicals. Toxicol Pathol. 2006;34:827–852. doi: 10.1080/01926230601042494. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Mattyasovszky P, Jeszenszky Z. Determination of caramels in wines by gel chromatography and gas chromatography. Borgazdasag. 1985;33:105–110. Abstr. [Google Scholar]
- Moree-Testa P, Saint-Jalm Y, Testa A. Identification and determination of imidazole derivatives in cigarette smoke. J Chromatogr. 1984;290:263–274. [Google Scholar]
- Morgan SE, Edwards WC. Bovine bonkers: New terminology for an old problem. A review of toxicity associated with ammoniated feeds. Vet Hum Toxicol. 1986a;28:240–242. [PubMed] [Google Scholar]
- Morgan SE, Edwards WC. Pilot studies in cattle and mice to determine the presence of 4-methylimidazole in milk after oral ingestion. Vet Hum Toxicol. 1986b;28:240–242. [PubMed] [Google Scholar]
- National Toxicology Program (NTP) Toxicity Studies of 2- and 4-Methylimidazole (CAS Nos. 693-98-1 and 822-36-6) Administered in Feed to F344/N Rats and B6C3F1 Mice. Toxicity Report Series No. 67. NIH Publication No. 04-4409. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; Research Triangle Park, NC: 2004a. [Google Scholar]
- National Toxicology Program (NTP) NTP Technical Report on the Toxicology and Carcinogenesis Studies of 2-Methylimidazole (CAS No. 693-98-1) in F344/N Rats and B6C3F1 Mice (Feed Studies). Technical Report Series No. 516. NIH Publication No. 05-4456. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; Research Triangle Park, NC: 2004b. [PubMed] [Google Scholar]
- National Toxicology Program (NTP) NTP Technical Report on the Toxicology and Carcinogenesis Studies of 4-mMethylimidazole (CAS No. 822-36-6) in F344/N Rats and B6C3F1 Mice (Feed Studies). Technical Report Series No. 535. NIH Publication No. 05-4471. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; Research Triangle Park, NC: 2006. [Google Scholar]
- Nielsen P, Friis C, Kraul I, Olsen CE. Disposition of 4-methylimidazole in goats and heifers. Res Vet Sci. 1993;54:72–79. doi: 10.1016/0034-5288(93)90014-7. [DOI] [PubMed] [Google Scholar]
- Nishie K, Waiss AC, Jr, Keyl AC. Toxicity of methylimidazoles. Toxicol Appl Pharmacol. 1969;14:301–307. doi: 10.1016/0041-008x(69)90111-2. [DOI] [PubMed] [Google Scholar]
- Nishie K, Waiss AC, Jr, Keyl AC. Pharmacology of alkyl and hydroxyalkylpyrazines. Toxicol Appl Pharmacol. 1970;17:244–249. doi: 10.1016/0041-008x(70)90149-3. [DOI] [PubMed] [Google Scholar]
- Perdok HB, Leng RA. Hyperexcitability in cattle fed ammoniated roughages. Animal Feed Sci Technol. 1987;17:121–143. [Google Scholar]
- Piegorsch WW, Bailer AJ. Statistics for Environmental Biology and Toxicology, Section 6.3.2. Chapman and Hall; London: 1997. [Google Scholar]
- Portier J, Bailer AJ. Testing for increased carcinogenicity using a survival-adjusted quantal response test. Fundam Appl Toxicol. 1989;12:731–737. doi: 10.1016/0272-0590(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Ray C, Raisor MJ, Herd DB, Murphy MJ, Reagor JC. Methylimidazole content of ammoniated forages associated with toxicity in cattle. American Association of Veterinary Laboratory Diagnosticians 27th Annual Proceedings. 1984:337–348. [Google Scholar]
- Sakuma H, Kusama M, Yamaguchi K, Matsuki T, Sugawara S. The distribution of cigarette smoke components between mainstream and sidestream smoke. II. Bases. Beitr Tabakforsch Int. 1984;22:199–209. Abstr. [Google Scholar]
- Sanders JM, Griffin RJ, Burka LT, Matthews HB. Disposition of 2-methylimidazole in rats. J Toxicol Environ Health A. 1998;54:121–132. doi: 10.1080/009841098158953. [DOI] [PubMed] [Google Scholar]
- Tarone RE. Tests for trend in life table analysis. Biometrika. 1975;62:679–682. [Google Scholar]
- Tierney WJ. A four-week dose range-finding study of Caramel Colour No 3, sample 3-1, in mice (1987). Cited in WHO Food Additive Series 20, The 29th Meeting of the Joint FAO/WHO Expert Committee on Food Additives; 1987; 1979. p. 147. [Google Scholar]
- Weiss WP, Conrad HR, Martin CM, Cross RF, Shockey WL. Etiology of ammoniated hay toxicosis. J Animal Sci. 1986;63:525–532. doi: 10.2527/jas1986.632525x. [DOI] [PubMed] [Google Scholar]
- Wiggins LF. Some recent studies on ammoniated molasses. Sugar J. 1956;18:18–20. [Google Scholar]
- Wong J, Bernhard RA. Effect of nitrogen source on pyrazine formation. J Agric Food Chem. 1988;36:123–129. [Google Scholar]
- Yoshikawa S, Fujiwara M. Determination of 4(5)-methylimidazole in food by thin layer chromatography. J Food Hyg Soc Jpn. 1981;22:189–196. Abstr. [Google Scholar]


